Abstract
The objective of current study was to fabricate PEG based hydrogels cross linked with monomer i.e. acrylic acid and 2, Acrylamide-2-methyl propane sulfonic acid (AMPS) in aqueous media. N,N'-Methylenebisacrylamide (MBA) was used as cross linker while ammonium per sulfate was added in reaction as initiator. Sodium bicarbonate was used as to create pores in hydrogel making the formulations superporous. Ivabradine hydrochloride as model drug was loaded into prepared hydrogel formulation. FTIR confirmed the compatibility of Polyethylene glycol with acrylic acid and AMPS. Thermal stability of PEG based hydrogels was confirmed by DSC and TGA techniques. Prepared hydrogels showed pH dependent swelling behavior. Presence of AMPS caused excellent swelling of hydrogels due to its polyelectrolyte nature while in case of acrylic acid dense cross linking cause comparatively less swelling of acrylic acid based hydrogels. Sol–gel fraction was determined to find out unreacted and uncross linked portion of each formulation. Invitro release study confirmed controlled release of loaded drug from PEG based polymeric matrices. Release kinetics suggested that drug release from hydrogel matrix followed solvent diffusion swelling mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Controlled drug delivery system that deliver the drug at predetermined rate, overcomes the lacking of the conventional drug delivery system. Drug delivery system would be more ideal if system deliver drug in response to specific stimuli and release the active drug in response to that stimuli. Such stimuli to which delivery systems show response and that greatly influence the release of active drug mainly include pH, temperature, light or pressure [1]. Hydrogels are considered as smart drug delivery system. Hydrogels are three dimensional network system, hydrophilic in nature, having ability to imbibe excess amount of water. Hydrogels have swelling mechanism and ability to maintain their structure and integrity during swelling process [2, 3]. Moreover these networks don’t dissolve because of chemical or physical cross linking [4, 5]. In hydrogel formulations, different methods of crosslinking have been used. Generally chemical and physical methods have been used in hydrogel formulations [4, 6]. Furthermore, chemically crosslinked gels can be produced by radical polymerization, chemical reaction with complementary groups, high energy irradiation and by using enzymes. While physically crosslinked gels can be produced through crystallization, ion interactions and from amphiphilic block and graft copolymers. Hydrogels show visco-eleastic and pure elastic behavior due to the presence of crosslinks present in polymeric chains. Chemically crosslinked hydrogels have covalent bonds in polymeric structures and physically crosslinked hydrogels have physical interactions in different polymeric chains [2, 7]. Crosslinking in hydrogels give stability and prevent them from dissolving in surrounding aqueous solution. Chemical crosslinking can be used to enhance the resistance of hydrogels to break [8].
[1]. Synthetic hydrogels dominate over natural hydrogels because of their more ability of imbibing water, their maximum stability as well as defined structure. Hydrogels can be prepared by bulk polymerization [6], solution polymerization [7], suspension polymerization, graft polymerization [7, 8] and photo polymerization as well as polymerization by irradiation. In the preparation of hydrogels, either natural polymers are used or synthetic materials are utilized [9]. Khan. et al. [10] prepared pH-sensitive biocompatible gelatin/carboxymethyl cellulose based hydrogels by free radical polymerization technique via chemical crosslinking approach and sustained the release of 5-Fluorouracil for 48 h. Similarly Pal. et al. [11] successfully prepared a graft copolymer based on polyacrylamide and polyvinyl pyrrolidone and used as wound healing agent. In another advanced study, Pal. P et el utilized microwave-induced process for surface modification and graft copolymerization of quaternary ammonium ion on psyllium. Authors reported that the synthesized water soluble product via surface modification resulted good antibacterial activity against Gram positive Staphylococcus aureus and Bacillus anthracis [12]. Pal. P et el in another study developed novel, non-toxic, eco-friendly grafted product via microwave assisted technique by incorporation of naturally occurring cinnamic acid into the structure of sesbania gum. The developed grafted copolymer was used for sugarcane juice clarification [13]. It has been reported in literature that natural polymers exhibit comparatively low mechanical strength than synthetic polymers.
Synthetic polymer such as polyethylene glycol (PEG) are water soluble and have high swelling rate. They also show good gelation. PEG also has ability to minimize accumulation of red blood cells. PEG is also biocompatible, water soluble and less toxic [14]. Poly (2-acrylamido-2-methylpropane sulfonic acid) (AMPS) [(CH2–CH–CONH–C–(CH3)2–CH2SO3H)n] which is a strongly acidic ionic monomer behaves as polyelectrolyte and is easily dissolved in water. Due to the polyelectrolyte nature of AMPS, the hydrogels prepared from AMPS show pH independent swelling behavior and exhibit complete dissociation in entire pH range [15, 16].
Acrylic acid (AA) is sensitive to pH due to ionic repulsion between (–COOH) groups. Acrylic acid shows high swelling properties in basic medium and low swelling properties in acidic medium. AA is bio-compatible due to presence of (–COOH) groups [17, 18]. Polyacrylic acid having good bioadhesive characteristics. That’s why acrylic acid is mostly use to increase the retention period of formulation.
Poly (2-acrylamido-2-methylpropane sulfonic acid) (AMPS) [(CH2–CH–CONH–C–(CH3)2–CH2SO3H)n] which is a strongly acidic ionic monomer behaves as polyelectrolyte and is easily dissolved in water. AMPS received huge attention in recent years due to the presence of strongly ionizable sulfonate groups in its chemical structure. Due to the polyelectrolyte nature of AMPS, the hydrogels prepared from AMPS show pH-independent swelling behavior and exhibit complete dissociation in entire pH range [19].
The Ivabradine hydrochloride has been loaded as model drug which has potential applications in angina [20]. Ivabradine hydrochloride performs its action by inhibiting \({I}_{f}\) current. It decreases oxygen demand. Ivabradine hydrochloride specifically lower heart rate. Ivabradine hydrochloride has effective half-life of 2 h. Drugs with short half- life are excreted fastly from the body. To maintain therapeutic level of drug in body repeated administration of drug is done. But this practice leads to noncompliance especially in elders and children. Short half-life of drug makes it suitable to be loaded in hydrogels.
Keeping in view the concept of high mechanical stability of synthetic polymeric materials, in recent work, polyethylene glycol (PEG) based porous and superporous hydrogels [5, 21] have been fabricated with different monomers i.e. acrylic acid (AA) and 2-acrylamido-2-methylpropane sulfonic acid (AMPS) using graft polymerization technique. Comparative swelling kinetics of developed formulations was investigated by conducting swelling experiments in various buffer solutions. Ivabradine was loaded as a model drug in synthesized hydrogels and release of drug was investigated in various buffer solutions. Various kinetic models were applied on release data to predict the best drug release mechanism from hydrogels. Fourier transform infrared spectroscopic study (FTIR), thermogravimetric analysis (TGA), differential spectroscopic (DSC), x-ray diffraction (XRD) and scanning electronic microscopy was performed to investigate the interpenetrating structure formation, thermal stability analysis, crystallinity and morphological analysis.
