Abstract
Our previous results showed that β-cyclodextrin–hemin complex (CDH) exhibited a vital protective role against cadmium-induced oxidative damage and toxicity in alfalfa seedling roots by the regulation of heme oxygenase-1 (HO-1) gene expression. In this report, we further test whether CDH exhibited the hormonal-like response. The application of CDH and an inducer of HO-1, hemin, were able to induce the up-regulation of cucumber HO-1 gene (CsHO1) expression and thereafter the promotion of adventitious rooting in cucumber explants. The effect is specific for HO-1 since the potent HO-1 inhibitor zinc protoporphyrin IX (ZnPP) blocked the above responses triggered by CDH, and the inhibitory effects were reversed further when 30 % saturation of CO aqueous solution was added together. Further, molecular evidence showed that CDH triggered the increases of the HO-1-mediated target genes responsible for adventitious rooting, including one DnaJ-like gene (CsDNAJ-1) and two calcium-dependent protein kinase (CDPK) genes (CsCDPK1 and CsCDPK5), and were inhibited by ZnPP and reversed by CO. The calcium (Ca2+) chelator ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) and the Ca2+ channel blocker lanthanum chloride (LaCl3) not only compromised the induction of adventitious rooting induced by CDH but also decreased the transcripts of above three target genes. However, the application of ascorbic acid (AsA), a well-known antioxidant in plants, failed to exhibit similar inducible effect on adventitious root formation. In short, above results illustrated that the response of CDH in the induction of cucumber adventitious rooting might be through HO-1-dependent mechanism and calcium signaling.
Key message Physiological, pharmacological and molecular evidence showed that β-cyclodextrin–hemin complex (CDH) was able to induce cucumber adventitious rooting through heme oxygenase-1 (HO-1)-dependent mechanism and calcium signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Normally, adventitious roots are derived from the stems and leaves, and from non-pericyclic tissues in old roots. It was further suggested that adventitious root formation plays important roles in providing the absorption area for plants to absorb water and other nutrients as well as to anchor the plants in the soil (Sorin et al. 2005; Lanteri et al. 2006; Den Herder et al. 2010). Therefore, understanding the regulation of adventitious root formation is of vital agronomic importance.
It was well established that many environmental factors, endogenous modulators including the second messengers are able to regulate adventitious root formation. Some of these endogenous factors have been elucidated, such as auxin (Sorin et al. 2005), hydrogen peroxide (H2O2) (Li et al. 2009), nitric oxide (NO) (Pagnussat et al. 2002), heme compounds (hematin and hemin) and carbon monoxide (CO) (Xu et al. 2006), hydrogen sulfide (H2S) (Lin et al. 2012), phosphatidic acid (Lanteri et al. 2008), calcium (Ca2+) and calcium-dependent protein kinase (CDPK) (Bellamine et al. 1998; Lanteri et al. 2006; Chen and Kao 2012), cyclic GMP (Pagnussat et al. 2003), mitogen-activated protein kinase (MAPK) (Pagnussat et al. 2004), and N-acyl homoserine-lactones (AHLs) (Bai et al. 2012). However, the molecular, biochemical and physiological pathways participating between corresponding signal transduction and adventitious root formation are less understood.
Heme oxygenase (HO, EC 1.14.99.3) catalyzes the degradation of heme into biliverdin IXα (BV), a well-known antioxidant, with the concomitant release of CO and iron (Fe2+), in a reaction requiring molecular oxygen and electrons from NADPH (Dulak and Józkowicz 2003; Otterbein et al. 2003; Bauer et al. 2008). There are three HO isozymes existing in mammals: the inducible form HO-1, the constitutively expressed HO-2, and HO-3 isozyme with a very low activity. Until now, much attention has been paid to HO-1 in mammals because it is associated with heme degradation and the antioxidant machinery (Ryter et al. 2002, 2006). Meanwhile, ample evidence in plants has recently confirmed that HO-1 mediates various physiological and biochemical processes (Shekhawat and Verma 2010), including phytochrome chromophore biosynthesis (Davis et al. 2001), senescence (Huang et al. 2011), programmed cell death (Wu et al. 2011), seed germination (Liu et al. 2010), stomatal closure (Cao et al. 2007a), adventitious root (Xu et al. 2006; Xuan et al. 2008; Li et al. 2011) and lateral root formation (Cao et al. 2007b; Chen and Kao 2012; Chen et al. 2012). Some of HO-1 functions could be attributed to its antioxidant behaviors (Noriega et al. 2004; Han et al. 2008; Xie et al. 2008, 2011a; Zilli et al. 2009; Xu et al. 2011a). Interestingly, we recently identified BnHO-1, a HO-1 gene from Brassica napus that is required for salinity and osmotic stress-induced lateral rooting, with a possible interaction with auxin signaling (Cao et al. 2011). More recently, similar function of HO-1 from Zea mays in the induction of lateral root formation was also reported (Han et al. 2012).
