Abstract
Ankylosing Spondylitis (AS) stands as a chronic inflammatory arthritis within the spondyloarthritis spectrum, notably increasing cardiovascular (CV) risk and mortality through accelerated atherosclerosis compared to the non-affected population. While evidence in some studies supports a higher cardiovascular morbidity in AS patients, results from other studies reveal no significant disparities in atherosclerotic markers between AS individuals and healthy controls. This discrepancy may arise from the complex interaction between traditional CV risk factors and AS inflammatory burden. Endothelial dysfunction, a recognized antecedent of atherosclerosis prevalent among most individuals with AS, demonstrates the synergistic impact of inflammation and conventional risk factors on endothelial injury, consequently hastening the progression of atherosclerosis. Remarkably, endothelial dysfunction can precede vascular pathology in AS, suggesting a unique relationship between inflammation, atherosclerosis, and vascular damage. The role of adhesion molecules in the development of atherosclerosis, facilitating leukocyte adherence and migration into vascular walls, underscores the predictive value of soluble intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) levels for cardiovascular events. Despite significant progress in comprehending the pathogenesis of AS and its associated cardiovascular implications, the interplay among inflammation, endothelial dysfunction, and atherosclerosis remains partially elucidated. Investigations into the efficacy of therapeutic approaches involving angiotensin receptor blockers and statins have demonstrated reduced cardiovascular risk in AS patients, underscoring the imperative for additional research in this domain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease stands as a significant contributor to morbidity and mortality within rheumatic conditions, notably diseases from the spectrum of chronic inflammatory arthritis [1]. Much of the evidence base derives from studies conducted on rheumatoid arthritis, with diseases from the spondyloarthritis (SpA) group being comparatively less represented.
SpA encompasses a range of chronic inflammatory conditions, divided primarily into axial SpA (AxSpA), affecting the sacroiliac joints and spine, and peripheral SpA, marked by symptoms like arthritis, enthesitis, and dactylitis [2,3,4]. AxSpA is further categorized into two forms: ankylosing spondylitis (AS) or radiographic AxSpA, where radiographic changes in the sacroiliac joints are evident, and non-radiographic axial SpA (nr-axSpA), where such changes are not found [5,6,7]. The body of evidence substantiating the heightened cardiovascular (CV) risk and the escalating cardiovascular morbidity and mortality in this demographic is continuously evolving [8].
Patients diagnosed with AS exhibit elevated standardized mortality ratios (SMRs) compared to the general population (1.6–1.9), with cardiovascular events often cited as a predominant contributing factor [9, 10]. In a meta-analysis of 12 longitudinal studies, Mathieu et al. uncovered a noteworthy increase in the risk of myocardial infarction (MI) among AS patients compared with controls (RR = 1.44; 95% CI 1.25 to 1.67). Moreover, the same study group conducted a meta-analysis focusing on incidents of strokes, revealing a significant elevation in stroke occurrence among AS patients compared with controls, with a risk ratio of 1.37 (95% CI 1.08 to 1.73) [11].
The increased cardiovascular morbidity observed in SpA patients primarily stems from accelerated atherosclerosis compared to the general population. [12, 13]. However, this hypothesis remains controversial in certain studies, which present no difference in outcomes in terms of established markers of atherosclerosis in patients with SpA compared with the healthy population [14, 15]. This discrepancy may arise from the intricate interplay between the overexpression of traditional risk factors in these patients and the inflammatory burden of the disease [16]. Efforts to understand this phenomenon have largely focused on the endothelium, which is thought to represent an initial step in the pathogenesis and maintenance of all stages of atherogenesis, from plaque formation to plaque rupture and thrombogenesis [17, 18].
The endothelium is a layer of cells that line the inner surfaces of blood vessels, serving as a key regulator in maintaining vascular homeostasis through the modulation of arterial tone, coagulation processes, and the proliferation of smooth muscle cells. The integrity of the endothelium is essential for maintaining optimal cardiovascular function. Conversely, endothelial dysfunction is mediated by increased expression of pro-inflammatory cytokines, increased oxidative stress, pro-thrombotic factors, abnormal regulation of vascular tone and adhesion molecules. This dysfunction signifies an early phase in the advancement of vascular pathologies, potentially culminating in arterial stiffening, subclinical atherosclerosis, and eventual onset of arterial disease.