Materials and methods
Materials
Polyethylene glycol (4000 g/mol), Merck (Germany), 2-Acrylamido-2-methylpropane sulfonic acid (AMPS) (MW = 207.25 g/mol, Sigma-Aldrich, UK), Acrylic acid (72.06 g/mol, Sigma-Aldrich, UK), Ivabradine hydrochloride, Benzoyl peroxide (242.23 g/mol, Sigma-Aldrich, UK), N, N-methylenebisacrylamide (154.17 g/mol, Merck Germany), Sodium hydrogen sulfite (190.11 g/mol, Sigma-Aldrich, UK), Ammonium peroxodisulfate (228.19 g/mol, Sigma-Aldrich, UK), Sodium hydroxide (40 g/mol, Sigma-Aldrich UK), Potassium dihydrogen phosphate (136.09 g/mol, Sigma-Aldrich, UK). All the solvents used were of analytical grade.
Synthesis of crosslinked PEG based polymeric matrices
Hydrogels based on poly ethylene glycol (PEG) with various monomers i.e. 2-acrylamide-2-methylpropane sulfonic acid (AMPS) and acrylic acid were prepared using graft polymerization technique as per composition given in Table 1. A brief methodology for their preparation is given below;
Synthesis of PEG-co-Acrylic acid hydrogels
PEG-co-Acrylic acid porous hydrogels were prepared by graft polymerization technique reported previously with little modification [22]. Briefly an accurately weighed amount of PEG 4000 was dissolved in pre weighed amount of distilled water with continuous stirring until a clear viscous solution is obtained. Then acrylic acid in predetermined amount was weighed accurately and stored for further use. A weighed amount of ammonium peroxo-disulphate (APS) and N,N’-methylenebisacrylamide (MBA) were dissolved separately in distilled water and kept on stirring till clear solutions obtained. Now APS solution was added drop wise to acrylic acid under continuous stirring. Then PEG solution previously prepared was added to the acrylic acid mixture drop wise at room temperature to get clear co-polymeric mixture. Finally MBA solution was added drop wise with continuous stirring into above reaction mixture. Reaction mixture was immediately transferred to labeled test tubes and placed in preheated water bath initially at 60 \(^\circ{\rm C} \) for 1 h and then at 65 \(^\circ{\rm C} \) for 24 h. Figure 1a indicates the presumptive proposed structure of PEG-co-AA based hydrogels.
Synthesis of PEG-co-(AMPS) hydrogels
An accurately weighed amount of PEG 4000 was dissolved in pre weighed amount of distilled water with continuous stirring on until a clear viscous polymer solution obtained. Then AMPS was weighed accurately, dissolved in distilled water and stored for further use. A weighed amount of ammonium peroxo-disulphate (APS) and N,N’-methylenebisacrylamide (MBA) were dissolved separately in distilled water and kept on stirring till clear solutions obtained. Now firstly AMPS solution was added into APS solution under constant stirring and then PEG solution previously prepared was added drop by drop to AMPS mixture. Finally MBA solution was added drop wise and final mixture was kept on stirring for 5 to 10 min. Then immediately the solution was transfer into pre labeled test tubes and kept them in pre heated water bath at 70 \(^\circ{\rm C} \) for 24 h. Figure 1b indicates the presumptive proposed structure of PEG-co-AA based hydrogels.
Synthesis of Superporous PEG-co-(AMPS) based hydrogel
For the preparation of AMPS based Superporous hydrogels, similar procedure was used as for PEG-co-poly (AMPS) hydrogels with little modifications. After the addition of MBA solution to co-polymeric mixture and kept on stirring for 2–3 min, sodium bicarbonate was sprinkled (1 g) over the prepared solutions and quickly transferred to test tubes so that bubbles can entrapped in gel. Finally the mixture was transferred to test tubes and placed them in water bath at 70 \(^\circ{\rm C} \). Sprinkling of sodium bicarbonate will lead to bubbles entrapment and make it effervescent.
Washing and drying
Test tubes were removed from the water bath after 24 h and cool them at room temperature. Solid, cylindrical, compact and smooth hydrogels were taken out from tubes. Hydrogels were cut into cylindrical disc of 8 mm thickness. These discs were washed by ethanol–water (70/30) to remove impurities, unreacted monomer and extraneous material. The discs were placed in the petri dishes properly labeled and placed them in oven at 40–45 \(^\circ{\rm C} \) for 3–4 days. Fully dried hydrogels were removed from oven. Kept the dried discs in sampling bags and properly labeled for further characterization [22]. Table 1 represents the composition of PEG based synthesized hydrogels.
Physical appearance
The synthesized hydrogel samples were inspected for physical appearance by visual examination along with manual examination of mechanical strength to determine physical stability of hydrogels.
Swelling studies
Dynamic and equilibrium swelling experiments of dried hydrogel discs were conducted in media of different pH buffer solutions to determine the effect of pH on swelling behavior. Accurately pre weighed dried hydrogel discs were immersed in 0.1 M HCl buffer pH 1.2, phosphate buffer solution of pH 4.5 and 6.8 at 37 °C in 250 ml beaker. At predetermined time intervals, the swollen gels were removed from buffer solution, placed on bloating paper to remove superficial solvent and then weighed on an analytical balance to determine weight gain and finally placed back into their respective buffer solutions. The swelling experiments were continued until hydrogel discs attained equilibrium weight [23]. Triplicate data were obtained for each measurement.
Following equations were used to determine degree of swelling and equilibrium water content respectively.
where \({{\varvec{M}}}_{{\varvec{s}}}\) is mass of swelling at predetermined time interval,\({{\varvec{M}}}_{{\varvec{d}}}\) indicates the weight of dried discs, and \({{\varvec{M}}}_{{\varvec{e}}{\varvec{q}}}\) is the mass of gels after equilibrium weight gained.
Structural analysis
FTIR spectroscopy
Fourier transform infrared spectroscopy was used to evaluate the presence of functional group, effective network formation of hydrogel, extent of cross linking and structural changes that occur during polymerization. Before analysis, samples were properly triturated. Bruker FTIR (Shimadzu, Japan) with Attenuated total reflectance (ATR) technology was employed to analyze the sample of formulation components and hydrogel formulations. FTIR spectra were obtained in the range of 4000–650 cm−1[22].
Thermogravimetric analysis
Thermogravimetric analysis is a technique that measures the amount and rate of change in the weight of a material as a function of temperature or time in controlled environment to investigate its thermal stability, oxidative stability and compositional analysis. TGA was performed by using TA instruments Q600 series Thermal Analysis System (TA instruments, WestSussex, UK). Samples were properly milled to powder before the analysis. About 0.5 to 5 mg sample of polymer (PEG), monomer (IA), cross-linker (MBA) and all formulated hydrogels without drug were placed in the sample pan for analysis over a temperature range of 20 °C to 500 °C. Decomposition thermograms were recorded at hating rate of 20 °C/min under continuous flow rate at 10 ml/min of nitrogen gas [24].