Hemin, a heme (ferroprotoporphyrin IX) compound, is a potent HO-1 inducer. It was well established that hemin was able to exert numerous beneficial physiological functions in animals, including inhibiting lipid peroxidation (Jung et al. 1997), preventing D-galactosamine and lipopolysaccharide-induced acute hepatic injury (Wen et al. 2007), controlling liver allograft failure (Dellon et al. 2002), and the induction of a host defense response against HIV infection (Devadas and Dhawan 2006). Similarly, some recent investigations of the functional roles of hemin in plants, including the improvement of salinity tolerance (Xu et al. 2011b), the induction of root segments elongation (Xuan et al. 2007), adventitious root and lateral root formation (Xuan et al. 2008; Cao et al. 2011), have greatly extended our understanding of physiological roles of hemin and corresponding signaling as a cellular defense mechanism against abiotic stresses-related as well as various other developmental processes in plants. However, the poor solubility of hemin in neutral aqueous solution and organic solvents limits its application. To cope with this problem, a soluble complex by combining an outstanding embedding medium β-cyclodextrin (β-CD) with hemin (β-cyclodextrin–hemin, β-CD-hemin, CDH) was successfully synthesized and applied (Huang et al. 1999; Liu et al. 1999).
Previously, we showed that the pretreatment of alfalfa seedling with CDH enhanced the capacity of alfalfa plants to withstand cadmium (Cd) toxicity and its derived oxidative damage partly by lowering the Cd accumulation in alfalfa seedling roots (Fu et al. 2011). In this context, we expanded our former findings, and further showed that the soluble CDH was able to induce cucumber adventitious rooting by the up-regulation of HO-1 gene expression. This is also in accordance with our previous work reporting the involvement of HO-1/CO in the auxin-induced adventitious root formation (Xuan et al. 2008). Furthermore, evidence is provided to show that calcium signaling is involved in CDH-induced adventitious root formation in cucumber explants.
Materials and methods
Chemicals
All chemicals were obtained from Sigma (St Louis, MO, USA) unless stated otherwise. According to previous report with minor modification (Liu et al. 1999), hemin and β-CD with a suitable molar ratio were mixed by grinding for 60 min after the addition of de-ionized water. The mushy mixture was then freeze-dried, and all products were sieved and used. The brown powder was named as β-CD-hemin (CDH). Afterwards, a stock standard solution (10−4 M, calculated based on the concentration of hemin) was prepared by simply dissolving 1.63 g of CDH (0.1 % hemin) in 25 mL of de-ionized water. The stock solution was used immediately. The corresponding hemin (0.1 μM) or β-CD (50 μM) was, respectively, regarded as the control of 0.1 μM CDH (complex of 0.1 μM hemin and 50 μM β-CD), which displayed the maximal inducible effect in the induction of adventitious root formation. Zinc protoporphyrin IX (ZnPP), a specific inhibitor of HO-1 (Xuan et al. 2008; Cao et al. 2011), was used at 10 μM. Naphthaleneacetic acid (NAA) was used at 10 μM (Lanteri et al. 2008). N-1-Naphthylphthalamic acid (NPA) from Chem Service (West Chester, PA, USA) was regarded as the auxin transport inhibitor at 10 μM (Xuan et al. 2008). Both bilirubin (BR) and FeSO4·7H2O (Fe2+) were used as the catalytic by-products of HO at a concentration of 10 μM, respectively. The Ca2+ chelator ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) and the Ca2+ channel blocker lanthanum chloride (LaCl3; Lanteri et al. 2006) were also used. Ascorbic acid (AsA), a well-known antioxidant, was applied at concentrations of 1, 10, and 100 μM.
CO aqueous solution preparation
The preparation of CO aqueous solution was carried out according to the method described previously (Xuan et al. 2008). The saturated stock solution (100 % saturation) was diluted immediately with distilled water to the concentration required with a maximal inducible response (30 % saturation [v/v]).
Plant material and growth conditions
Cucumber (Cucumis sativus ‘Lufeng’) seeds were kindly supplied by Jiangsu Agricultural Institutes, Jiangsu Province, China. Selected identical seeds were germinated in petri dishes on filter papers imbibed in distilled water, then transferred to an illuminating incubator and maintained at 25 ± 1 °C for 5 d with a 14-h photoperiod at 200 μmol m−2 s−1 intensity. Cucumber seedlings were used decapitated by excising the apical bud immediately above the cotyledons and incubated in the presence of 10 μM NPA (auxin-depleted) for 48 h, before removing primary root. Cucumber explants were then maintained under the same conditions of temperature and photoperiod for another 4 days or the indicated time points in the presence of different media as indicated.
Explant treatments
After primary roots were removed, every eight cucumber explants were put into a petri dish containing 10 mL of distilled water, varying concentrations of CDH or AsA, 10 μM NAA, 10 μM NPA, 0.1 μM or 100 μM hemin, 50 μM β-CD, 10 μM ZnPP, 30 % saturation of CO aqueous solution, 10 μM BR, 10 μM FeSO4·7H2O (Fe2+), 1 mM CaCl2, 100 μM EGTA, 100 μM LaCl3, either alone or in combination, and kept at 25 ± 1 °C for 4 days or different time periods according to the experimental design. Previous studies (Pagnussat et al. 2002, 2003, 2004; Lanteri et al. 2006, 2008; Xuan et al. 2008; Cao et al. 2011) and our pilot experiments showed that the concentrations and the time of treatments with these chemicals are suitable for investigating the role of HO-1/CO in the root developmental signaling. Finally, excised cucumber hypocotyls (5-mm long segments of the hypocotyl base, where adventitious root develops; Lanteri et al. 2008) were used for the following determination.