Endothelial dysfunction, quantifiable through noninvasive techniques, precedes manifestations of arterial disease in AxSpA patients, often combined with traditional risk factors. Increased prevalence of endothelial dysfunction in AS has been demonstrated in several studies [19,20,21] along with increased subclinical atherosclerosis [22]. Patients with AS often exhibit endothelial dysfunction much earlier in the course of the disease, before the manifestation of vascular pathology [23]. Emerging findings indicate a substantial link between AxSpA and endothelial dysfunction, marked by irregular expression of adhesion molecules and inflammatory mediators linking this inflammatory arthritis to endothelial dysfunction, accelerated atherosclerosis and cardiovascular complications. Understanding and addressing endothelial dysfunction in AS patients may offer new avenues for therapeutic interventions aimed at mitigating the heightened cardiovascular risk in this population [24], which was done in a systematic review addressing patients with rheumatoid arthritis [25]. The current review aims to explore the complex interplay between endothelial dysfunction, atherosclerosis, and AS, shedding light on potential mechanisms and therapeutic targets for this population.
Methods
Search strategy
A thorough review of the literature was conducted until February 2024 using Scopus and Web of Science databases. The primary search strategy employed involved a combination of pertinent MeSH keywords and subject headings: (“ankylosing spondylitis” OR “axial spondyloarthritis”) AND (“ICAM-1” OR “VCAM-1” OR “PECAM-1” OR “E-selectin” OR “P-selectin*” OR “cadherins” OR “adhesion molecules”). Additionally, various relevant keywords were employed in different permutations to increase the search strategy’s sensitivity. Moreover, the reference lists of the chosen articles were scrutinized to ensure comprehensive coverage and avoid overlooking any relevant studies.
Inclusion criteria and study selection
To be eligible for inclusion in the final review, studies had to meet several predetermined criteria. First, the study design needed to be either cross-sectional or randomized controlled trial (RCT) that investigated adhesion molecules in patients with AS as potential markers of inflammatory activity and endothelial dysfunction. Second, the population studied had to involve human subjects. Third, the intervention examined was the levels of adhesion molecules in patients with ankylosing spondylitis. Finally, the outcome of interest was the evaluation of the relationship between adhesion molecules as potential markers of endothelial dysfunction and subclinical atherosclerosis in these patients. Only full-text articles meeting these criteria were considered for inclusion.
Data extraction
One reviewer (MM) assessed the titles and abstracts of all identified records to determine their compliance with the predetermined inclusion criteria for the systematic review. Following the initial screening phase, the reviewer reassessed the remaining articles based on pre-established inclusion and exclusion criteria. In instances where essential details were unclear, attempts were made to contact the authors of the original papers to acquire additional information. The following data were extracted from each report: authors’ names, publication year, study design, statistical methodology, risk factors (including risk or odds ratios), study findings (including relevant 95% confidence intervals), and the number of cases and controls. Specifically, data pertaining to the study of adhesion molecule levels and their association with inflammatory activity, as well as endothelial dysfunction in patients with AS, were of interest.
Reporting method
In preparing the manuscript, we meticulously adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and implemented the extensive search strategies recommended by Gasparyan et al. [26], ensuring thorough coverage across multiple databases.
Results
The search strategy resulted in 298 records, with no extra relevant articles found through manual bibliography checks. After an initial screening of titles and abstracts, 82 duplicate records were removed, and 149 studies were excluded based on the predefined criteria. Data were then collected from the remaining 19 studies. Ten of these were excluded due to incorrect outcomes, unsuitable study designs, or lack of p-values, leaving nine studies that met the inclusion criteria for the final review (Fig. 1). Table 1 provides a detailed overview of studies investigating adhesion molecules in ankylosing spondylitis, noting the associations between these molecules and disease severity.
Discussion
Atherosclerosis and adhesion molecules
Atherosclerosis is an inflammatory disease with a complex pathogenesis characterized by different stages from onset to progression [27, 28]. One of the early stages of atherosclerosis involves the recruitment of inflammatory cells from the bloodstream and their passage across the endothelial barrier. This mechanism mainly relies on cell adhesion molecules that are activated on endothelial cells lining blood vessels and on circulating white blood cells following various inflammatory signals [29,30,31,32]. Among these molecules, selectins (in particular P, E and L) and their respective binding partners (in particular P-selectin ligand) play a major role in facilitating the initial rolling and attachment of leukocytes along the vascular endothelium [30, 33]. Subsequently, intercellular adhesion molecules (ICAMs) [34], vascular cell adhesion molecules (VCAM-1) and some integrins promote robust adhesion of inflammatory cells to the vascular endothelium, while platelet endothelial cell adhesion molecule-1 (PECAM-1) promotes extravasation of cells from the bloodstream into the vascular wall and adjacent tissues [35]. Although soluble forms of most cell adhesion molecules, with the exception of integrins, have been detected in blood, their exact origin remains incompletely understood.