Differential scanning calorimetry
Differential scanning calorimetry is a thermal analysis method to measure the rate of heat flow. Amount of energy absorbed or released by a sample upon heating or cooling can be measured by this method. Qualitative and quantitative data on endothermic and exothermic processes can be obtained. DSC is a thermo analytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. The samples were scanned from 0 to 600 \(^\circ{\rm C} \) with increase in heating rate of 20 \(^\circ{\rm C} \)/min under rate of 20 ml/min rate of stream nitrogen gas [25].
Scanning electron microscopy
The structural analysis of formulated hydrogels was analyzed using scanning electron microscopy (SEM). JEOL analytical scanning electron microscopy (JSM-6490A, Tokyo Japan) was used for the examination of hydrogel samples for surface morphology. Dried discs of hydrogel were cut into optimum size particles and mounted on an aluminum stub with double adhesive tape. The stubs were coated with gold under argon atmosphere using gold sputter coater. Surface morphology of hydrogels was analyzed by taking photomicrographs randomly [26].
Sol–gel fraction
Sol and gel contents were measured to evaluate the amount of reactants consumed in the reactive mixture of hydrogels preparation. In order to measure the sol–gel fraction dried disc of 5 mm thickness was accurately weighed and immersed in deionized distilled water in glass beaker for one week. The disc was shaken occasionally to remove the water-soluble unreacted part. Water insoluble part of dried hydrogel was removed from water after one week and placed in labeled petri dish and dry in vacuum oven until a constant weight is obtained [4].
Following equations were used to determine sol and gel %.
where \({{\varvec{m}}}_{{\varvec{c}}}\) represent weight of dried disc and \({{\varvec{m}}}_{{\varvec{d}}}\) represents weight of water insoluble portion of hydrogel.
Drug loading of hydrogel samples
All hydrogel formulations were loaded with Ivabradine hydrochloride through swelling diffusion process. For drug loading 1\(\%\) solution of Ivabradine hydrochloride was prepared in phosphate buffer of pH 6.8. Dried discs of hydrogels of known weight were soaked in 100 ml of drug solution in labeled glass jars at room temperature. The discs were kept in drug solution for 72 h until the equilibrium weight was achieved at room temperature. After 72 h, the discs were dried at room temperature after washing with distilled water. Then the loaded discs were placed in oven at 40 °C until constant weight obtained [4, 25].
Drug loaded in hydrogel discs was found out by using following formula;
where \({{\varvec{W}}}_{{\varvec{D}}}\) indicates weight of dried loaded discs and \({{\varvec{W}}}_{{\varvec{d}}}\) presents the weight of dried hydrogel disc before drug loading.
Loaded drug contents determination
The preformed drug loaded hydrogel discs were evaluated for drug loaded contents. For this purpose, drug loaded discs of hydrogels were crushed thoroughly and then placed in 500 ml phosphate buffer solution of pH 6.8 for 24 h with continuous stirring at 37 ± 0.5 °C and then centrifuged at 3000 rpm. The supernatant layer was separated, filtered through 0.45 µm filter paper and assayed for drug content via UV–visible spectrophotometer (UV-1700 Shimadzu). Analysis was performed at λmax 286 nm [27]. The loaded drug contents (mg/g) of hydrogel samples were determined by using following formula:
In-vitro drug release studies
To investigate the drug delivery from cross-linked polymeric hydrogel, in-vitro drug release experiments were conducted. Drug release from hydrogels at various pH values were evaluated to confirm the pH dependent and controlled release of Ivabradine hydrochloride from hydrogel polymeric matrices. Dissolution studies were performed in 900 ml solution of pH 1.2 and 6.8 using USP dissolution apparatus-II at 37 ± 0.5 °C and 50 rpm equipped with UV–visible spectrophotometer (UV-1700 Shimadzu). Aliquots (5 ml) of samples were withdrawn at equal intervals of time and analyzed using UV–visible spectrophotometer (UV-1700 Shimadzu) at \({\lambda }_{\mathrm{max}}\) 286 nm [4, 28]. The withdrawn samples were replaced with fresh buffer to maintain the sink conditions.
Drug release kinetics
Drug release kinetics was assessed by fitting the in vitro experimental data obtained to various mathematical models [4].
Zero order kinetic model
where Qt is the amount of drug dissolved in time t, Q0 is the initial amount of drug in the solution and K0 is zero order release constant.
First order kinetic model
Mo is the initial amount of drug (5-FU) and Mt is the remaining amount of drug (5-FU) at time t and k1 is the first order rate constant.
Higuchi model.
The model relates cumulative drug release versus square root of time as shown in Eq. (10).
where M is the amount of 5-FU released at time t and KH is the Higuchi rate equation.
Power Law or Korsmeyer–Peppas model.
This model relates exponentially the drug release to the elapsed time. The equation is given as
(Mt/M∞) is the fraction of drug released at time t and n is the exponent which determines the mechanism of drug release diffusion from the hydrogel matrix.
Statistical analysis
Data is presented as percentage or mean ± standard deviation (SD). The difference of parameters are statistically tested for significance by one-way analysis of variance (ANOVA) using Graph Pad InStat (GraphPad Software, Inc., La Jolla, CA, USA) or Origin (v6.0; Microcal™ Software, Inc., Northampton, MA, USA) programs. Statistically significant values were defined as P < 0.05.
Results and discussion
Physical appearance
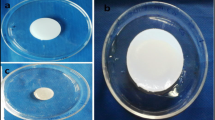
Polyethylene glycol based hydrogels were prepared after successful polymerization and proper cross-linking with different monomers i.e. acrylic acid and 2-acrylamido-2-methylepropane sulfonic acid (AMPS). PEG-co-acrylic acid hydrogel was stable cross-linked network. Pre-formulated hydrogels with low polymer contents were white in color, smooth and compact gel with good mechanical strength, rubbery in nature and very difficult to grind. Freshly prepared hydrogels were transparent in appearance. PEG-co-Acrylic acid based hydrogel has less swelling rate as compared to hydrogels having AMPS based hydrogel. PEG-co-Acrylic acid based hydrogel has more mechanical strength and strong cross-linking. It has excellent smooth texture. PEG-co-AMPS based hydrogel and Superporous PEG-co-AMPS based hydrogels became transparent upon swelling. While PEG-co-AMPS based hydrogel was crystal white in color, soft to touch and flexible and stretchable. It was transparent, compact and sticky in nature. Super porous PEG-co-AMPS based hydrogel was transparent, properly gelled, soft structure and have uniformly distributed pores. Superporous hydrogel also has good mechanical strength, flexible, rubbery and sticky in nature. All fabricated hydrogels were stable in network structure and have adequate mechanical strength. They retain their shape even after swelling. Physical appearance of PEG based hydrogel formations are shown in Supplementary Fig. 1.
