Abstract
This study aimed to develop new thermally stable synthetic polyester network containing β-cyclodextrin (β-CD) cavities with good absorbent behavior to remove organic pollutants such as parabens derivatives (methyl and propyl parabens) from aqueous solution. β-Cyclodextrin polyester network (β-CDPN) was synthesized by the reaction of β-CD with N,N′-(4,4′-diphenylether) bis trimellitimide diacid chloride as a cross-linker agent in the presence of sodium hydride. Diimide acid chloride as a synthetic cross-linker agent was prepared by two-step reactions. The sorbent process was optimized by four different parameters, such as pH, the temperature of the solution, contact time, β-CDPN ratio. After adsorbed of parabens in aqueous solution, the β-CDPN precipitates were gathered, and the solution was analyzed by using the high-performance liquid chromatography (HPLC) technique. The results show the high absorbent capacity of parabens (about 99%) by β-CDPN cavities. Furthermore, the kinetic data were measured using the pseudo-first-order and pseudo-second-order equation. The pseudo-second-order showed the best fit for the kinetic studies (methyl and propyl parabens R2: 0.9979 and 0.9998, respectively). The adsorption equilibrium of methyl paraben using β-CDPN could be well defined with the Langmuir isotherm model. The adsorption ability of β-CDPN kept nearly unchanged after five filtration-regeneration cycles, further TGA and DTG experiments show β-CDPN has excellent thermal stability and can be used in a wide range of temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water has an important role in our life. It is also used in domestic, industrial and agricultural domains. However, biological activities lead to a series of organic pollutants such as dyes, aromatic phenol derivatives (such as parabens derivatives) and metals into water [1]. Parabens are a group of alkyl esters of p-hydroxybenzoic acid that includes methylparaben (MP), ethylparaben (EP), propylparaben (PP), butylparaben (BP), isobutyl paraben (IBP), isopropyl paraben (IPP), benzyl paraben (BeP), heptyl paraben (HP) and their sodium salts. Recently, studies have shown that a small percentage of the general population suffers from paraben allergies [2]. Parabens can be absorbed by dermal penetration or oral. A new investigation has also shown that parabens possess estrogen agonist activity [3], and they have detected in human breast tumor tissue with an average concentration of 20 ng/g of tissue [4]. Parabens may also cause male infertility, as they may cause testis mitochondrial dysfunction. Parabens, especially methyl and propyl parabens, are widely used as cosmetic maintainers in a wide variety of products such as face, body and hand creams, shampoos, lotions, moisturizers and underarm deodorants foundation [5]. These consumer products are used daily in different human activities. Therefore, due to their continued distribution through recreational waters (liberation from the skin in swimming pools, resorts, etc.), and domestic, urban and industrial wastewaters, parabens might achieve the aquatic media and particularly drinkable water sources [6]. For this reason, lately, the existence and transfer pathways of these compounds into the aquatic ecosystems have been receiving particular attention [7, 8]. A growing concern has arisen concerning their potential long-term effects not only on humans but also on aquatic organisms. The increase in water pollutions as the result of these compounds has led to research development in the water purification industry. Different methods of water purification are such as ion-exchange chromatography, activated carbon adsorption, reverse osmosis, molecular sieves and zeolite absorption techniques. However, some methods cannot remove trace amounts of organic pollutants in the range of ppm to ppb levels. Recently reported literature indicated that nonporous networks with cyclodextrin cavities are capable of absorbing organic pollutants into the water at favorable levels [9]. β-CD, as cyclic oligosaccharide, has seven glucose units linked by α-(1, 4) bonds. β-CD as molecular hosts can interact with different range of guest molecules and show non-covalent interactions such as hydrophobic, van der waals and hydrogen bonding within various organic compounds by formation inclusion complex into cylindrical cavity [10, 11]. However, the high solubility of β-CD into an aqueous medium limits their applications in the removal of organic pollutants from water resources [12]. Preparation of β-CDPN is a proper way to solve this problem [12, 13]. Polymerization of β-CD by cross-linked agents is one of the most important methods to change its properties, such as stability against pH, heat and chemical agents, as well as solubility in common solvents [14]. β-CDPN show to have many applications in several areas such as pharmacology [15], chromatography [16, 17], catalysts [18], environment [19], nutrition [20] and digressing of softener immigration [21,22,23]. So β-CDPN that was prepared by cyclic imide cross-linker agent the same polymer containing cyclic imide moiety [24, 25] have also thermal and oxidative stability into different natural media in comparison with other synthetic polymeric networks using commercial cross-linker agents [26, 27].