Clinical implications of adhesion molecules in atherosclerosis
Numerous studies provide compelling evidence for the significant involvement of adhesion molecules in the development of atherosclerosis and plaque vulnerability. In particular, the consistent expression of VCAM-1, ICAM-1, and L-selectin has been observed in atherosclerotic plaques. Furthermore, accumulating evidence from prospective studies suggests a predictive association between elevated circulating levels of soluble ICAM-1 (sICAM-1) in initially asymptomatic individuals and elevated sVCAM-1 in individuals at high risk or with established coronary artery disease [31, 36,37,38,39,40]. Increased expression and levels of soluble serum adhesion molecules are associated with various diseases such as cardiovascular, inflammatory, malignant, psychiatric, autoimmune, etc. [41,42,43,44]. A number of studies are known involving the investigation of adhesion molecule levels as potential markers of atherosclerosis and risk of future cardiovascular events [45,46,47,48,49,50]. The link between chronic inflammation and the intersection of atherosclerosis has long been established. In this direction, there are a large number of studies studying this relationship in patients with inflammatory joint diseases [51,52,53,54,55].
Adhesion molecules, endothelial dysfunction and treatment response
Activation of the renin-angiotensin system (RAS) plays a key role in cardiovascular pathophysiology. Immune cells express various components of RAS, while lymphocytes and macrophages present angiotensin II (Ang II) receptors on their surface [56]. Upon binding to the angiotensin type 1 receptor (AT1R), Ang II initiates signaling pathways that activate nuclear factor kappa B (NF-κB). This activation triggers the release of pro-inflammatory cytokines, chemokines, and cell adhesion molecules from endothelial cells, promoting the migration of inflammatory cells to injured tissue and sustaining inflammation. These cytokines enter the systemic circulation, leading to oxygen radical formation, metabolic and lipid imbalances [57]. These factors collectively cause endothelial activation, resulting in leukocyte extravasation, increased expression of adhesion molecules (e.g., ICAM-1 and VCAM-1), oxygen radical generation, and impaired nitric oxide-mediated muscle relaxation and vascular dilation [56].
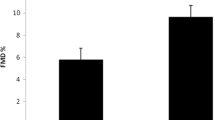
In addition, numerous studies have highlighted the significant immunomodulatory properties of angiotensin receptor blockers (ARBs), leading to lower systemic cytokine levels and improved endothelial dysfunction in individuals at increased cardiovascular risk. Garg et al. found that AS patients treated with Olmesartan showed significant improvement in flow-mediated dilation (FMD) and decreased VCAM-1 levels after 24 weeks, but no significant change in ICAM-1 levels [58]. Similarly, rosuvastatin treatment significantly improved FMD and reduced ICAM-1 levels in AS patients, though VCAM-1 levels did not improve notably [59]. These findings suggest adhesion molecules as independent markers of endothelial dysfunction in AS patients post-treatment. Additionally, a significant correlation was found between FMD and inflammatory markers like CRP, IL-6, and ICAM-1 after rosuvastatin treatment. This underscores rosuvastatin’s positive impact on endothelial dysfunction through its anti-inflammatory and immunomodulatory effects, irrespective of its cholesterol-lowering properties [60,61,62,63,64]. The study establishes adhesion molecules as crucial markers for assessing subclinical atherosclerosis risk and progression in AS individuals. Given the link between adhesion molecules and inflammation markers, controlling disease activity is pivotal for reducing cardiovascular risk, aligning with European Alliance of Associations for Rheumatology (EULAR) recommendations for managing cardiovascular risk in inflammatory arthritis [65].
Liu, R. et al. examined the relationship between ICAM-1,2,3 levels and inflammatory activity [66]. They found that plasma levels of ICAMs were significantly higher in patients with ankylosing spondylitis compared to healthy controls. Correlation analysis demonstrated associations between ICAM-1 and ICAM-2 levels with the proinflammatory cytokines TNF-α and IL-6, as well as with CRP and ESR. While ICAM-1 levels showed no significant associations with disease activity, ICAM-2 levels correlated positively with Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI) and Ankylosing Spondylitis Disease Activity Score (ASDAS). Wendling et al. did a similar study to assess sICAM-1 levels in patients with spondylarthritis and correlate them with inflammatory markers. sICAM-1 levels were assessed in SpA patients, healthy controls and RA patients [67]. Although sICAM-1 levels were found to be elevated in SpA patients, the increase was not significant. However, 28% of SpA patients were found to have particularly high levels above the defined cut-off (> 400 ng/ml). Correlations were found between sICAM-1 and inflammatory markers in SpA patients but not in RA patients. This could imply that ICAM-1 correlating with markers of inflammatory activity may indirectly provide information about activity in the endothelium and thus the initiation of the leukocyte adhesion process as part of the initiation of the atherosclerosis process.