Swelling experiments
Swelling dynamics of PEG-co-Acrylic acid based hydrogels
Swelling kinetics of hydrogels was evaluated in buffer solution of pH 1.2, pH 4.5 and pH 6.8 respectively. The swelling behavior of hydrogels at low and high pH can predict the behavior of drug-loaded hydrogels in different parts of gastrointestinal tract. The pH values in the physiological environment highly change from acidic conditions in the stomach (pH 1–3) to the almost neutral environment in the small intestine (6.22–7.10) and colon (6.59–7.89). Swelling behavior is dependent upon polymer as well as nature of solvent. Swelling dynamics of PEG based hydrogels are given in Fig. 2. Comparison of swelling rate at different pH demonstrates that swelling rate of PEG-co-Acrylic acid based hydrogel was higher at pH 6.8 as compared to at pH 1.2 and pH 4.5. Hydrogels containing AA exhibit low swelling degree at gastric pH, but as they pass into the gastrointestinal tract (GI), the swelling degree increases with increase in pH. This is because AA has carboxyl groups in its network and swelling of the gel is greatly affected due to the presence of ionized charges on the polymer network. At higher pH, the presence of similar ionized charges causes an electrostatic repulsion along the chain which in turn causes the expansion of the originally coiled molecules and lead to swelling of gel network. PEG 4000 consist of long chains of hydroxyl (-OH) groups that made this polymer highly attractive towards water and showed hydrophilic properties. It is known that at high pH hydroxyl and carboxylic groups get ionized and cause production of electrostatic repulsive forces among negatively charged groups. The presence of these repulsive forces cause chains expansions and in turn swelling of the gel matrix. Figure 2a indicates the response of PEG-co-AA based hydrogels to swelling kinetics at various pH values.
Swelling index of PEG-co-AMPS and super porous PEG-co-AMPS based hydrogels
Figure 2b, c demonstrates the swelling kinetics of PEG-co-AMPS and super porous PEG-co-AMPS based hydrogels respectively. It was observed from swelling profile, that hydrogels demonstrate high swelling rate at pH 6.8. Higher swelling of AMPS based hydrogels is justified because of presence of strong sulfonate groups in its structure. This is because with increasing AMPS contents, the presence of highest number of strong hydrophilic sulfonate groups (–SO3H) become ionized which in turn lead to electrostatic repulsions and swelling. The presence of these groups in the gel structure gives enhanced swelling capacity due to increase interaction and uptake of water molecules. While acrylic acid having low viscosity show less swelling. Swelling of acrylic acid based hydrogel is much lower than AMPS based hydrogel. Swelling index of acrylic acid based hydrogel discs at 30 h was 2.107 at pH 1.2, 2.278 at pH 4.5 while 9.978 at pH 6.8. Such swelling index clearly describe that there is approximately significant difference in swelling index at pH 1.2, pH 4.5 and 6.8 pH. Similarly swelling index of PEG-co-AMPS based hydrogel was also compared and it represent that swelling index of AMPS based discs at 30 h at pH 1.2, pH 4.5 and pH 6.8 was 16.00, 24.69 and 30.85. It is also clearly shown that a difference has been seen at pH 1.2, 4.5 and pH 6.8. Same is in the case of superporous hydrogels. At 30 h, swelling index of disc at pH 1.2 was 18.99, at pH 4.5 swelling index was 29.01 and at pH 6.8 it was 29.74. It was obvious that no difference of swelling index was between pH 4.5 and 6.8. However, a significant difference was seen between pH 1.2 and 6.8. Such difference was associated with special combination of PEG polymer and AMPS monomer.
Effect of foaming agent on swelling
Slow swelling indicates slow diffusion of water into dried hydrogels. However, if hydrogels contain pores then water will enter into hydrogel from pores via capillary action. Such system provides rapid swelling. Gas blowing method is used to create pores. Sodium bicarbonate was added into monomer. Sodium bicarbonate work by increasing pH of the system so that polymerization process increases. Volume of prepared super porous hydrogel was 2–10 times more than original volume of solution. Most of superporous hydrogel swell to such an extent that makes them too difficult to handle. Superporous hydrogel after swelling become too fragile.
In present study, superporous hydrogel have clear, visible pores. Addition of sodium bicarbonate results in release of carbon dioxide. Pores of hydrogel results in rapid swelling of hydrogel. Pores also make hydrogel fragile because of loose polymer structure formed. Figure 2C shows the swelling kinetics of superporous hydrogels.
Structural analysis
Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared spectroscopy of hydrogels was performed to evaluate the structure formation. FTIR was performed to find out chemical structural relationship of hydrogels. FTIR spectra of Polyethylene glycol indicated in Supplementary Fig. 2A show bands around 2888 \({\mathrm{cm}}^{-1}\) and 2920 \({\mathrm{cm}}^{-1}\) refers to of alkyl group of PEG. The characteristic band at 1342 \({\mathrm{cm }}^{-1}\) refers to C‒H bending. The bands at 1100 \({\mathrm{cm}}^{-1}\) is assumed to C‒O stretching. While the bands at 1242 \({\mathrm{cm}}^{-1}\) assigned to C‒H twisting vibration [29]. ‒OH stretching vibration was confirmed by band at 3272 \({\mathrm{cm}}^{-1}\). Sharp band at 2882 \({\mathrm{cm}}^{-1}\) depicts alkyl stretching, while band in the region of 1095 \({\mathrm{cm}}^{-1}\) represent stretching of ether group (C–O–C).
In the FTIR spectrum of AMPS, band around 2,986.69 \({\mathrm{cm}}^{-1}\) observed refers to deformation vibration of –NH group. The characteristic band around 1233.25 \({\mathrm{cm}}^{-1}\) represent symmetrical stretching vibration of \({\mathrm{SO}}_{2}\) group. Two bands observed at 1666.69 and 1612.62 \({\mathrm{cm}}^{-1}\) represent absorption of ‒\({\mathrm{CO}}_{2}\mathrm{NH}\)‒ group. AMPS spectra also represent a broad band at 3448 \({\mathrm{cm}}^{-1}\). This band is assumed to represent collapse of ‒NH and ‒OH groups respectively. The characteristic band around 3039 \({\mathrm{cm}}^{-1}\) and 2992 \({\mathrm{cm}}^{-1}\) show stretching of ‒\( {\text{CH}}_{3} \;{\text{and}} - {\text{CH}}_{2} \;{\text{groups}} \) respectively. The band around 1662 \({\mathrm{cm}}^{-1}\) represent C\(=\)O stretching and band at 1613 \({\mathrm{cm}}^{-1}\) represent N‒H bending. Peak at 1379 \({\mathrm{cm}}^{-1}\) and 1300 \({\mathrm{cm}}^{-1}\) show presence of S\(=\)O group. A broad region in the range of 1089 \({\mathrm{cm}}^{-1}\)-950 \({\mathrm{cm}}^{-1}\) assumed strong stretching absorption due to S‒O‒C group. Wide band in the region of 3600–3100 \({\mathrm{cm}}^{-1}\) depicts stretching vibration of -OH and N–H respectively. The presence of multiple weak peaks around 1300–1550 \({\mathrm{cm}}^{-1}\) represent C‒H bending of methyl and methylene groups respectively. The characteristic band around 1226 \({\mathrm{cm}}^{-1}\) refers to –S = O functional group. While the characteristic band around 1043 \({\mathrm{cm}}^{-1}\) refers to ‒C‒N group stretching vibration [30].