In our study, we prepared a new synthetic thermally stable polyester network (β-CDPN) (6a–d) by the reaction of β-CD (5) with diimide acid chloride (DACl) (4) as a cross-linked agent. β-CDPN (6c) can form an inclusion complex between β-CD cavities with parabens, so we investigated the efficiency of β-CDPN (6c) as an adsorbent for the removal of methyl and propyl parabens from various aqueous resources. Besides, the effects of various experimental parameters such as pH, temperature, time and amount of β-CDPN (6c) on the solution were studied. The sorption properties of various β-CDPN (6c) were studied in aqueous solution by using the HPLC method.
Materials and method
Materials
β-cyclodextrin (β-CD), phosphate buffered saline (PBS), acetonitrile and thionyl chloride were obtained from Sigma-Aldrich Chemical Company. Trimellitic anhydride, 4,4′-diaminodiphenyl ether, acetone, acetic acid and methanol were purchased from Merck Chemical Company and used without previous purification. Methyl paraben (MP), propyl paraben (PP), N,N-dimethyl formamide (DMF) and tetrahydro furan (THF) were obtained from Fluka Chemical Company. Potassium phosphate tribasic anhydrous (K3PO4), phosphoric acid (H3PO4), potassium phosphate tribasic anhydrous (K3PO4), potassium phosphate dibasic trihydrate (K2HPO4. 3H2O) and mono potassium phosphate (KHPO4) were purchased from Acros Organics (USA).
Equipment and experimental procedure
Fourier transform infrared (FT-IR) data was recorded by using Galaxy series FT-IR 5000 spectrophotometer (England) at wavenumber range of 400–4000 cm−1 via KBr pellets. 1H-NMR spectra were recorded on a Brucker Avance 300 MHz spectrometer into DMSO-d6 with TMS as an internal standard. Thermal behaviors of the resulting samples were measured by thermogravimetric analysis (TGA, TA instruments Q 5000) under N2 atmosphere at a heating rate of 10 °C/min. X-ray diffraction (XRD) was carried out on Philips X-Pert in the range of 2θ = 5°–80° by using 0.04° as the step length. Sorption of paraben samples was carried out by the Shimadzu HPLC system consisting of a pump, degasser, auto-injector, column oven, ultraviolet detector, guard column and chromolith C18 column (100 mm × 4.6 mm, Merck, Germany). HPLC gradient conditions used to separate samples by using acetonitrile and deionized water with a flow rate of 0.7 mL min−1 and detection at 254 nm. The gradient elution was performed as follows: 30% acetonitrile (0–8 min), ramped to 50% acetonitrile (8–14 min), and then ramped to 30% acetonitrile (14–16 min).
Preparation of cross-linker agent (4)
N,N′-(4,4′-diphenylether) bistrimellitimide diacid (3)
N,N′-(4,4′-diphenylether) bistrimellitimide (3) as diacid was prepared according to a published article [28]. Into a 250 mL, round-bottom flask, 3.84 g (20 mmol) of trimellitic anhydride (1), 2.00 g (10 mmol) of 4,4′-diaminodiphenyl ether (2), 80 mL of a mixture of acetic acid and pyridine (3:2) were mixed and the mixture was stirred at room temperature overnight, then was refluxed for 5 h. The solvent was removed under reduced pressure, and the residue was dissolved in 100 mL of cold water (Scheme S1). Then, the solid yellow product was filtered off and dried in vacuo; this yielded 4.95 g (90.3%) of yellow crystals (3). mp: 392–394 °C Anal. Calcd for C30H16N2O9: C, 65.71%; H, 2.92%; N, 5.10%. Found: C, 65.50%; H, 3.10%; N, 4.80%.
N,N′-(4,4′-diphenylether) bistrimellitimide diacid chloride (DACl)(4)
First, 3.00 g (5.47 mmol) of diacid (3) and 20 mL of thionyl chloride were added into a 100-mL round-bottom flask. The combination was heated in an oil bath up to 65 °C until the suspension mixture converted into a clear solution. Formerly, the solution stirred overnight at room temperature (Scheme S1). Unreacted thionyl chloride was separated under reduced pressure, and the deposit (DACl)(4) washed with dry n-hexane; this yielded 2.98 g (95.5%) of reed crystals (4) mp: 270–273 °C, Anal. Calcd for C30H14N2O7Cl2: C, 61.53%; H, 2.39%; N, 4.75%. Found: C, 61.42%; H, 2.48%; N, 4.54%.