Another study examined how IL-8 and biomarkers of endothelial dysfunction, like VCAM and ICAM, relate to disease activity in AS patients who are not receiving biologic treatments [68]. The findings revealed that VCAM levels are elevated in patients with hypertension; however, these elevated levels do not correlate with CRP levels or BASDAI scores, which are other measures of disease activity. Furthermore, ICAM was positively associated with factors such as high blood pressure, dyslipidemia, smoking, and history of coronary disease, but not with disease activity. VCAM-1 has been identified as a reliable marker of high blood pressure, whereas ICAM-1 is indicative of diastolic blood pressure, smoking, high blood pressure, dyslipidemia, and history of coronary disease but is not associated with specific assessments of AS disease activity [68].
Przepiera-Będzak et al. examined inflammation in the course of arthritis, as well as traditional risk factors for atherosclerosis, along with cytokines and adhesion molecules that influence endothelial activation and dysfunction [69]. They studied 3 groups with AS, PsA and SAPHO. In patients with AS, serum sICAM-1 showed positive correlations with CRP, ESR and age. Patients with CRP ≥ 5 mg/L had higher serum sICAM-1 compared with those with CRP < 5 mg/L. These findings suggest that serum sICAM-1 may correlate with disease activity in SpA and potentially influence endothelial dysfunction.
Genre et al. investigated correlations between biomarkers of endothelial cell activation and characteristics of patients with AS, and the effect of anti-TNF-α therapy on these biomarkers [70]. Serum levels of sE-selectin, Monocyte Chemoattractant Protein-1 (MCP-1), and sVCAM-1 were measured in nondiabetic patients undergoing anti-TNF-α therapy before and after infliximab infusion. It was found that sE-selectin correlated positively with CRP and sVCAM-1. The sVCAM-1 correlated negatively with BMI, diastolic blood pressure and serum glucose, but positively correlated with VAS (Visual Analogue Scale) spine pain. Furthermore, an infliximab infusion significantly reduced sE-selectin and sVCAM-1 levels. These findings highlight the link between endothelial dysfunction, inflammation and metabolic syndrome in patients with AS, with evidence suggesting a beneficial effect of anti-TNF-α blockade on endothelial dysfunction by reducing biomarkers of endothelial cell activation. On the other hand, Orum et al. performed a study evaluating platelet parameters and endothelial activation in patients with AS on a background of anti-TNF therapy [71]. They found no significant changes in platelet and endothelial activation parameters in patients with AS after anti-TNF therapy. Soluble P-selectin (sP-selectin) serves as a plasma marker of platelet activation; it is part of the selectins, which are adhesion molecules. Zhou et al. set out to evaluate the relationship between sP-selectin and clinicopathological characteristics [72]. While ankylosing spondylitis is associated with microvascular dysfunction such as atherosclerosis, the specific relationship between sP-selectin and AS remains unclear. The authors found a correlation between sP-selectin levels and disease stage, HLA-B27, erythrocyte sedimentation rate (ESR), and C-reactive protein.
Sari et al. compared three groups of ankylosing spondylitis (AS) patients: those receiving conventional treatment, those on anti-TNF-α treatment, and a control group, to assess soluble cell adhesion molecules (CAMs) and platelet activation markers [73]. They found no significant differences in CAMs and platelet activation markers among the groups. However, Pearson correlation analysis revealed significant associations between several markers, including sE-selectin, sP-selectin, sCD40L, BASFI, and BASDAI. Notably, soluble P-selectin showed significant correlations with sE-selectin, sCD40L, BASFI, and BASDAI, highlighting the complex relationship between these markers and disease activity in AS patients. These findings offer valuable insights into AS pathophysiology and could guide future research on targeted therapies to reduce cardiovascular risk in AS patients.
Strengths and limitations
Our review underscores the key role of adhesion molecules such as VCAM-1, ICAM-1, and selectins in the pathogenesis of atherosclerosis, particularly in the context of AS. These molecules facilitate leukocyte adherence and migration into the vascular walls, contributing to the inflammatory processes that accelerate atherosclerosis. This comprehensive examination highlights the potential of adhesion molecules as predictive biomarkers for cardiovascular risk and as therapeutic targets. The positive effects of interventions such as angiotensin receptor blockers and statins on reducing endothelial dysfunction and cardiovascular risk in AS patients further emphasize their importance.