FTIR spectra of acrylic acid indicated in Supplementary Fig. 2B show a band at 2972 \({\mathrm{cm}}^{-1}\) refers to \({\mathrm{CH}}_{2}\) stretching vibration. The characteristic band at 1296 \({\mathrm{cm}}^{-1}\) refers to C‒C stretching, while C=O stretching was assumed to be shown by band at 1635 \({\mathrm{cm}}^{-1}\). The band around 1173 \({\mathrm{cm}}^{-1}\) represents C‒O stretching vibration of carboxylic acid group.
The FTIR spectra of Ivabradine hydrochloride indicates band at 3595 \({\mathrm{cm}}^{-1}\) represent presence of alkenes group i.e. R‒\({\mathrm{CH}}_{2}\) ‒\({\mathrm{CH}}_{3}\). While the band at 2994 \({\mathrm{cm}}^{-1}\) indicate stretching of C‒N group. The characteristic band at 1631 \({\mathrm{cm}}^{-1}\) indicate stretching of aromatic C‒C group while band at 1631 \({\mathrm{cm}}^{-1}\) represent stretching of aromatic C‒C. Stretching of C=C bond and presence of alkenes was assumed by strong band at 1203 \({\mathrm{cm}}^{-1}\). Aromatic C‒H group is observed by presence of band at 1484 \({\mathrm{cm}}^{-1}\) [31, 32]. The characteristic band in the region of 1640–1690 \({\mathrm{cm}}^{-1}\) depicts presence of C=O of amide group.
FTIR spectra of PEG-co-Acrylic acid based hydrogel indicated in Supplementary Fig. 2C represent a broad band between 3651.25 \(\mathrm{and} \)2974.23 \({\mathrm{cm}}^{-1}\) represent stretching of OH and NH groups. The characteristic band at 1590 \({\mathrm{cm}}^{-1}\) refers to presence of aromatic ring in spectrum. A broad region between 1743.65 \({\mathrm{cm}}^{-1}\mathrm{ and }1502.55{\mathrm{ cm}}^{-1}\) represent vibrational stretching of C=C, C=N and C=O respectively. Another band at 1110 \({\mathrm{cm}}^{-1}\) presenting asymmetrical stretching of C–O–C of ethoxy group, indicating presence of PEG group in formulation. FTIR spectra of PEG-co-AMPS based hydrogel indicated in Supplementary Fig. 2D show sharp bands 3480 \({\mathrm{cm}}^{-1}\) indicate presence of NH bond. A broad region between 1475.54 \({\mathrm{cm}}^{-1}\) and 1741.72 \({\mathrm{cm}}^{-1}\) indicating N–H and C=O stretching respectively. This band region for superporous PEG-co-AMPS hydrogel indicated in Supplementary Fig. 2E was shifted to 1508.33–1753.29 \({\mathrm{cm}}^{-1}.\) The end of the peak show 95.5 percent transmittance while the Porous PEG-co-AMPS based shift the band to 82.5 percent transmittance. A very clear band in the region of 3300–\(2978.09 {\mathrm{cm}}^{-1}\) indicate presence of OH, NH groups respectively.
Thermogravimetric analysis
Thermogravimatric analysis is most robust technique in comparison to differential scanning calorimetery. Thermograms of each of PEG based hydrogels has been shown in Fig. 3. TGA characterize material by measuring loss in mass as a function of temperature. It measures absorbed moisture content, degradation or combustion. Thermal analysis was performed at 500 \(^\circ{\rm C} \) temprature and heat at 20 \(^\circ{\rm C} \)/min. Flow rate was 10 ml/min as purge gas.
PEG-co-acrylic acid based hydrogel showed a thermograms indicated in Fig. 3 that clearly indicate alteration in thermal stability. It has been shown that slight weight change was there with loss of moisture and decomposition of hydrogel start earlier then decomposition of polymer as well as monomer. Such hydrogel has a series of degradation. Rapid loss in mass of hydrogel has been shown until complete degradation has been obtained from 555 to 567 \(^\circ{\rm C} .\) Thermogram show another different behavior, where combustion temperature of PEG increase in hydrogel as compared to pure PEG. While degradation temperature of acrylic acid in hydrogel has been lowered as compared to pure acrylic acid. In PEG-co-AMPS based hydrogel indicated in Fig. 3, initial loss of water occurred at 70–77 \(^\circ{\rm C} \) indicating evaporation of solvent. Incorporation of AMPS into PEG polymer shifted the initial and maximum decomposition temperature symmetrically towards much higher temperature i.e. 567–578 \(^\circ{\rm C} \). Super porous PEG-co-AMPS based formulation indicated in Fig. 3 having addition of sodium bicarbonate to introduce pores. Evaporation of solvent start at much lower temperature as compared to pure ingredients of hydrogel. However complete thermal degradation of formulation was observed at about 542 \(^\circ{\rm C} \).
Differential scanning calorimetry (DSC)
Differential scanning calorimetry estimate thermal transition of samples. During heating endothermic as well as exothermic reaction occur. Endothermic reaction indicate melting, boiling or evaporation of sample. Exothermic reaction indicates crystallization or degradation. In DSC, curve we get are crystalline peak representing exothermic process and melting dip indicate endothermic process.
In DSC curve of PEG-co-Acrylic acid based hydrogel indicated in Fig. 4, a number of endothermic peaks were starting melting at 263 \(^\circ{\rm C} \), 395 \(^\circ{\rm C} \) and 498 \(^\circ{\rm C} .\) DSC curve of PEG-co-AMPS based hydrogel indicated in Fig. 4 showed melting dips indicating melting of formulation at 222 \(^\circ{\rm C} \), 324 \(^\circ{\rm C} \) and 473 \(^\circ{\rm C} \). In comparison to superporous PEG-co-AMPS based hydrogel, there was decrease in endothermic peak temperature. This formulation show melting at slightly lower temperature i.e. 219 \(^\circ{\rm C} \), 330 \(^\circ{\rm C} \) and 434 \(^\circ{\rm C} \) as compared to PEG-co-AMPS based hydrogel. A cross linking curve at about 518 \(^\circ{\rm C} \) has been obtained. A sharp exothermic peak at about region between 570 and 600 \(^\circ{\rm C} \). All these finding elaborate thermal stability of hydrogels as compared to pure ingredients. A symmetrical shift towards increase thermal stability has been indicated.
a Surface morphology of PEG-co-Acrylic acid based hydrogels at a magnification × 100 b magnification × 250 c cross-section morphology at magnification × 30 d cross-section morphology at magnification × 1000. b Surface morphology of PEG-co-AMPS based hydrogels at a magnification × 100 b magnification × 250 c cross-section morphology at magnification × 30 d cross-section morphology at magnification × 1000. c Surface morphology of Superporous PEG-co-AMPS based hydrogels at a magnification × 100 b magnification × 250 c cross-section morphology at magnification × 30 d cross-section morphology at magnification × 1000
Scanning electron microscopy
For analysis of surface morphology, SEM was performed by crushing dried hydrogels and place crushed hydrogel on aluminum stub. Coat the sample with gold coater for at least two minutes with 20 mA. SEM micrographs of hydrogels are presented in Fig. 5a–c.