Western blot analysis for CsHO1
Rabbit polyclonal antibody was made against the mature cucumber HO-1 (mCsHO1) expression in E. coli (Li et al. 2011). Sixty micrograms of protein from homogenates was subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis using a 12.5 % acrylamide resolving gel (Mini Protean II System, BioRad, Hertz, UK). Separated proteins were then transferred to PVDF membranes and non-specific binding of antibodies was blocked with 5 % non-fat dried milk in PBS (pH 7.4) for 2 h at room temperature. Membranes were then incubated overnight at 4 °C with primary antibody raised against mCsHO1 diluted 1:3,000 in PBS buffer. Immune complexes were detected using horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG. The color was developed with a solution containing 3,3′-diaminobenzidine tetrahydrochloride (DAB) as the HRP substrate.
Real-time RT-PCR analysis
Total RNA was isolated from 100 mg (fresh-weight) of excised cucumber hypocotyls by grinding with mortar and pestle in liquid nitrogen until a fine powder appeared and using Trizol reagent (Invitrogen, Gaithersburg, MD) according to the manufacturer’s instructions. DNA-free total RNA (5 μg) from different treatments was used for first-strand cDNA synthesis in a 20-μL reaction volume containing 2.5 U of AMV reverse transcriptase XL (TaKaRa, Bio Inc., China) and 2.5 μM random primer.
Real-time quantification RT-PCR reactions were performed using a Mastercycler® ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) with SYBR® Premix Ex Taq™ (TaKaRa Bio Inc., China) according to the manufacturer’s instructions. The cDNA was amplified using the following primers: for CsActin (accession number AB010922.1), forward 5′-AGATGACGCAGATAATGTTT-3′ and reverse 5′-ATCACCAGAATCCAGCAC-3′; for CsHO1 (accession number HQ198046.1), forward 5′-GGAGTCACCTATGCTCGTTA-3′ and reverse 5′-CTTTCGCCCAATCATTCTAC-3′; for CsDNAJ-1 (accession number X67695), forward 5′-CGACACTGTTACTGGGGACA-3′ and reverse 5′-GACGAGAGACAAGGTATGCT-3′; for CsCDPK1 (accession number AJ312239), forward 5′-GTAAGACCATCCCCAAG-3′ and reverse 5′-CTCTCCACCCTCACAAA-3′; and for CsCDPK5 (accession number AY027885), forward 5′-TTCTGGCTCGTCCCTTTTC-3′ and reverse 5′- CCTGTTTCGTTTCCTTGTG-3′. Relative expression levels were presented as values relative to that of corresponding control sample at the indicated times, after normalization to CsActin transcript levels.
Statistical analysis
Where indicated, results were expressed as the mean values ± SE of at least three independent experiments (n = 24). Statistical analysis was performed using SPSS Statistics 17.0 software. For statistical analysis, t test (P < 0.05) was chosen as appropriate.
Results
Effects of CDH, hemin and β-CD on cucumber adventitious rooting
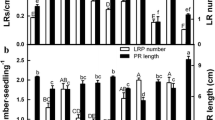
Two parameters, the number and length of adventitious roots per explant, were used to detect adventitious root formation in controls and the explants treated with CDH, hemin and β-CD 4 days after the primary root removal. The results in Fig. 1 illustrated that exogenous CDH with the concentrations of 0.01 and 0.1 μM could promote the formation of adventitious rooting and its effects were dose-dependent, with a maximal response at 0.1 μM CDH (P < 0.05). Meanwhile, a slight but no significant increase in adventitious rooting was observed when 1 μM CDH was applied. However, 10 μM CDH could obviously inhibit adventitious root formation (P < 0.05), and 100 μM CDH was toxic to the explants. To assess the nature of the CDH-triggered adventitious root formation, the responses of hemin and β-CD were investigated. Interestingly, similar concentration of hemin (0.1 μM, data not shown) or β-CD (50 μM) compared with those in 0.1 μM CDH (complex of 0.1 μM hemin and 50 μM β-CD) had no significant effect on the induction of adventitious root formation. Meanwhile, a similar dose-dependent induction of adventitious rooting was observed when hemin was added exogenously (Xuan et al. 2008), and with a maximal response at 100 μM (some data not shown). We also noticed that 100 μM hemin response was greater in comparison with the effect of 0.1 μM CDH.
Effects of β-CD-hemin (CDH), hemin and β-CD on the induction of cucumber adventitious root formation. Cucumber seedlings were used immediately after decapitated by excising the apical bud above the cotyledons, and incubated in the presence of 10 μM NPA (auxin-depleted) for 2 days before removing the primary root. Then explants were maintained for another 4 days in the presence of different concentrations of CDH, hemin (100 μM) and β-CD (50 μM). Photographs were then taken (a). Bar = 0.5 cm. Meanwhile, the root number and length per explants were recorded (b). Mean and SE values were calculated from at least three independent experiments (n = 24). ND none detected. Within each set of experiments, bars with different letters were significantly different at P < 0.05 according to t test
Time course of CsHO1 transcripts in response to CDH, hemin, β-CD and NAA
To assess if HO-1 is associated with the CDH and hemin response leading to adventitious rooting, the expression level of the CsHO1 transcripts was analyzed by real-time RT-PCR. Figure 2 showed that the application of CDH and hemin brought about the increases of CsHO1 transcripts at 12 h of treatments, followed by decrease until 24 h of incubation. However, no significant difference in β-CD-treated sample was observed, in comparison with the control sample during the similar treatment period. As expected (Xuan et al. 2008), similar inducible pattern of CsHO1 transcript was observed when NAA was added, suggesting a possible link between auxin and CsHO1 in the induction of adventitious rooting.