Preparing of β-CDPN network (6a–d)
β-CD (5) (1.135 g, 1 mmol) was dissolved in DMF (25 mL) and NaH (1–7 mmol) dissolved in DMF and then was slowly added into the mixture and stirring continued over 24 h at room temperature to produce cyclodextrin oxyanion. Then, DACl (4) (cross-linker) (1–11 mmol) was dissolved into DMF (10 mL), added dropwise and the mixture was stirred over 24 h at (70–100 °C) under N2 atmosphere. The prepared mixture was cooled to room temperature and poured into 80-ml acetone until β-CDPN network (6a–d) was precipitated and washed with a large quantity of acetone (typically 3 × 100 ml) (in order to remove residual DMF) and finally was dried as brown powder into vacuum at room temperature (Scheme 1).
Water solubility measurements of β-CDPN (6a–d)
Water solubility’s of β-CDPN (6a–d) was prepared by different molar ratios of β-CD: NaH: cross-linker (according to Table 1) were measured as described in the followings procedure: Each sample (0.2 g) was dispersed into distillate water (1.0 ml) at room temperature within 1 h, and the mixture was centrifuged at 10,000 rpm about 15 min. The precipitate was taken, and the dried, into the vacuum oven, and dry mass was measured to calculate of its solubility [13, 29].
Batch method of parabens adsorptions
Adsorption experiments were performed by using plastic tubes. A specified 0.05 g of β-CDPN (6c) was added into plastic tubes containing a solution of parabens (100 mg L−1) with buffer phosphate. Then, the tubes were shaken by orbital shaker (YIHDER TS-560, Taiwan) at 150 rpm. Parabens adsorptions were measured by changing different parameters, such as pH, temperature of the solution, shaking time and β-CDPN (6c) amount. Finally, β-CDPN (6c) was centrifuged, and the concentration of adsorbed parabens was determined by HPLC (Fig. S1A). In fact, HPLC was used to determine the concentration of parabens before and after removal. The mobile phase consisted of acetonitrile (eluent A) and deionized water (eluent B), the gradient elution: 30% acetonitrile (0–8 min), ramped to 50% acetonitrile (8–14 min) and then ramped to 30% acetonitrile (14–16 min). Removal were accomplished at a flow rate of 0.7 mL min−1 and detection at 254 nm.
The highest adsorption capacity of parabens by each parameter was chosen for further investigation. The adsorption capacity and removal percentages (removal %) were calculated by using Eqs. (1) and (2) that follow.
Qe (mg g−1), is the adsorbed mass per gram of adsorbent, C0 and Ce are the initial and the equilibrium concentrations of parabens in (mg L−1) the solution, V is the volume of paraben solutions in a liter and m is the dry mass of β-CDPN (6c) in gram [30].
Desorption and reusability
Desorption studies were carried out by 100-mL methanol (96%) to desorb MP and PP by adsorbents (β-CDPN (6c)). The adsorbent (5 mg) added to 10 ml of paraben solutions (150 mg L−1), and the resulting mixture was stirred at room temperature for 3 h. The mixture was centrifuged, and the upper solution was separated. Then, 10 ml of methanol was added to the mixture, and they were stirred for 3 h. The mixture was centrifuged; the adsorbent was retained and reused in other steps. Concentrations of MP and PP solutions into the upper layer were measured using HPLC analysis (Fig. 3b) according to Eq. 3. HPLC gradient conditions used to separate samples by using acetonitrile and deionized water with a flow rate of 0.7 mL min−1 and detection at 254 nm. The gradient elution was performed as follows: 30% acetonitrile (0–8 min), ramped to 50% acetonitrile (8–14 min), and then ramped to 30% acetonitrile (14–16 min).
These adsorption–desorption cycles were successively conducted over five times by using the same adsorbents to each experiment Fig. 5a, b [31].
In order to test the reusability of the prepared β-CDPN (6c), the following equation was used.
where, Cd is the parabens (MP, PP) concentration in the desorption solutions (mg L−1) and Vd is the volume of the desorption solution in a liter, respectively, and V is the volume of the solution in a liter.
Results and discussions
Instrumental analysis
FT-IR spectroscopy
The chemical structure and purity of diacid (3) were confirmed by FT-IR and elemental analyses. FT-IR spectrum of diacid (3) showed a broad peak between 2500 and 3400 cm−1, which assigned to the COOH groups, peaks at 1775 cm−1 (C=O asymmetric imide stretching), 1722 cm−1 (C=O acid and symmetric imide stretching), 1390 and 728 cm−1 (imide characteristic ring vibration) that confirmed the presence of imide rings and carboxylic groups in this compound (Fig. 1a). The elemental analysis confirmed the molecular structure of diacid (3).