SEM of acrylic acid based hydrogel show slightly rough surface shown in Fig. 5a. Brittle nature of gel shows its tough texture. Moreover, rough and tough surface of hydrogel indicates strong cross linking between monomer and polymer. SEM of PEG-co-AMPS based hydrogel clearly showed smooth and compact texture as shown in Fig. 5b. Surface of hydrogel show efficient crosslinking between polymer and monomer. A constant even surface was obtained. SEM results of super porous hydrogel shown in Fig. 5c show excellent texture of hydrogel and water channels for the absorption of fluids.
Sol–gel fraction
Sol–gel fraction of all samples was calculated to elaborate the effect of different concentrations of polymers, monmers and crosslinker on gel strength. The unreacted components of hydrogels e.g. cross linker and monomers make sol fraction. It was concluded from the results that negligible sol fraction was seen in each formulation. So most of the reactants are consumed and hydrogels are stable.
Drug loading of hydrogels
For loading of drug in selected hydrogel samples, swelling diffusion process was used. It was observed from the results reported in Table 2 that, PEG-co-AA based hydrogels showed maximum drug loading at higher pH (6.8) due to presence of negatively charged protonated groups i.e. carboxylic group of AA and hydroxyl group of polyethylene glycol. These protonated groups cause electrostatic repulsion which leads to swelling and pores opening for drug diffusion. Similalry for PEG-co-AMPS and superporous hydrogels, maximum drug loading was noted to high pH (pH 6.8). This increased in drug loading at higher pH was assemued to be due to hydrophilic nature of AMPS and presence of increase number of protonated carboxyl groups in AMPS. This in turn increase the degree of interaction between PEG and AMPS, which in turn lead to swelling and drug diffusion. Table 2 indicates the amount of drug loaded in hydrogel samples.
In-vitro release studies
Three dimensional structure of hydrogel make it special to deliver the drug by controlling release kinetics of drug. So hydrogels are actually swelling based drug delivery systems. Thermodynamic compatibility of polymer changes as it come in contact with water, it leads to swelling of polymer. Drug within hydrogel, dissolve and start to diffuse out against concentration gradient. Polymer stress gradient, concentration gradient and osmotic force are driving force that cause release of drug. Release of hydrophilic drug from hydrogel follow simultaneously absorption of water. All components of hydrogel contribute to affect release of drug. Most commonly release mechanism of drug from PEG based hydrogels follow swelling-controlled, diffusion-controlled and chemically controlled. USP dissolution apparatus II was used for in-vitro drug release study of hydrogel formulation. Release of drug from hydrogel follow swelling controlled diffusion mechanism against concentration gradient i.e. from higher to lower concentration. The dissolution media used consisted of phosphate buffer of pH 1.2 and pH 6.8. Volume of dissolution media was 900 ml. Dissolution study of each formulation was performed at temperature 37 \(\pm 0.5^\circ{\rm C} \) with constant stirring at 50 rpm. Aliquots of dissolution media were taken after specified interval of time and immediately we replaced it with fresh media to maintain the volume. These aliquots were filter and then observed by UV visible spectroscopy. Amount of drug released was analyzed by UV spectroscopy technique. It was observed from the drug release profile of hydrogel samples that all hydrogel formulation showed initial burst release which is suggested to be due to the presence of drug particles on hydrogel samples surface layers. So when the samples came in contact with fluids, initially surface drug was released in a burst manner. It was also concluded that burst release was followed by sustained profile owing to the release of encapsulated drug from underlying polymer layers. It has also been observed that PEG-co-AA based hydrogels showed more sustained behavior with highest T50 values (at pH 6.8). While on the other hand comparatively fastest drug release has been observed with PEG-co-AMPS porous and Superporous based hydrogels owing to high hydrophilic nature of these formulations with corresponding lesser T50 values (at pH 6.8) indicated in Table 2.
Hydrogels response to pH of release media
From drug release profiles of all hydrogel samples, it was observed that hydrogel samples showed maximum drug release in PBS of higher pH (6.8), while subsequent drug release was also observed by at lower pH (1.2). It can be explained by the fact that drug release from hydrogel samples occurred through solvent diffusion swelling controlled mechanism. As it is known that PEG contain abundant of hydroxyl groups (–OH) in its side chains, while AA and AMPS has large of carboxyl (–COOH) and sufonic (SO3H) groups. Both type of these groups are hydrophilic in nature and also get protonated in basic buffer (6.8) solutions. So in buffer solution of higher pH (6.8), the hydrophilic groups absorb enough of solvent molecules and also get protonated. Due to the presence of these charged groups, electrostatic repulsion occur causing chains expansion and pores opening in hydrogel network. As a result of opening these pores, solvent molecules get diffused inside gel matrix and lead to the dissolution of encapsulated drug and in turn lead to the release of drug from gel network.
On the other hand in buffer solution of lower pH (1.2), hydrogel samples also showed drug release as observed from their drug release profile. It can be explained by the fact that since hydroxyl groups (–OH) from PEG and sufonic (SO3H) and carboxyl groups (–COOH) from AA and AMPS remained deprotonated at lower pH, but release from gel network occurred due to the hydrophilic nature of these groups and materials. As it has explained earlier that both type of these functional groups are hydrophilic in nature. So due to increased hydrophilicity, hydrogels samples also showed some sort of drug release from gel network following solvent diffusion swelling controlled mechanism. However as whole drug release was found dominant in higher buffer solutions (6.8). The drug release profile of all PEG based hydrogels is presented graphically in Fig. 6.
Effect of PEG on drug release
It is known that hydrogels followed solvent diffusion swelling controlled mechanism for drug release. Swelling of hydrogel depends upon nature of polymer used in gel network. Since PEG is a hydrophilic polymer containing a number of hydroxyl groups (–OH) in its side chains. PEG incorporation in hydrogels increased the hydrophilicity of gel network, lead to increase diffusion of water molecule into hydrogel and release of drug molecule from gel matrix.
So the all formulation that based on PEG polymer showed good release of drug from hydrogels. Moreover PEG also imparts the hydrophilic properties to components that are linked with it. PEG based hydrogels were stable over extended period and released drug in continuous manner over prolonged period of time.
Effect of AMPS on release of drug
2-Acrylamido-2-methylpropane sulfonic acid (AMPS) is hydrophilic monomer containing abundant of sulfonate groups in its side chain. By increasing concentration of AMPS, we can enhance swelling index and drug release of hydrogels due to electrostatic repulsion of sulfonic group. It has been seen that PEG-co-AMPS showed significant drug loading and release of drug at both pH but was found lower in comparison to PEG-co-AA hydrogels as shown in Table 2. It can be explained by reason that AMPS monomer is more active as it contain negatively charged sulfonic group. Moreover due to the polyelectrolyte nature of AMPS, the hydrogels prepared from AMPS show pH-independent swelling behavior and exhibit complete dissociation in entire pH rangeSo slow release of drug was observed because of possible interaction between drug and AMPS. Similarly in case of Superporous hydrogels, drug loading and release was also found lower because of possible interaction between drug and AMPS.