Time course of CsHO1 transcripts in auxin-depleted cucumber explants. Explants were treated with water (Con), NAA (10 μM), CDH (0.1 μM), hemin (100 μM) and β-CD (50 μM) for 24 h. The expression levels of the CsHO1 transcripts were analyzed by real-time RT-PCR, and presented as values relative to the control at 0 h. Mean and SE values were calculated from three independent experiments
CDH-, hemin- and CO-induced adventitious rooting were sensitive to NPA
To further confirm above possibility, NPA, the auxin transport inhibitor, was also used together with CDH, hemin or CO in auxin-depleted explants. Results of Fig. 3 showed that in comparison with the auxin-depleted explants (Con), the addition of NPA brought about the obvious reduction in adventitious root length, although a slight but no significant decrease was observed in the changes of adventitious root number. However, although the auxin-depletion-induced inhibition of adventitious root formation was significantly restored by CDH, hemin (in particular) and CO, the simultaneously added NPA was able to differentially reverse above responses, suggesting that the possible role of auxin in CsHO1 mediated the induction of adventitious rooting process.
Effects of NPA on CDH-, hemin-, and CO-induced cucumber adventitious rooting. Auxin-depleted explants were incubated with water (Con), CDH (0.1 μM), hemin (100 μM), CO aqueous solution (30 % saturation) and NPA (10 μM), either alone or in the combination for 4 days. Then, photographs were also taken (a), bar = 0.5 cm. Meanwhile, adventitious root number and length per explant were recorded (b). Mean and SE values were calculated from at least three independent experiments (n = 24). Within each set of experiments, bars with different letters were significantly different at P < 0.05 according to t test
Induction of adventitious root formation, CsHO1 transcripts and its protein levels were sensitive to ZnPP, but only reversed by CO
To test the hypothesis that HO-1 was involved in CDH-induced adventitious rooting process, the potent HO-1 inhibitor ZnPP confirmed in plants recently (Xuan et al. 2008; Cao et al. 2011) was applied exogenously. In the subsequent experiment, treating cucumber explants with 10 μM ZnPP was able to prevent the induction role of CDH on adventitious root formation (Fig. 4), whereas the addition of 30 % saturated aqueous solution of CO could block the response of ZnPP. For example, the inhibition of the number and length of adventitious root triggered by ZnPP together with CDH was relieved and returned to a similar extent to that exhibited in cucumber explants treated with CDH or CO alone, respectively. However, no significant responses of BR and Fe2+ regardless of whether applied alone or together with CDH plus ZnPP were observed (Fig. 4), suggesting that CDH-induced response was in a CO-dependent, but BR- and Fe2+-independent fashion. Interestingly, a slight but no significant decrease of adventitious rooting appeared in ZnPP-treated alone explants.
Effects of ZnPP, Fe2+, BR and CO on CDH-induced cucumber adventitious rooting. Auxin-depleted explants were incubated with water (Con), CDH (0.1 μM), ZnPP (10 μM), Fe2+ (FeSO4·7H2O, 10 μM), BR (10 μM) and CO aqueous solution (30 % saturation), either alone or in combination for 4 days. Then, photographs were also taken, bar = 0.5 cm (a). Meanwhile, adventitious root number and length per explant were recorded (b). Mean and SE values were calculated from at least three independent experiments (n = 24). Within each set of experiments, bars with asterisks were significantly different at P < 0.05 according to t test
In order to study a possible link between CsHO1 and CDH-mediated adventitious root formation signaling, real-time RT-PCR combined with immunoblot analysis was performed. In the presence of ZnPP added exogenously, CDH-induced CsHO1 transcript and corresponding protein level in cucumber explants were significantly lower than those of CDH-treated alone samples (Fig. 5a, b and c). However, when a 30 % saturated aqueous solution of CO was applied together with ZnPP in 0.1 μM CDH-treated sample, the decreases of CsHO1 transcripts and its protein level were blocked significantly. As a positive control, the inducible expression of CsHO1 gene expression was observed when hemin (especially) or CO was added exogenously, separately. In addition, application of ZnPP alone for 12 h brought about an obvious decrease in the CsHO1 gene expression, both in mRNA and protein levels. Interestingly, all above results match adventitious root formation in cucumber explants treated for 4 days as mentioned previously (Fig. 4).