This review highlights substantial limitations and gaps in the current body of research. Despite their critical importance, adhesion molecules are insufficiently studied in the context of AS. Most existing studies suffer from cross-sectional designs, small sample sizes, and inconsistent methodologies, which undermine the generalizability and reliability of their findings. Additionally, the reliance on correlational data hinders the ability to determine causative relationships between adhesion molecule levels and cardiovascular outcomes in AS patients. This underrepresentation may stem from the complex interaction between traditional cardiovascular risk factors and the inflammatory burden of AS, which can obscure the specific contributions of adhesion molecules. Furthermore, research has often prioritized well-known inflammatory markers and traditional cardiovascular risk factors, potentially overlooking the nuanced role of adhesion molecules. Future studies should focus on longitudinal designs with larger, more diverse cohorts to clarify the evolving role of adhesion molecules in the progression of cardiovascular disease in AS patients.
Conclusion
In summary, the evidence surrounding the heightened cardiovascular risk in patients with AxSpA continues to evolve, with a consensus emerging regarding the association between AS and increased cardiovascular morbidity and mortality. This augmented risk primarily stems from accelerated atherosclerosis compared to the general population. However, controversy exists in certain studies where outcomes related to established markers of atherosclerosis do not differ significantly between AS patients and healthy individuals. This discrepancy may be attributed to the intricate interplay between the overexpression of traditional cardiovascular risk factors in AS patients and the inflammatory burden of the disease. Efforts to elucidate this phenomenon have predominantly focused on endothelial dysfunction, which is believed to represent an initial step in the pathogenesis and maintenance of atherosclerosis. Studies have consistently shown an increased prevalence of endothelial dysfunction and subclinical atherosclerosis in AS patients, often preceding the manifestation of vascular pathology. The relationship between inflammation, atherosclerosis, vascular injury, and AS remains multifactorial and not yet fully understood.
Simplified representation of the role of adhesion molecules mediating leucocyte adhesion to the endothelium and endothelial transmigration. Abbreviations: platelet endothelial cell adhesion molecule 1 (PECAM-1), vascular cell adhesion molecule 1 (VCAM-1, intercellular adhesion molecule 1 (ICAM-1), E-selectin (endothelial), P-selectin (platelets), Integrin α4β1, junctional adhesion molecules (JAM)
References
Zimba O, Gasparyan AY (2023) Cardiovascular issues in rheumatic diseases. Clin Rheumatol 42:2535–2539. https://doi.org/10.1007/s10067-023-06656-y
Sieper J, Poddubnyy D (2017) Axial spondyloarthritis. Lancet 390:73–84. https://doi.org/10.1016/s0140-6736(16)31591-4
Ivanova M, Zimba O, Dimitrov I, Angelov AK, Georgiev T (2024) Axial spondyloarthritis: an overview of the disease. Rheumatol Int. https://doi.org/10.1007/s00296-024-05601-9
Raychaudhuri SP, Deodhar A (2014) The classification and diagnostic criteria of ankylosing spondylitis. J Autoimmun 48–49:128–133. https://doi.org/10.1016/j.jaut.2014.01.015
Rudwaleit M, van der Heijde D, Landewe R et al (2009) The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68:777–783. https://doi.org/10.1136/ard.2009.108233
Rudwaleit M, Landewe R, van der Heijde D et al (2009) The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 68:770–776. https://doi.org/10.1136/ard.2009.108217
Rudwaleit M, Khan MA, Sieper J (2005) The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 52:1000–1008. https://doi.org/10.1002/art.20990
Wright KA, Crowson CS, Michet CJ et al (2015) Time trends in incidence, clinical features, and cardiovascular disease in ankylosing spondylitis over three decades: a population-based study. Arthritis Care Res (Hoboken) 67:836–841. https://doi.org/10.1002/acr.22512
Lehtinen K (1993) Mortality and causes of death in 398 patients admitted to hospital with ankylosing spondylitis. Ann Rheum Dis 52:174–176. https://doi.org/10.1136/ard.52.3.174
Zochling J, Braun J (2008) Mortality in ankylosing spondylitis. Clin Exp Rheumatol 26