Effect of AA on drug release
Acrylic acid has carboxylic group in its chain and has good water solubility. A burst effect has been shown in case of hydrogel with acrylic acid monomer. Increase in pH cause ionization of carboxylic group of acrylic acid. Such ionization leads to repulsion between acrylic acid and PEG backbone. This repulsion is source of breakdown of hydrogen bonds and cause decomplaxation of hydrogel and release of drug at much faster rate occur.
Effect of cross linker (MBA) on drug loading
In current study N,N-methylene-bis-acrylamide (MBA) was used as crosslinking agent for the fabrication of PEG based hydrogels with various monomers. It was concluded from the results that with increasing MBA contents in gel structure, loading of drug in hydrogels decreases accordingly as per results reported in Table 2. This decrease in drug loading is suggested because of growing cross-linking density, lower pore size and compact gel structure at higher cross-linker concentrations.
Comparative analysis of drug release
Drug release from acrylic acid based hydrogels was abrupt. Loading time for acrylic acid based hydrogel was 12 h while loading time for PEG-co-AMPS based hydrogels and superporous PEG-co-AMPS based hydrogel was 8 h because AMPS based hydrogels started to degrade if these were kept for longer duration in drug solution. Moreover, higher swelling rate of AMPS based hydrogels allow increased rate of diffusion of drug into hydrogels. While acrylic acid based hydrogels remain stable over 12 h. Because these hydrogels show lower swelling index. All formulations retain drug and release drug upto 26 h. Drug release pattern represents that drug release was independent of pH as well as swelling index. Swelling index of acrylic acid based hydrogel was less than that of AMPS based hydrogels. 80% drug release was seen at 8 h in case of acrylic acid based hydrogel at pH 1.2 and at 9 h at pH 6.8. At 7th hour in case of AMPS based hydrogel, 80% drug was released at pH 1.2 and at 6th hour at pH 6.8. Superporous hydrogels show approximately 80% of drug release at 8th hour at both pH. The release pattern of drug is not consistent with swelling behavior of hydrogels. All formulations show pH independent drug release.
Drug release rate and kinetics
From drug release profile shown in Fig. 6, it can be seen that cumulative release and release rate is affected mainly by hydrogel matrix composition. From drug release plots it was observed that release rate from hydrogel matrix occurred through solvent diffusion swelling controlled mechanism. However drug release rate from all hydrogels matrix composed of PEG, AA and AMPS followed three steps. First step is the penetration of the dissolution medium in the hydrogel matrix causing hydration. Second step is the swelling with concomitant or subsequent dissolution or erosion of the hydrogel matrix and third step is the transport of the dissolved drug, either through the hydrated matrix or from the parts of the eroded gel component to the surrounding dissolution medium. So it was concluded that release rate of drug from gel matrix was mainly controlled by matrix composition.
The invitro release data obtained in various dissolution medias was used to predict the drug release kinetics and release mechanism by fitting to various mathematical models. Evaluation of method that best fits the release data was done by considering regression coefficient. Table 1 shows the values of regression coefficient (r) for all the hydrogel formulations prepared. These samples showed zero order release kinetics because values of “r” for zero order were slightly greater than first order and Higuchi model. This indicates that drug release from gel network occur through pore diffusion in controlled fashion and the release is independent of the drug concentration at the application site. Korsmeyer–Pappas or power model was applied to predict the release mechanism from the values of release exponent ‘n’ for all hydrogel samples as displayed in Table 3. It was found that both the samples followed fickian diffusion with “n” values less than 0.5. This phenomena indicates that release from hydrogel system occur through swelling and relaxation of the polymers.
Conclusion
The objective of current research study was to develop a stable polymeric hydrogel system with increased loading capacity, swelling and efficient release mechanism.
Polymeric hydrogels were prepared by using a synthetic polymer, Polyethylene glycol cross linked with 2-Acrylamido-2-methyl-1-propane sulfonic acid (AMPS) and Acrylic acid through free radical polymerization process. N,N'-Methylenebisacrylamide (MBA) was used as cross linker and Ammonium per sulfate (APS) as initiator. Sodium bicarbonate was used to create pores by evolving \({\mathrm{CO}}_{2}\) gas. All formulations showed efficient mechanical strength, increase swelling and loading of drug. Hydrogels also showed maximum stability and drug release. FTIR analysis confirmed the copolymer structure formation and thermal stability was confirmed by TGA and DSC. It showed Model drug was loaded successfully into each formulation. However, loading time for PEG-co- acrylic acid based hydrogel was longer then PEG -co-AMPS based hydrogel because sulfonic group of AMPS cause expansion of network that allow maximum diffusion of drug into hydrogel system. PEG-co-AMPS based hydrogels pH dependent swelling at pH 1.2, 4.5 and 6.8 while superporous PEG-co-AMPS based hydrogels showed different swelling index at pH 1.2 and 6.8 but non-significant difference between swelling at pH 4.5 & 6.8. Acrylic acid based hydrogels show pH dependent swelling and higher drug loading time because of dense cross linked network. Based on results it was concluded that PEG based hydrogels could be effectively used as controlled drug delivery carriers.