Effects of CDH, ZnPP, CO, hemin and β-CD on CsHO1, CsDNAJ-1 and CsCDPK1/5 transcripts and CsHO1 protein levels. Auxin-depleted cucumber explants were incubated with water (Con), CDH (0.1 μM), ZnPP (10 μM), CO aqueous solution (30 % saturation) and hemin (100 μM) alone, or in combination for 12 h (a, b, c) or 24 h (d). Then, corresponding gene expression was analyzed by real-time RT-PCR (a and d), and presented as values relative to the control. Mean and SE values were calculated from three independent experiments. Within each set of experiments, bars with different letters were significantly different in comparison with the control at P < 0.05 according to t test. b CsHO1 protein level was determined by western blot. The number below the band illustrates the relative abundance of the CsHO1 protein compared with that of the control sample (100 %). The blot was representative of three blots with a similar tendency. c Coomassie Brilliant Blue-stained gels were present to show that equal amounts of proteins were loaded
Expression profiles of CsDNAJ-1 and CsCDPK1/5 genes
According to a previous report, the auxin-induced adventitious root formation is mediated by HO-1/CO signaling system and required the participation of up-regulation of several target genes, including CsDNAJ-1 and CsCDPK1/5 (Xuan et al. 2008). In the subsequent text, molecular evidence illustrated that CDH and hemin could induce higher expression of the CsDNAJ-1 and CsCDPK1/5 transcripts after 24 h of treatments (Fig. 5d), and these changes were consistent with the number and length of adventitious root observed after another 3-day treatment (Figs. 1, 4), whereas the CDH-induced expression of CsDNAJ-1 and CsCDPK1/5 was prevented in explants treated with the specific inhibitor of HO-1 ZnPP. When a 30 % saturated aqueous solution of CO was applied together with ZnPP in CDH-treated explants, the decreases of CsDNAJ-1 and CsCDPK1/5 transcripts were restored. Meanwhile, the inhibition of adventitious root formation was also relieved (Fig. 4), further strengthening the hypothesis that CO produced by CsHO1 might be involved in CDH-induced adventitious root formation. In addition, the contrasting responses (down-regulation or up-regulation) in above target genes were confirmed when ZnPP or CO (especially) was applied alone. However, no significant differences were found in β-CD-treated samples with respect to the control samples.
Ca2+ might be required for CDH-induced adventitious rooting, and the up-regulation of CsDNAJ-1 and CsCDPK1/5 transcripts
In order to investigate whether Ca2+ is related to the CDH-induced adventitious rooting, and the up-regulation of CsDNAJ-1 and CsCDPK1/5 transcripts, the Ca2+ chelator ethylene glycol-bis (2-aminoethylether)-N,N,N’,N’-tetraacetic acid (EGTA) and the Ca2+ channel inhibitor lanthanum chloride (LaCl3) were used. Figure 6a showed that in comparison with the CDH-treated alone samples, the two compounds significantly blocked the CDH-induced adventitious rooting. Meanwhile, the up-regulation of CsDNAJ-1 and CsCDPK1/5 gene expression was also reversed (Fig. 6b). However, only a slight but no significant decrease in adventitious root formation was observed when EGTA or LaCl3 was applied alone (Fig. 6a), and the responses of LaCl3 were consistent with the significant down-regulation of CsDNAJ-1 and CsCDPK1/5 (Fig. 6b). Further experiments showed that the addition of CaCl2 plus CDH could strength the induction of adventitious root development as well as the increases of CsDNAJ-1 and CsCDPK1/5 transcripts. Comparatively, the weaker but also significant responses were observed when CaCl2 was applied alone with respect to the control samples. These results suggested the involvement of Ca2+ in CDH-induced adventitious rooting.
Effects 1 of CDH, CaCl2, EGTA and LaCl3 on cucumber adventitious rooting, CsDNAJ-1 and CsCDPK1/5 transcripts. Auxin-depleted cucumber explants were incubated with water (Con), CDH (0.1 μM), CaCl2 (1 mM), EGTA (100 μM) and LaCl3 (100 μM) alone, or in combination for 4 days (a) or 24 h (b). Then, the adventitious root number and length per explant were recorded (a). The amount of corresponding genes expression was analyzed by real-time RT-PCR, and presented as values relative to the control (b). Mean and SE values were calculated from three independent experiments. Within each set of experiments, bars with different letters were significantly different at P < 0.05 according to t test
Ascorbic acid fails to influence adventitious root formation
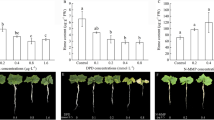
Previous evidence confirmed that HO-1 in plants was induced by a variety of oxidative stress, and thus exhibited antioxidant behaviors (Shekhawat and Verma 2010). To evaluate the possible role of antioxidant or prooxidant in adventitious root formation, ascorbic acid (AsA), a non-enzymatic antioxidant or scavenger of free radicals and peroxides, was used in our experiment conditions. As expected, AsA with various concentrations ranging from 1 to 100 μM was not able to influence adventitious root formation in the presence of 0.1 μM CDH (Fig. 7). Meanwhile, no significant difference was observed in the AsA-treated alone explants, in comparison with the control sample.