The chemical structure of DACl (4) was confirmed by FT-IR and elemental analyses. The FT-IR spectrum of diacid (3) showed peaks at 1780 cm−1 (C=O asymmetric imide stretching), 1724 cm−1 (C=O acid and symmetric imide stretching), 827 and 690 cm−1 (C–Cl) confirmed the presence of acyl chloride in this compound (Fig. 1a). The elemental analysis confirmed the molecular structure of DACl (4). The FT-IR spectrum of β-CDPN (6c) exhibited a new absorption peak at 1716 cm−1 related to ester groups and absorption bands in 1780, 1725, 1380 and 720 cm−1 related to stretching vibrations of the imide rings. The results indicate that esterification successfully took placed, and ester linkages were formed by the reaction of some of alkoxy anionic groups of β-CD (5) and DACl (4) moiety into cross-linker agent. FT-IR spectra of β-CD (5) and β-CDPN (6c) are shown in Fig. 1a. The β-CD (5) spectrum showed a broad peak between 3000 and 3600 cm−1, which was assigned to hydroxyl groups in this compound. Also, sharp peaks were found between 1027 and 1154 cm−1 related to primary and secondary C–OH stretching and C–O–C unsymmetrical stretching [32].
1H-NMR spectroscopy
The 1H-NMR characterization of β-CD (5) and β-CDPN (6c) were carried out in DMSO-d6. In the 1H spectrum (Fig. 1d), the six peaks at 5.66, 4.63, 4.46, 3.58, 3.46, 3.96 ppm were identified the protons of β-CD (5). Resulting peaks of β-CDPN (6c) confirmed the presence of imide and aromatic hydrogen’s at 7.22, 7.56, 8.02 ppm into the network, and the three peaks at 5.06, 3.96, 3.28 ppm could be assigned the protons of β-CD (5). DACl (4) is the most important product in order further functionalize the primary hydroxyl groups of diacid (3), since cl is a good leaving group and can be easily substituted by other nucleophiles. β-CDPN (6c) was obtained from reaction of β-CD (5) and DACl (4). The chemical shift of Hf (β-CDPN (6c)) on the compared with the original Hf (β-CD (5)) indicates a downshift and the esterification might cause the chemical shift difference on the different hydroxyl groups β-CDPN (6c), so confirmed the formation of ester moieties into the network.
X-ray diffraction technique
XRD figures of β-CD (5) and β-CDPN (6c) are shown in Fig. 1b. The diffraction peaks of β-CD (5) and β-CDPN (6c) located at 2θ = 13°, 18° and 18°–19°, respectively. The XRD patterns of β-CD (5) revealed several diffraction peaks, which are indicative of its crystalline character, and they showed a regular structure. The wide peak of β-CDPN (6c) approved an amorphous structure, and it can show a grainy and cavity structure, so β-CDPN (6c) had the ability formed the inclusion complex by MP and PP.
Thermal analysis
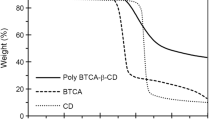
TGA thermogram of neat β-CD (5) at Fig. 1c displays multiple weight loss steps containing a weight loss around 110 °C due to the release of free and freeze-bound water (± 12%) into the β-CD (5) cavity. Second weight loss around 300–350 °C due to deoligomerization of β-CD (5) macromolecular chains and glucosidic rings cleavage (± 6%) and last weight loss around 450 °C due to degradation of C–C and C-O bond, release CO2 and H2O and finally formation of char yield.
TGA thermogram of β-CDPN (6c) networks displayed two weight loss steps above 350 °C and did not show any weight loss until 350 °C. It means β-CDPN (6c) shown thermal stability until 350 °C due to the formation of the ester linkage between some hydroxyl moiety into β-CD (5) and DACl (4). The formation of β-CDPN (6c) highly decreased the amount of water absorption of β-CD (5) moiety and so did not show any weight loss steps under 350 °C. First weight loss appeared around 350 °C (7%) due to the deoligomerization of β-cyclodextrin macromolecular moiety and glycoside rings cleavage same β-CD (5) thermogram and second weight loss appears around 480 °C due to the degradation of C–C and C–O bond, release CO2 and H2O and finally the formation of char yield. Thermogram of β-CDPN (6c) showed final thermal degradation taking place at higher temperature and more char yield formation due to its stability and more linkages into network (Fig. 1c).