Mathieu S, Soubrier M (2019) Cardiovascular events in ankylosing spondylitis: a 2018 meta-analysis. Ann Rheum Dis 78. https://doi.org/10.1136/annrheumdis-2018-213317
Yuan Y, Yang J, Zhang X et al (2019) Carotid intima-media thickness in patients with ankylosing spondylitis: a systematic review and updated meta-analysis. J Atheroscler Thromb 26:260–271. https://doi.org/10.5551/jat.45294
Bai R, Zhang Y, Liu W et al (2019) The relationship of ankylosing spondylitis and subclinical atherosclerosis: a systemic review and meta-analysis. Angiology 70:492–500. https://doi.org/10.1177/0003319718814309
Hatipoğlu E, Şengül İ, Kaya T et al (2019) Assessment of subclinical atherosclerotic cardiovascular disease in patients with ankylosing spondylitis. Anatol J Cardiol 22:185–191. https://doi.org/10.14744/AnatolJCardiol.2019.13367
Kaplanoglu H, Özişler C (2019) Evaluation of subclinical atherosclerosis using ultrasound radiofrequency data technology in patients diagnosed with ankylosing spondylitis: evaluation of subclinical atherosclerosis in ankylosing spondylitis. J Ultrasound Med 38:703–711. https://doi.org/10.1002/jum.14754
Hamdi W, Maatallah K (2019) Subclinical atherosclerosis: a hidden threat for patients with ankylosing spondylitis. Anatol J Cardiol 22:192–193. https://doi.org/10.14744/AnatolJCardiol.2019.78703
Davignon J, Ganz P (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109(23):27–32. https://doi.org/10.1161/01.CIR.0000131515.03336.f8
Gimbrone MA Jr, García-Cardeña G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118:620–636. https://doi.org/10.1161/CIRCRESAHA.115.306301
Sari I, Okan T, Akar S et al (2006) Impaired endothelial function in patients with ankylosing spondylitis. Rheumatology (Oxford) 45:283–286. https://doi.org/10.1093/rheumatology/kei145
Bodnar N, Kerekes G, Seres I et al (2011) Assessment of subclinical vascular disease associated with ankylosing spondylitis. J Rheumatol 38:723–729. https://doi.org/10.3899/jrheum.100668
Angelov AK, Markov M, Ivanova M et al (2023) The genesis of cardiovascular risk in inflammatory arthritis: insights into glycocalyx shedding, endothelial dysfunction, and atherosclerosis initiation. Clin Rheumatol 42:2541–2555. https://doi.org/10.1007/s10067-023-06738-x
Sharma SK, Prasad KT, Handa R et al (2015) Increased prevalence of subclinical atherosclerosis in ankylosing spondylitis. Indian J Rheumatol 10:53–55
Castañeda S, Nurmohamed MT, González-Gay MA (2016) Cardiovascular disease in inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. https://doi.org/10.1016/j.berh.2016.10.006
Gasparyan AY (2023) Cardiovascular manifestations and comorbidities in rheumatic diseases: perspectives on timely diagnosis, prevention, and treatment. Clin Rheumatol 42(10):2531–2533. https://doi.org/10.1007/s10067-023-06762-x
Gerganov G, Georgiev T, Dimova M et al (2023) Vascular effects of biologic and targeted synthetic antirheumatic drugs approved for rheumatoid arthritis: a systematic review. Clin Rheumatol 42:2651–2676. https://doi.org/10.1007/s10067-023-06587-8
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Ross R (1999) Atherosclerosis–an inflammatory disease. N Engl J Med 340(2):115–126. https://doi.org/10.1056/NEJM199901143400207
Falk E (2006) Pathogenesis of atherosclerosis. J Am Coll Cardiol 47(8 Suppl):C12. https://doi.org/10.1016/j.jacc.2005.09.068
Springer TA (1990) Adhesion receptors of the immune system. Nature 346:425–434. https://doi.org/10.1038/346425a0
Davies MJ, Gordon JL, Gearing AJ et al (1993) The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol 171:223–229. https://doi.org/10.1002/path.1711710311
Price DT, Loscalzo J (1999) Cellular adhesion molecules and atherogenesis. Am J Med 107:85–97. https://doi.org/10.1016/s0002-9343(99)00153-9
Blankenberg S, Barbaux S, Tiret L (2003) Adhesion molecules and atherosclerosis. Atherosclerosis 170:191–203. https://doi.org/10.1016/s0021-9150(03)00097-2
Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN (1994) Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol 144:952–961
Bui TM, Wiesolek HL, Sumagin R (2020) ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol 108:787–799. https://doi.org/10.1002/jlb.2mr0220-549r
Dangerfield J, Larbi KY, Huang MT, Dewar A, Nourshargh S (2002) PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J Exp Med 196:1201–1211. https://doi.org/10.1084/jem.20020324
O’Brien KD, Allen MD, McDonald TO et al (1993) Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest 92:945–951. https://doi.org/10.1172/jci116670