Effects of ascorbic acid (AsA) and CDH on cucumber adventitious root formation. Auxin-depleted cucumber explants were treated with water, CDH (0.1 μM) and various concentrations of AsA, either alone or in combination for 4 days. The root number and length per explant were recorded. Mean and SE values were calculated from three independent experiments (n = 24). Within each set of experiments, bars with different letters were significantly different at P < 0.05 according to t test
Discussion
Root system development is crucial for the plants to reach optimal growth and is sure to contribute to the levels of crop yield. Thus, root architecture, including adventitious rooting, was regarded as one of the promising features of crops enabling a very much needed new green revolution (Den Herder et al. 2010). In this study, we provide evidence supporting the involvement of HO-1 in CDH-induced adventitious root formation. The following results obtained from biochemical, pharmacological and physiological experiments support this conclusion: (1) exogenously applied CDH and hemin induced adventitious root formation (Fig. 1); (2) similar to the action of NAA, the up-regulation of HO-1 in response to CDH and hemin was rapid and transient (Fig. 2), and we also noticed that the up-regulation of CsHO1 apparently preceded adventitious root formation; (3) induction of adventitious rooting as well as CsHO1 transcripts and its protein levels triggered by CDH were sensitive to the potent inhibitor of HO-1 ZnPP, but reversed further only by the addition of CO aqueous solution (Figs. 4 and 5a, b). Therefore, this work expanded our previous investigation concerning the antioxidative behavior of CDH in Cd-treated alfalfa seedling roots (Fu et al. 2011), and showed for the first time, as we know, that CDH exhibits the hormone-like effect in plants. In view of the fact that no such inducible response was discovered using β-CD (Fig. 1), we further deduced that the hormone-like effect of CDH was hemin dependent and β-CD independent. In addition, the possibility of auxin’s role (transport, etc.) in CsHO1 that mediated the induction of adventitious rooting process could not be easily ruled out (Fig. 3).
As expected, we demonstrated that, similar to NAA response, both hemin and CDH could induce CsHO1 transcripts (Fig. 2), which could be explained by the fact that hemin is an inducer of HO-1, and CDH is a soluble complex of hemin and β-CD. This finding is in accordance with our recent work reporting that the up-regulation of HO-1 gene expression was triggered by the application of CDH in Cd-treated alfalfa seedling roots (Fu et al. 2011). Some evidence in animals showed that hemin could prevent oxidative damage by up-regulating the HO-1 gene expression (Wen et al. 2007). Amounting evidence further suggested that HO serves as a protective gene by virtue of the anti-inflammatory, anti-apoptotic and anti-proliferative actions of one or more of three catalytic by-products, CO, BV and free iron (Otterbein et al. 2003). As a signaling molecule, CO was confirmed to play an important role in auxin-triggered adventitious root formation (Xuan et al. 2008). We also discovered that AsA failed to influence adventitious root formation in cucumber explants (Fig. 7). In view of the fact that the application of CO could reverse the inhibitory roles of ZnPP in the induction of adventitious root formation (Fig. 4), we further deduced that the induction of cucumber adventitious root formation is specific for CDH-mediated up-regulation of HO-1 and, at least partially, its catalytic product (CO), rather than its induced antioxidant behaviors.
It is well known that DnaJ-like gene was induced during root initiation and formation triggered by auxin, suggesting its phase-specific changes during the cell cycle in G2/M (Frugis et al. 1999). In cucumber, there have been cloned and characterized one full-length cDNA (CsCDPK5) encoding a putative CDPK isoform (Kumar et al. 2004). The isolation of five cDNAs encoding cucumber CDPK (CsCDPK1-4) was also previously reported (Ullanat and Jayabaskaran 2002). Furthermore, Lamattina research group provided evidence that Ca2+ and CDPK activity are downstream messengers in the signaling pathway triggered by auxin and NO to promote cucumber adventitious root formation (Lanteri et al. 2006). In the subsequent work, we observed that CDH treatment for 24 h induced higher expression of CsDNAJ-1 and CsCDPK1/5 (Fig. 5d), the target genes responsible for HO-1/CO-induced adventitious rooting (Xuan et al. 2008), and these were in agreement with the number and length of adventitious root observed after another 3-d treatment (Fig. 4b). Further evidence illustrated that the addition of the specific inhibitor of HO-1 ZnPP not only inhibited the induction of adventitious root formation induced by CDH but also down-regulated the transcripts of CsDNAJ-1 and CsCDPK1/5 in cucumber explants (Fig. 5). However, above inhibitory effects could be reversed when CO aqueous solution was added together with ZnPP. Therefore, we further deduced that CsDNAJ-1 and CsCDPK1/5 might be the target genes for the induction of adventitious rooting triggered by CDH.
The involvement of Ca2+ in auxin and NO signaling responsible for adventitious root or lateral root formation in plants have been well elucidated (Bellamine et al. 1998; Lanteri et al. 2006; Chen and Kao 2012). Ca2+ was confirmed to be involved fundamentally in cell division and in the root primordial elongation process. Our subsequent results demonstrated that membrane-impermeable Ca2+ chelator EGTA and plasma membrane Ca2+ LaCl3 channels, which were expected to decrease cytosolic levels of Ca2+, not only resulted in a significant reduction in CDH-induced CsDNAJ-1 and CsCDPK1/5 transcripts but also compromised the thereafter induction of adventitious root formation (Fig. 6), suggesting an essential role for calcium. Notwithstanding the limitations of the pharmacological approach used in this study, these data collectively indicated that extracellular Ca2+ pools and plasma membrane Ca2+ channels might be required for the induction responses of CDH. However, the interrelationship between HO-1 and calcium signaling triggered by CDH to promote adventitious rooting should be elucidated further.
Taken together, this study illustrated a vital role of HO-1 in the CDH-induced cucumber adventitious root formation by the modulation of DnaJ-like and CDPK genes. Further pharmacological and molecular evidence indicated that CDH responses might be through calcium signaling. Certainly, our results illustrated that HO-1 might play central roles in not only various plant responses against abiotic stresses (Shekhawat and Verma 2010; Xie et al. 2011b) but also plant growth and development processes. In fact, our results really confirmed that HO-1 possesses a central role in determining adventitious rooting in plants (Xuan et al. 2008).