Water solubility
Four samples of β-CDPN (6a–d) were prepared with different cross-linker agent contents (Table 1S). The results show β-CDPN (6C) prepared with higher cross-linker agent content is insoluble in water but has good solubility in polar aprotic solvents such as DMSO, DMAc and DMF [29]. Solubility behavior of four prepared β-CDPN (6a–d) indicated that the polymerization reaction of β-CD (5) with cross-linker agents occurred by the different percentage of cross-linking (Table 1S). β-CDPNs (6a and 6b) were soluble in water because the cross-linking agent is not enough, and cross-linking ratio is low.
Batch adsorption
MP is particularly crucial because it has high solubility (2.7 mg/ml in phosphate buffer) compared to other parabens. MP has also excellent chemical stability, is resistant to hydrolysis in hot and cold water and stable in air. In this research, we chose MP and PP because they have been used more as antimicrobial preservatives in foods, drugs and cosmetics for over the past 50 years and have high industrial value.
Effect of pH on the extraction recovery of parabens
The adsorption process mainly depended on pH of the solution. The adsorption experiments were measured in different pH values in the range of 2–12. The pH value has a vital role in surface charge of adsorbent, degree of ionization and paraben structure (Scheme 2a). In the paraben molecules, two sequences and competitor processes occur. Firstly, oxygen atoms of the ester group (C=O) are protonated into pH < 3. Then the hydroxyl groups are deprotonated into pH > 9. Parabens exist mainly as a neutral form between pH 4–9, and strongly hydrophilic interaction occurred between parabens and β-CDPN (6c) cavities that led to maximum complexion (Fig. 2a). Parabens mostly exit as deprotonated form above pH > 9, and there are not firmly hydrophilic interaction between parabens and β-CDPN (6c) cavities leading to minimum complexion (Fig. 2a).
Effect of temperature on the extraction recovery of parabens
The results show a little influence on the recovery extent (about 8 percent) due to changing temperature change (Fig. 2b). Therefore, optimum temperature was set at 30 °C because a high recovery extent accrued at 30 °C. Besides, at this temperature, efficient separation of phases was achieved [33].
Effect of time on the extraction recovery of parabens
The effect of time on recovery extent was studied between 0.5 and 24 h (Fig. 2c). The results show a strong relation between time of extraction and recovery extent in the range of 3–18 h. MP was extracted about 99.0% percent after 3 h and 100% percent after 18 h into pH = 9 at 30 °C (Fig. 3a). The highest degree of extraction value (about 98.0%) of PP was accrued after 18 h into pH = 9 at 30 °C. MP has highest extraction value due to its small size that easily trapped into β-CDPN (6c) cavities. Therefore, in order to find a higher value of extracting parabens, all of experiments were measured after 18 h.
Effect of β-CDP amounts on the extraction recovery of parabens
The effect of the β-CDPN (6c) amount on the recovery extent of parabens was tested. The results show there is a slight relation (about five percent) between the β-CDPN (6c) amount and the recovery extent of parabens. The optimum amount of β-CDPN (6c) was about 40 ppm (Fig. 2d) [30].
Adsorption behavior of β-CDPN (6c) toward parabens
Table 1 shows the adsorption capacities of each paraben by β-CDPN (6c). When β-CDPN (6c) swelled into an aqueous solution of paraben molecules in water, parabens penetrated apolar β-cyclodextrin’s cavities [34, 35] and host-quest complexes were formed. Because apolar β-cyclodextrin’s cavities show lipophilic character, water molecules will be easily substituted by paraben molecules as organic lipophilic compounds [36, 37]. The results show that adsorption capacity increased within small parabens such as methyl paraben. Small molecules can easily penetrate into non-covalent cavities of β-CDPN (6c), and they formed better interactions (hydrophobic interactions, van der waals forces and hydrogen bonds) between β-CDPN (6c) and parabenes (Scheme 2b).
Adsorption isotherms
There are several equilibrium relation models between sorbent and sorbet. The Freundlich and Langmuir models are the most frequently employed models. Here, both models are used to describe the relationship between parabenes and its equilibrium concentrations. The linear form of the Freundlich isotherm given by the following relation:
The qe parameter is the adsorbed amount at equilibrium (mg g−1), Ce is the equilibrium concentration of the adsorbent (mg L−1), k and \( \frac{1}{n} \) are the Freundlich constants related to capacity and intensity adsorption, respectively, of the adsorbent. Freundlich isotherm constants are determined by plot of log qe versus log Ce (Fig. 3a).