O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE (1996) Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation 93:672–682. https://doi.org/10.1161/01.cir.93.4.672
Bowden RA, Ding ZM, Donnachie EM et al (2002) Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ Res 90:562–569. https://doi.org/10.1161/01.res.0000013835.53611.97
DeGraba TJ, Siren AL, Penix L et al (1998) Increased endothelial expression of intercellular adhesion molecule-1 in symptomatic versus asymptomatic human carotid atherosclerotic plaque. Stroke 29:1405–1410. https://doi.org/10.1161/01.str.29.7.1405
Haim M, Tanne D, Boyko V et al (2002) Soluble intercellular adhesion molecule-1 and long-term risk of acute coronary events in patients with chronic coronary heart disease. Data from the bezafibrate infarction prevention (BIP) study. J Am Coll Cardiol 39:1133–1138. https://doi.org/10.1016/s0735-1097(02)01728-x
Nielsen HH, Soares CB, Høgedal SS et al (2020) Acute neurofilament light chain plasma levels correlate with stroke severity and clinical outcome in ischemic stroke patients. Front Neurol 11:448. https://doi.org/10.3389/fneur.2020.00448
Santos LF, Stadtlober NP, Costa Dall’Aqua LG et al (2018) Increased adhesion molecule levels in systemic lupus erythematosus: relationships with severity of illness, autoimmunity, metabolic syndrome and cortisol levels. Lupus 27:380–388. https://doi.org/10.1177/0961203317723716
Macías C, Villaescusa R, del Valle L et al (2003) Moléculas De adhesión endoteliales ICAM-1, VCAM-1 y E-selectina en pacientes con síndrome coronario agudo [Endothelial adhesion molecules ICAM-1, VCAM-1 and E-selectin in patients with acute coronary syndrome]. Rev Esp Cardiol 56(2):137–144. https://doi.org/10.1016/s0300-8932(03)76837-7
Troncoso MF, Ortiz-Quintero J, Garrido-Moreno V et al (2021) VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim Biophys Acta Mol Basis Dis 1867(9):166170. https://doi.org/10.1016/j.bbadis.2021.166170
Haught WH, Mansour M, Rothlein R et al (1996) Alterations in circulating intercellular adhesion molecule-1 and L-selectin: further evidence for chronic inflammation in ischemic heart disease. Am Heart J 132:1–8. https://doi.org/10.1016/s0002-8703(96)90383-x
Ridker PM, Hennekens CH, Roitman-Johnson B et al (1998) Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 351:88–92. https://doi.org/10.1016/s0140-6736(97)09032-6
Hwang SJ, Ballantyne CM, Sharrett AR et al (1997) Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the atherosclerosis risk in communities (ARIC) study. Circulation 96:4219–4225. https://doi.org/10.1161/01.cir.96.12.4219
de Lemos JA, Hennekens CH, Ridker PM (2000) Plasma concentration of soluble vascular cell adhesion molecule-1 and subsequent cardiovascular risk. J Am Coll Cardiol 36:423–426. https://doi.org/10.1016/s0735-1097(00)00742-7
Mulvihill NT, Foley JB, Murphy R et al (2000) Evidence of prolonged inflammation in unstable angina and non-Q wave myocardial infarction. J Am Coll Cardiol 36:1210–1216. https://doi.org/10.1016/s0735-1097(00)00824-x
Gearing AJ, Newman W (1993) Circulating adhesion molecules in disease. Immunol Today 14:506–512. https://doi.org/10.1016/0167-5699(93)90267-o
Haroon NN, Paterson JM, Li P, Inman RD, Haroon N (2015) Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med 163:409–416. https://doi.org/10.7326/m14-2470
Szabo SM, Levy AR, Rao SR et al (2011) Increased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: a population-based study. Arthritis Rheum 63(11):3294–3304. https://doi.org/10.1002/art.30581
del Rincón I, Williams K, Stern MP et al (2001) High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 44:2737–2745. https://doi.org/10.1002/1529-0131(200112)44:12%3C2737. ::aid-art460%3E3.0.co;2-%23
Avina-Zubieta JA, Choi HK, Sadatsafavi M et al (2008) Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 59:1690–1697. https://doi.org/10.1002/art.24092
Szabo SM, Levy AR, Rao SR et al (2011) Increased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: a population-based study. Arthritis Rheum 63:3294–3304. https://doi.org/10.1002/art.30581
Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM (2007) Renin-angiotensin system and cardiovascular risk. Lancet 369(9568):1208–1219. https://doi.org/10.1016/s0140-6736(07)60242-6
Han C, Liu J, Liu X, Li M (2010) Angiotensin II induces C-reactive protein expression through ERK1/2 and JNK signaling in human aortic endothelial cells. Atherosclerosis 212(1):206–212. https://doi.org/10.1016/j.atherosclerosis.2010.05.020