References
Bai X, Todd CD, Desikan R, Yang Y, Hu X (2012) N-3-oxo-decanoyl-l-homoserine-lactone activates auxin-induced adventitious root formation via H2O2- and nitric oxide-dependent cyclic GMP signaling in mung bean. Plant Physiol 158:725–736
Bauer M, Huse K, Settmacher U, Claus RA (2008) The heme oxygenase–carbon monoxide system: regulation and role in stress response and organ failure. Intensive Care Med 34:640–648
Bellamine J, Penel C, Greppin H, Gaspar T (1998) Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. Plant Growth Regul 26:191–194
Cao ZY, Huang BK, Wang QY, Xuan W, Ling TF, Zhang B, Chen X, Nie L, Shen W (2007a) Involvement of carbon monoxide produced by heme oxygenase in ABA-induced stomatal closure in Vicia faba and its proposed signal transduction pathway. Chinese Sci Bull 52:2365–2373
Cao ZY, Xuan W, Liu ZY, Li XN, Zhao N, Xu P, Wang Z, Guan RZ, Shen WB (2007b) Carbon monoxide promotes lateral root formation in rapeseed. J Integr Plant Biol 49:1070–1079
Cao Z, Geng B, Xu S, Xuan W, Nie L, Shen W, Liang Y, Guan R (2011) BnHO1, a haem oxygenase-1 gene from Brassica napus, is required for salinity and osmotic stress-induced lateral root formation. J Exp Bot 62:4675–4689
Chen YH, Kao CH (2012) Calcium is involved in nitric oxide- and auxin-induced lateral root formation in rice. Protoplasma 249:187–195
Chen YH, Chao YY, Hsu YY, Hong CY, Kao CH (2012) Heme oxygenase is involved in nitric oxide- and auxin-induced lateral root formation in rice. Plant Cell Rep. doi:10.1007/s00299-012-1228-x
Davis SJ, Bhoo SH, Durski AM, Walker JM, Vierstra RD (2001) The heme-oxygenase family required for phytochrome chromophore biosynthesis is necessary for proper photomorphogenesis in higher plants. Plant Physiol 126:656–669
Dellon ES, Szczepiorkowski ZM, Dzik WH, Grame-Cook F, Ades A, Bloomer JR, Cosimi AB, Chung RT (2002) Treatment of recurrent allograft dysfunction with intravenous haematin after liver transplantation for erythropoietic protoporphyria. Transplantation 73:911–915
Den Herder G, Van Isterdael G, Beeckman T, De Smet I (2010) The roots of a new green revolution. Trends Plant Sci 15:600–607
Devadas K, Dhawan S (2006) Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol 176:4252–4257
Dulak J, Józkowicz A (2003) Carbon monoxide–a “new” gaseous modulator of gene expression. Acta Biochim Pol 50:31–47
Frugis G, Mele G, Giannino D, Mariotti D (1999) MsJ1, an alfalfa DnaJ-like gene, is tissue-specific and transcriptionally regulated during cell cycle. Plant Mol Biol 40:397–408
Fu GQ, Zhang LF, Cui WT, Wang YQ, Shen WB, Ren Y, Zheng TQ (2011) Induction of heme oxygenase-1 with β-CD-hemin complex mitigates cadmium-induced oxidative damage in the roots of Medicago sativa. Plant Soil 345:271–285
Han Y, Zhang J, Chen X, Gao Z, Xuan W, Xu S, Ding X, Shen W (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol 177:155–166
Han B, Xu S, Xie YJ, Huang JJ, Wang LJ, Yang Z, Zhang CH, Sun Y, Shen WB, Xie GS (2012) ZmHO-1, a maize haem oxygenase-1 gene, plays a role in determining lateral root development. Plant Sci 184:63–74
Huang YP, Cai RX, Mao LY, Liu ZH, Huang HP (1999) Spectrophotometric determination of hydrogen peroxide using β-CD-Hemin as a mimetic enzyme of peroxidase. Anal Sci 15:889–894
Huang J, Han B, Xu S, Zhou M, Shen W (2011) Heme oxygenase-1 is involved in the cytokinin-induced alleviation of senescence in detached wheat leaves during dark incubation. J Plant Physiol 168:768–775
Jung YD, Chay KO, Song DU, Moon JS, Yang SY, Lee MW, Ahn BW (1997) Hemin inhibits lipid peroxidation induced by ascorbate/FeSO4 and 2,2′-azobis-2-amidino-propane hydrochloride (ABAP). Exp Mol Med 29:171–175
Kumar KG, Ullanat R, Jayabaskaran C (2004) Molecular cloning, characterization, tissue-specific and phytohormone-induced expression of calcium-dependent protein kinase gene in cucumber (Cucumis sativus L.). J Plant Physiol 161:1061–1071
Lanteri ML, Pagnussat GC, Lamattina L (2006) Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J Exp Bot 57:1341–1351
Lanteri ML, Laxalt AM, Lamattina L (2008) Nitric oxide triggers phosphatidic acid accumulation via phospholipase D during auxin-induced adventitious root formation in cucumber. Plant Physiol 147:188–198
Li SW, Xue L, Xu S, Feng H, An L (2009) Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ Exp Bot 65:63–71
Li MY, Cao ZY, Shen WB, Cui J (2011) Molecular cloning and expression of a cucumber (Cucumis sativus L.) heme oxygenase-1 gene, CsHO1, which is involved in adventitious root formation. Gene 486:47–55