References
Qiu Y, Park K (2001) Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev 53:321–339
Khan S, Anwar N (2019) Highly porous pH-responsive carboxymethyl chitosan- grafted -poly (acrylic acid) based smart hydrogels for 5-fluorouracil controlled delivery and colon targeting. Int J Polym Sci. https://doi.org/10.1155/2019/6579239
Khan S, Minhas MU, Ahmad M (2017) Self-assembled supramolecular thermoreversible β -cyclodextrin/ethylene glycol injectable hydrogels with difunctional Pluronic 127 as controlled delivery depot of curcumin. Development, characterization and in vitro evaluation. J Biomater Sci Polym Ed 5063:1–34. https://doi.org/10.1080/09205063.2017.1396707
Khan S, Ranjha NM (2014) Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly(vinyl alcohol) hydrogels. Polym Bull 71:2133–2158. https://doi.org/10.1007/s00289-014-1178-2
Bukhari SMH, Khan S, Rehanullah M, Ranjha NM (2015) Synthesis and characterization of chemically cross-linked acrylic acid/gelatin hydrogels: effect of pH and composition on swelling and drug release. Int J Polym Sci. https://doi.org/10.1155/2015/187961
Ahmed EM (2015) Hydrogel: preparation, characterization, and applications: a review. J Adv Res 6:105–121
Mathur AM, Moorjani SK, Scranton AB (1996) Methods for synthesis of hydrogel networks: a review. J Macromol Sci Rev Macromol Chem Phys 36:405–430
Akhtar MF, Hanif M, Ranjha NM (2016) Methods of synthesis of hydrogels a review. Saudi Pharm J 24:554–559
Khan S, Ullah A, Ullah K, Rehman N (2016) Insight into hydrogels. Des Monomers Polym 19:456–478. https://doi.org/10.1080/15685551.2016.1169380
Khan S, Anwar N (2021) Gelatin/carboxymethyl cellulose based stimuli-responsive hydrogels for controlled delivery of 5-fluorouracil, development, in vitro characterization, in vivo safety and bioavailability evaluation. Carbohydr Polym 257:117617. https://doi.org/10.1016/j.carbpol.2021.117617
Pal P, Rangra N, Samanta S et al (2020) Graft copolymer of PVP—A sutureless, haemostatic bioadhesive for wound healing application. Polym Bull 77:5191–5212. https://doi.org/10.1007/s00289-019-03013-5
Pal P, Banerjee A, Soren K et al (2019) Novel biocide based on cationic derivative of psyllium: surface modification and antibacterial activity. J Polym Environ. https://doi.org/10.1007/s10924-019-01419-w
Pal P, Pandey JP, Sen G (2018) Grafted sesbania gum: a novel derivative for sugarcane juice clarification. Int J Biol Macromol 114:349–356. https://doi.org/10.1016/j.ijbiomac.2018.03.123
Kadajji VG, Betageri GV (2011) Water soluble polymers for pharmaceutical applications. Polymers (Basel) 3:1972–2009. https://doi.org/10.3390/polym3041972
Saikia AK, Aggarwal S, Mandal UK (2013) Preparation and controlled drug release characteristics of thermoresponsive PEG/Poly (NIPAM-co-AMPS) hydrogels. Int J Polym Mater 62:39–44. https://doi.org/10.1080/00914037.2012.664208
Khan S, Akhtar N, Minhas MU et al (2020) A difunctional Pluronic®127-based in situ formed injectable thermogels as prolonged and controlled curcumin depot, fabrication, in vitro characterization and in vivo safety evaluation. J Biomater Sci Polym Ed. https://doi.org/10.1080/09205063.2020.1829324
Elliott JE, MacDonald M, Nie J, Bowman CN (2004) Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer (Guildf) 45:1503–1510. https://doi.org/10.1016/j.polymer.2003.12.040
Ijaz QA, Abbas N, Arshad MS et al (2018) Synthesis and evaluation of pH dependent polyethylene glycol-co-acrylic acid hydrogels for controlled release of venlafaxine HCl. J Drug Deliv Sci Technol 43:221–232. https://doi.org/10.1016/j.jddst.2017.10.010
Khan S, Akhtar N, Minhas MU et al (2021) A difunctional Pluronic®127-based in situ formed injectable thermogels as prolonged and controlled curcumin depot, fabrication, in vitro characterization and in vivo safety evaluation. J Biomater Sci Polym Ed 32:281–319. https://doi.org/10.1080/09205063.2020.1829324
Savelieva I, Camm AJ (2006) Novel I<sub>f</sub> current inhibitor ivabradine: safety considerations. In: Heart rate slowing by If current inhibition. KARGER, Basel, pp 79–96
Gupta NV, Shivakumar HG (2010) Preparation and characterization of superporous hydrogels as gastroretentive drug delivery system for rosiglitazone maleate. Daru 18:200–210
Barkat K, Ahmad M, Usman Minhas M et al (2018) Development and characterization of pH-responsive polyethylene glycol-co-poly(methacrylic acid) polymeric network system for colon target delivery of oxaliplatin: Its acute oral toxicity study. Adv Polym Technol 37:1806–1822
Abdullah O, Usman Minhas M, Ahmad M, et al. (2018) Synthesis, optimization, and evaluation of polyvinyl alcohol-based hydrogels as controlled combinatorial drug delivery system for colon cancer. Adv Polym Technol [Internet]. [cited 2021 Jan 11];37:3348–3363. Available from: http://doi.wiley.com/https://doi.org/10.1002/adv.22119.
Jalil A, Khan S, Naeem F, et al. (2017) The structural, morphological and thermal properties of grafted pH-sensitive interpenetrating highly porous polymeric composites of sodium alginate/acrylic acid copolymers for controlled delivery of diclofenac potassium. Des Monomers Polym [Internet]. [cited 2018 Sep 12];20:308–324. Available from: https://www.tandfonline.com/doi/full/https://doi.org/10.1080/15685551.2016.1259834.
Khan S, Minhas MU, Ahmad M, et al. (2018) Self-assembled supramolecular thermoreversible β-cyclodextrin/ethylene glycol injectable hydrogels with difunctional Pluronic ® 127 as controlled delivery depot of curcumin. Development, characterization and in vitro evaluation. J Biomater Sci Polym Ed [Internet]. [cited 2018 Dec 18];29:1–34. Available from: https://www.tandfonline.com/doi/full/https://doi.org/10.1080/09205063.2017.1396707.
Sohail M, Ahmad M, Minhas MU, et al. (2015) Controlled delivery of valsartan by cross-linked polymeric matrices: Synthesis, in vitro and in vivo evaluation. Int J Pharm [Internet]. [cited 2018 Sep 12];487:110–119. Available from: https://www.sciencedirect.com/science/article/pii/S0378517315003208.
Ranjha NM, Ayub G, Naseem S, et al. (2010) Preparation and characterization of hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J Mater Sci Mater Med [Internet]. [cited 2017 May 18];21:2805–2816. Available from: http://springerlink.bibliotecabuap.elogim.com/https://doi.org/10.1007/s10856-010-4134-1.
Hussain A, Khalid SH, Qadir MI et al (2011) Water uptake and drug release behaviour of methyl methacrylateco-itaconic acid [P(MMA/IA)] hydrogels cross-linked with methylene bis-acrylamide. J Drug Deliv Sci Technol 21:249–255
Khanum H, Ullah K, Murtaza G et al (2018) Fabrication and in vitro characterization of HPMC-g-poly(AMPS) hydrogels loaded with loxoprofen sodium. Int J Biol Macromol 120:1624–1631. https://doi.org/10.1016/j.ijbiomac.2018.09.184
León A, Reuquen P, Garín C, et al. (2017) FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl Sci [Internet]. [cited 2021 Jan 12];7:49. Available from: http://www.mdpi.com/2076-3417/7/1/49.
Ali L, Ahmad M, Usman M et al (2014) Controlled release of highly water-soluble antidepressant from hybrid copolymer poly vinyl alcohol hydrogels. Polym Bull 71:31–46
Nasir N, Ahmad M, Minhas MU et al (2019) pH-responsive smart gels of block copolymer [pluronic F127-co-poly(acrylic acid)] for controlled delivery of Ivabradine hydrochloride: its toxicological evaluation. J Polym Res 26:1–15
Acknowledgements
The authors highly acknowledge College of Pharmacy, University of Sargodha Pakistan for providing lab facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zahra, Q., Minhas, M.U., Khan, S. et al. Fabrication of polyethylene glycol hydrogels with enhanced swelling; loading capacity and release kinetics. Polym. Bull. 79, 5389–5415 (2022). https://doi.org/10.1007/s00289-021-03740-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03740-8