Garg N, Krishan P, Syngle A (2021) Angiotensin-receptor blockade improves inflammation and endothelial dysfunction in ankylosing spondylitis: ARB-AS study. Int J Angiol 30(4):262–270. https://doi.org/10.1055/s-0040-1722738
Garg N, Krishan P, Syngle A (2015) Rosuvastatin improves endothelial dysfunction in ankylosing spondylitis. Clin Rheumatol 34(6):1065–1071. https://doi.org/10.1007/s10067-015-2912-3
Jain MK, Ridker PM (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 4:977–987. https://doi.org/10.1038/nrd1901
Greenwood J, Mason JC (2007) Statins and the vascular endothelial inflammatory response. Trends Immunol 28:1–19. https://doi.org/10.1016/j.it.2006.12.003
Tan KCB, Chow WS, Tam VH et al (2002) Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilatation in type 2 diabetes mellitus. J Clin Endocrinol Metab 87:563–568. https://doi.org/10.1210/jcem.87.2.8249
Marchesi S, Lupattelli G, Siepi D et al (2000) Short-term atorvastatin treatment improves endothelial function in hypercholesterolemic women. J Cardiovasc Pharmacol 36:617–621. https://doi.org/10.1097/00005344-200011000-00011
Timár O, Szekanecz Z, Kerekes G et al (2013) Rosuvastatin improves impaired endothelial function, lowers high sensitivity CRP, complement and immune complex production in patients with systemic sclerosis—a prospective case-series study. Arthritis Res Ther 15:1–9. https://doi.org/10.1186/ar4285
Agca R, Heslinga SC, Rollefstad S et al (2017) EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 76(1):17–28. https://doi.org/10.1136/annrheumdis-2016-209775
Liu R, Yue Z, Peng X et al (2016) Association between intercellular adhesion molecule-1, -2, -3 plasma levels and disease activity of ankylosing spondylitis in the Chinese Han population. Spine 41–E624. https://doi.org/10.1097/brs.0000000000001328
Wendling D, Racadot E, Augé B et al (1998) Soluble intercellular adhesion molecule 1 in spondylarthropathies. Clin Rheumatol 17:202–204. https://doi.org/10.1007/BF01451047
Azevedo VF, Faria-Neto JR, Stinghen A et al (2013) IL-8 but not other biomarkers of endothelial damage is associated with disease activity in patients with ankylosing spondylitis without treatment with anti-TNF agents. Rheumatol Int 33:1779–1783. https://doi.org/10.1007/s00296-012-2631-x
Przepiera-Będzak H, Fischer K, Brzosko M (2016) Serum interleukin-18, fetuin-A, soluble intercellular adhesion molecule-1, and endothelin-1 in ankylosing spondylitis, psoriatic arthritis, and SAPHO syndrome. Int J Mol Sci 17:1255. https://doi.org/10.3390/ijms17081255
Genre F, López-Mejías R, Miranda-Filloy JA et al (2015) Anti-TNF-α therapy reduces endothelial cell activation in non-diabetic ankylosing spondylitis patients. Rheumatol Int 35:2069–2078. https://doi.org/10.1007/s00296-015-3314-1
Örüm H, Pamuk GE, Pamuk ÖN et al (2012) Does anti-tnf therapy cause any change in platelet activation in ankylosing spondylitis patients? J Thromb Thrombolysis 33:154–159. https://doi.org/10.1007/s11239-011-0663-9
Zhou X, Li W, Ding Q (2015) Up-regulation of soluble P-selectin predicates its prognostic value in patients with ankylosing spondylitis. Int J Clin Exp Pathol 8:7272–7276
Sari I, Alacacioglu A, Kebapcilar L et al (2010) Assessment of soluble cell adhesion molecules and soluble CD40 ligand levels in ankylosing spondylitis. Joint Bone Spine 77:85–87. https://doi.org/10.1016/j.jbspin.2009.07.005
Acknowledgements
Figure 2 was made in BioRender.com.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to the conception and design of the study, acquisition and analysis of data, and interpretation of results. MM and TG have been involved in drafting the manuscript, while MD and AA critically have revised it. All authors have provided final approval for its submission to this journal. Furthermore, they agree to take responsibility for all aspects of the work and are prepared to be held accountable for any related issues that may arise. Finally, all contributing authors have reviewed and endorsed the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest associated with this research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Markov, M., Georgiev, T., Angelov, A.K. et al. Adhesion molecules and atherosclerosis in ankylosing spondylitis: implications for cardiovascular risk. Rheumatol Int 44, 1837–1848 (2024). https://doi.org/10.1007/s00296-024-05693-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-024-05693-3