The linear form of the Langmuir isotherm model has been represented by the following relation:
Qe is also the adsorbed amount at equilibrium (mg g−1), Q (mg g−1) and b (L mg−1) are the Langmuir constants related to the maximum adsorption and energy of adsorption, respectively. Langmuir isotherm constants determined by plots of Ce/qe versus Ce (Fig. 3b). The isotherm parameters and the correlation coefficients are shown in Table 2. The sorption equilibrium data fitted Langmuir and Freundlich equations with correlation coefficients values of 0.9923 and 0.9932, respectively (Table 2).
Adsorption kinetics
Kinetics of adsorption method during the design of an adsorption system for practical applications is very important. For this purpose, the two well-known kinetic models, i.e., pseudo-first-order, pseudo-second-order, were applied to study the kinetics and mechanism of MP and PP sorption onto β-CDPN (6c) and the most suitable model was picked for the experimental kinetic data. The linear plots of the kinetic models for MP and PP presented in Fig. 4 and Table 3.
Lagergren pseudo-first-order model
This mathematical equation gives the kinetic form of the Lagergren pseudo-first-order model:
qe and qt are the amounts of the MP, and PP adsorbed at equilibrium (mg g−1) and at contact time t (min), respectively. K1 (min−1) is the rate constant for the first-order adsorption. As shown in Table 3 and Fig. 4, the obtained correlation coefficients values (R2) for β-CDPN (6c) at temperatures (30 °C) were in out of the unit form. Therefore, the pseudo-first-order kinetic could not describe the β-CDPN (6c) diffusion mechanism; hence, the pseudo-second-order kinetic as another model was applied.
Agergren pseudo-second-order model
This model is applied when the rate of occupation of sites is proportional to the square of the number of unoccupied sites on β-CDPN (6c) [38]. The mathematical equation gives the kinetic form of the agergren pseudo-second-order model:
K2 (g mg−1 min−1) is the rate constant of second-order adsorption. The values K2 and qe were calculated from the slope and intercept of the plot of t/qe versus t, according to the equation mentioned above. The obtained values of the correlation coefficients (R2) for the β-CDPN (6c) via the pseudo-second-order kinetic model were closer to the unit at temperature (30 °C). These results indicated the preference of using pseudo-second-order kinetic model for adsorption of the MP and PP of β-CDPN (6c) [39].
Desorption and reusability
The recovery and reusability of MP and PP of the sorbent are essential factors for evaluating potential application value of the β-CDPN (6c). The synthesized β-CDPN (6c) showed a good affinity for MP and PP, thus the sorbents used for the removal of MP and PP. Four various solvent eluents consisting of methanol, ethanol, acetone and H2O were applied to remove the MP and PP from sorbet samples at 3 h as shown in Fig. 5c. The preliminary results indicate that MP and PP full onto the samples can easily desorb with a single wash and by using methanol and acetone with a maximum desorption for MP and PP above 85.86%, 81.22%, 66.68% and 63.23%, respectively. The reusability of adsorbent can reduce the cost of processing because adsorbent is usually an expensive component for experiment. Methods and materials used in regeneration process are essential, and they should be simple and low cost. Besides, the results for five cycles of adsorption–desorption are shown in Fig. 5a, b. The β-CDPN (6c) maintained nearly 75.32% and 65.41% of MP and PP, respectively, after the five consecutive cycles of adsorption–desorption. These results suggested good performance and recyclability for β-CDPN (6c) prepared as adsorbents of MP and PP.
Comparison between our results and related literature
Table 4 lists the comparison of maximum adsorption capacity of MP and PP on different adsorbents. In comparison with these adsorbents, the β-CDPN (6c) adsorbent gave a higher adsorption capacity with faster rate of adsorption and a lower amount of the adsorbent. The main reason, the presence of diimide rings in the cross-linking were caused π–π interactions and hydrogen bonding by parabenes, and all the active sites of β-CD were entirely exposed and saturated. All these features account for establishing the efficacy of the β-CDPN (6c) adsorbent as a potential adsorbent for MP and PP [30, 31, 40].