Lin YT, Li MY, Cui WT, Lu W, Shen WB (2012) Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J Plant Growth Regul. doi:10.1007/s00344-012-9262-z
Liu ZH, Cai RX, Mao LY, Huang HP, Ma WH (1999) Highly sensitive spectrofluorimetric determination of hydrogen peroxide with β-cyclodextrin–hemin as catalyst. Analyst 124:173–176
Liu YH, Xu S, Ling TF, Xu LL, Shen WB (2010) Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J Plant Physiol 167:1371–1379
Noriega GO, Balestrasse KB, Batlle A, Tomaro ML (2004) Heme oxygenase exerts a protective role against oxidative stress in soybean leaves. Biochem Biophys Res Commun 323:1003–1008
Otterbein LE, Soares MP, Yamashita K, Bach FH (2003) Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24:449–455
Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129:954–956
Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132:1241–1248
Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L (2004) Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135:279–286
Ryter SW, Otterbein LE, Morse D, Choi AMK (2002) Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem 234\235(1–2):249–263
Ryter SW, Alam J, Choi AM (2006) Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86:583–650
Shekhawat GS, Verma K (2010) Haem oxygenase (HO): an overlooked enzyme of plant metabolism and defence. J Exp Bot 61:2255–2270
Sorin C, Bussell JD, Camus I, Ljung K, Kowalczyk M, Geiss G, McKhann H, Garcion C, Vaucheret H, Sandberg G, Bellini C (2005) Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell 17:1343–1359
Ullanat R, Jayabaskaran C (2002) Distinct light-, cytokinin-, and tissue-specific regulation of calcium-dependent protein kinase gene expression in cucumber (Cucumis sativus). Plant Sci 162:153–163
Wen T, Wu ZM, Liu Y, Tan YF, Ren F, Wu H (2007) Upregulation of heme oxygenase-1 with hemin prevents d-galactosamine and lipopolysaccharide-induced acute hepatic injury in rats. Toxicology 237:184–193
Wu MZ, Huang JJ, Xu S, Ling TF, Xie YJ, Shen WB (2011) Haem oxygenase delays programmed cell death in wheat aleurone layers by modulation of hydrogen peroxide metabolism. J Exp Bot 62:235–248
Xie YJ, Ling TF, Han Y, Liu KL, Zheng QS, Huang LQ, Yuan XX, He ZY, Hu B, Fang L, Shen ZG, Yang Q, Shen WB (2008) Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defence in wheat seedling roots. Plant, Cell Environ 31:1864–1881
Xie YJ, Cui WT, Yuan XX, Shen WB, Yang Q (2011a) Heme oxygenase-1 is associated with wheat salinity acclimation by modulating reactive oxygen species homeostasis. J Integr Plant Biol 53:653–670
Xie YJ, Xu S, Han B, Wu MZ, Yuan XX, Han Y, Gu Q, Xu DK, Yang Q, Shen WB (2011b) Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role in RbohD-derived reactive oxygen species synthesis. Plant J 66:280–292
Xu J, Xuan W, Huang BK, Zhou YH, Ling TF, Xu S, Shen WB (2006) Carbon monoxide-induced adventitious rooting of hypocotyl cutting from mung bean seedling. Chinese Sci Bull 51:668–674
Xu DK, Jin QJ, Xie YJ, Liu YH, Lin YT, Shen WB, Zhou YJ (2011a) Characterization of a wheat heme oxygenase-1 gene and its response to different abiotic stresses. Int J Mol Sci 12:7692–7707
Xu S, Lou TL, Zhao N, Gao Y, Dong LH, Jiang DJ, Shen WB, Huang LQ, Wang R (2011b) Presoaking with hemin improves salinity tolerance during wheat seed germination. Acta Physiol Plant 33:1173–1183
Xuan W, Huang LQ, Li M, Huang BK, Xu S, Liu H, Gao Y, Shen WB (2007) Induction of growth elongation in wheat root segments by heme molecules: a regulatory role of carbon monoxide in plants? Plant Growth Regul 52:41–51
Xuan W, Zhu FY, Xu S, Huang BK, Ling TF, Qi JY, Ye MB, Shen WB (2008) The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol 148:881–893
Zilli CG, Santa-Cruz DM, Yannarelli GG, Noriega GO, Tomaro ML, Balestrasse KB (2009) Heme oxygenase contributes to alleviate salinity damage in Glycine max L. leaves. Int J Cell Biol 2009:848516
Acknowledgments
This work was supported by the Doctoral Fund of Ministry of Education of China (grant no. 20100097110019), the Natural Science Foundation of Jiangsu Province of China (grant no. BK2009309), and the Technology Support Program in Jiangsu Province, China (grant no. BE2010382).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kumar.
Rights and permissions
About this article
Cite this article
Lin, Y., Li, M., Huang, L. et al. Involvement of heme oxygenase-1 in β-cyclodextrin–hemin complex-induced cucumber adventitious rooting process. Plant Cell Rep 31, 1563–1572 (2012). https://doi.org/10.1007/s00299-012-1270-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1270-8