Conclusions
In this study, we reported the synthesis of some β-CDPN (6a–d) containing imide rings into the backbone prepared by a facile polymerization reaction into DMF solution by reaction of β-CD (5) with DACl (4) as cross-linkages agent. The structure and properties of β-CDPN (6c) were determined by FT-IR, 1H NMR, TGA and XRD. β-CDPN (6c) used as an adsorbent for the removal of the MP and PP from different aqueous solutions. The adsorption of MP and PP found to be dependent on initial concentration, pH, temperature and time. β-CDPN (6c) showed remarkable adsorption capacities at optimum condition (pH = 9, T = 30 °C, time = 3 h, concentration = 0.05) and adsorb parabens by electrostatic attraction, host–guest supra-molecular interactions and π–π conjugation interactions. The MP and PP loaded on the β-CDPN (6c) could be removed above 86.2% and 84.1% with a single wash by methanol, whiles after five consecutive cycles of adsorption–desorption, 75.4% and 73.2% of removal efficiency were achieved. The thermogram of β-CDPN (6c) showed that final thermal degradation took placed at high temperatures and high char yield formation due to stability and more linkages into the network. These materials show several advantages compared to other adsorbents, including being environment friendly, effective adsorption and reusability. The kinetic method can predict by pseudo-second-order model, and rate constants for MP and PP sorption were found to be 0.0012 and 0.0063 g·mg−1·min−1, respectively, at 30 °C. Also, based on the highest R2 values, fitted with Langmuir model. These results show that β-CDPN (6c) can be used to remove other organic contaminants such as aromatic amines, phenol derivatives, dyes, pesticides and drugs. This study improves our understanding of rationally designing highly efficient adsorbents to remove MP and PP from aqueous solutions.
References
Crini G (2008) Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigm 77(2):415–426
Nagel JE, Fuscaldo JT, Fireman P (1977) Paraben allergy. JAMA 237(15):1594–1595
Harvey PW, Darbre P (2004) Endocrine disrupters and human health: could oestrogenic chemicals in body care cosmetics adversely affect breast cancer incidence in women? A review of evidence and call for further research. J Appl Toxicol Int J 24(3):167–176
Darbre P et al (2004) Concentrations of parabens in human breast tumours. J Appl Toxicol Int J 24(1):5–13
Márquez-Sillero I et al (2010) Determination of parabens in cosmetic products using multi-walled carbon nanotubes as solid phase extraction sorbent and corona-charged aerosol detection system. J Chromatogr A 1217(1):1–6
Juliano C, Magrini G (2017) Cosmetic ingredients as emerging pollutants of environmental and health concern. A mini-review. Cosmetics 4(2):11
Li W et al (2015) Occurrence, fate and risk assessment of parabens and their chlorinated derivatives in an advanced wastewater treatment plant. J Hazard Mater 300:29–38
Ramaswamy BR et al (2011) GC–MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J Hazard Mater 186(2–3):1586–1593
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30(1):38–70
Saenger W (1980) Cyclodextrin inclusion compounds in research and industry. Angew Chem Int Ed Engl 19(5):344–362
Al-Rawashdeh NA, Al-Sadeh KS, Al-Bitar MB (2013) Inclusion complexes of sunscreen agents with β-cyclodextrin: spectroscopic and molecular modeling studies. J Spectrosc 2013
Li X et al (2018) A water-insoluble viologen-based β-cyclodextrin polymer for selective adsorption toward anionic dyes. React Funct Polym 126:20–26
Girek T, Shin D-H, Lim S-T (2000) Polymerization of β-cyclodextrin with maleic anhydride and structural characterization of the polymers. Carbohyd Polym 42(1):59–63
Soni M, Carabin I, Burdock G (2005) Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 43(7):985–1015
Hostetler JS, Hanson L, Stevens D (1992) Effect of cyclodextrin on the pharmacology of antifungal oral azoles. Antimicrob Agents Chemother 36(2):477–480
Rodríguez-Bonilla P et al (2011) Development of a reversed phase high performance liquid chromatography method based on the use of cyclodextrins as mobile phase additives to determine pterostilbene in blueberries. J Chromatogr B 879(15–16):1091–1097
Guillaume Y et al (2003) Chromatographic determination of the association constant between 8-methoxypsoralen and modified β-cyclodextrin: protective effect of hydroxypropyl-β-cyclodextrin on 8-methoxypsoralen toxicity in human keratinocytes. J Chromatogr B 798(2):217–222
Zhang B, Breslow R (1997) Ester hydrolysis by a catalytic cyclodextrin dimer enzyme mimic with a metallobipyridyl linking group. J Am Chem Soc 119(7):1676–1681
Nandi N, Bagchi B (1996) Ultrafast solvation dynamics of an ion in the γ-cyclodextrin cavity: the role of restricted environment. J Phys Chem 100(33):13914–13919
Flurer CL et al (1995) Determination of ephedrine compounds in nutritional supplements by cyclodextrin-modified capillary electrophoresis. J Chromatogr B Biomed Sci Appl 669(1):133–139
Raeisi A, Faghihi K, Shabanian M (2017) Designed biocompatible nano-inhibitor based on poly (β-cyclodextrin-ester) for reduction of the DEHP migration from plasticized PVC. Carbohyd Polym 174:858–868
Raeisi A, Shabanian M, Faghihi K (2017) Preparation of β-cyclodextrin-ester network and new organo-modified LDH as dual additives of PVA: thermal, dynamic-mechanical and migration study. Prog Org Coat 111:402–415
Raeisi A et al (2019) A complete description on effect of β-cyclodextrin-ester as a bio-based additive for preparation of safe PVC: from synthesis to Computational study. Mater Today Commun 22:100736
Faghihi K, Hajibeygi M, Shabanian M (2010) Polyimide–silver nanocomposite containing phosphine oxide moieties in the main chain: synthesis and properties. Chin Chem Lett 21(11):1387–1390
Faghihi K et al (2011) Synthesis and thermal and photo behaviors of new polyamide/organocaly nanocomposites containing para phenylenediacrylic moiety. High Temp Mater Process (Lond) 30(3):223–228
Song Q et al (2014) Controlled thermal oxidative crosslinking of polymers of intrinsic microporosity towards tunable molecular sieve membranes. Nat Commun 5:4813
Hădărugă DI et al (2014) Thermal and oxidative stability of the Ocimum basilicum L. essential oil/β-cyclodextrin supramolecular system. Beilstein J Org Chem 10(1):2809–2820
Faghihi K, Hajibeygi M (2004) Synthesis and properties of new poly (amide imide) s containing trimellitic rings and hydantoin moieties in the main chain under microwave irradiation. J Appl Polym Sci 92(6):3447–3453
Girek T, Ciesielski W (2011) Polymerization of β-cyclodextrin with maleic anhydride along with thermogravimetric study of polymers. J Incl Phenom Macrocycl Chem 69(3–4):445–451
Noorashikin M, Mohamad S, Abas M (2014) Extraction and determination of parabens in water samples using an aqueous two-phase system of ionic liquid and salts with beta-cyclodextrin as the modifier coupled with high performance liquid chromatography. Anal Methods 6(2):419–425
Chin YP, Mohamad S, Abas MRB (2010) Removal of parabens from aqueous solution using β-cyclodextrin cross-linked polymer. Int J Mol Sci 11(9):3459–3471
Kono H, Fujita S (2012) Biodegradable superabsorbent hydrogels derived from cellulose by esterification crosslinking with 1, 2, 3, 4-butanetetracarboxylic dianhydride. Carbohyd Polym 87(4):2582–2588
Noorashikin M, Mohamad S, Abas M (2012) Ionic liquid with β-cyclodextrin based aqueous two phase extraction of parabens in water samples. In: Proceedings from the 3rd international conference on chemistry and chemical engineering. IACSIT Press, Singapore
Pariot N et al (2000) Cross-linked β-cyclodextrin microcapsules: preparation and properties. Int J Pharm 211(1):19–27
Dong H et al (2013) A versatile multicomponent assembly via β-cyclodextrin host-guest chemistry on graphene for biomedical applications. Small 9(3):446–456
Kono H, Teshirogi T (2015) Cyclodextrin-grafted chitosan hydrogels for controlled drug delivery. Int J Biol Macromol 72:299–308
Dodziuk H (2006) Cyclodextrins and their complexes: chemistry, analytical methods, applications. Wiley, New York
Dil NN, Sadeghi M (2018) Free radical synthesis of nanosilver/gelatin-poly (acrylic acid) nanocomposite hydrogels employed for antibacterial activity and removal of Cu (II) metal ions. J Hazard Mater 351:38–53
Rahangdale D, Kumar A (2018) Chitosan as a substrate for simultaneous surface imprinting of salicylic acid and cadmium. Carbohyd Polym 202:334–344
Noorashikin M, Mohamad S, Abas M (2012) Ionic liquid with β-cyclodextrin based aqueous two phase extraction of parabens in water samples. In: 3rd international conference on chemistry and chemical engineering
Acknowledgements
We gratefully acknowledge the financial support of this research by the Research Council of Arak University and thank experts at Food and Beverage Quality Control Laboratory of Arak.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faghihi, K., Soleimani, M. Synthesis of new polyester networks containing β-cyclodextrin cavities for removal of paraben derivatives from water resources by inclusion complexes. Polym. Bull. 79, 227–244 (2022). https://doi.org/10.1007/s00289-020-03416-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03416-9