Abstract
In this study the β-cyclodextrin (β-CD) polymer, synthesized by reaction of β-CD with maleic anhydride (MA) in anhydrous N,N-dimethylformamide (DMF) in the presence of NaH, was characterized by thermogravimetric (TG) and calorimetric (DSC) studies. The weight-average molecular weight (Mw) and the chemical structure of the polymers were described in earlier papers. The molecular weight of the polymer increased with reaction temperature. The molar ratio of the reagents was deciding in determination of the structure and molecular weight of the obtained β-CD polymers. The thermogravimetric measurements were made to explain the influence of ratio of substrates on thermal decomposition of β-cyclodextrin polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins and their derivatives are used in a very large number of applications. Cyclodextrins are cyclic oligosaccharides formed by α-(1–4) bonds of glucopyranose units. The α-, β- and γ-cyclodextrins consist of six, seven and eight glucopyranose units, respectively [1, 2]. In a CD molecule, the secondary hydroxyl groups at the C2 and C3 positions of the glucopyranosyl unit protrude from the wide opening of truncated cone structure of CD, whereas the primary hydroxyl groups at C6 are exposed from the opposite opening. It is widely acknowledged that CDs can form complexes with various organic and inorganic substances, included in their hydrophobic cavities [3, 4]. Because of this unique property, CDs are applied in pharmaceutical, food and cosmetic industries [5, 6] as well as in analytical chemistry [7, 8] and in separation techniques [9].
When CD is polymerized, it is less soluble and more stable, therefore CD polymers can be used to remove contaminants from foods or biological matrices [10, 11]. Two types of CD polymers are known. In first type polymers the CD molecules are attached as pendant groups on polymer chains. Such polymers are often prepared by radical polymerization of the functional CD monomers such as acryloyl cyclodextrin (CD-A) or N-acryloyl-6-aminocaprocyclodextrin (CD-NAC) [12, 13].
Formation of the second type polymers requires the reaction of CD molecules with bifunctional agents. The most common agent serving as a crosslinker is epichlorohydrin; this kind of a polymer is used e.g. for Hg(II) determination by a solid-phase spectrophotometric method [14]. The CD polymer crosslinked with toluene-2,4-diisocyanate is applied as stationary phase in HPLC. This polymer, synthesized using steroids as templates shows a good recognition mechanism for cholesterol from the steroids family [15].
The nucleophilic substitution reactions of β-CD with bifunctional agents usually occur under strong alkaline conditions required for the deprotonation of hydroxyl groups of glucose units. On the other hand, in anhydrous medium such as N,N-dimethylformamide, CDs easily react with sodium hydride (NaH) resulting in deprotonation of hydroxyl groups mainly at the C2-position to give oxoanions. In some cases such deprotonated CDs react with other CD molecules deprotonated in 3-position affording 2,3-epoxides. [16, 17] The epoxides can react with succinic and octenylsuccinic anhydrides to give CD polymers [18, 19].
In the present study, β-cyclodextrin polymers were prepared by crosslinking with maleic anhydride via the oxoanion or epoxide intermediate formed by treatment with sodium hydride.
Experimental
Reagents and solvents
Crystalline β-CD, maleic anhydride (MA), sodium hydride, N,N-dimethyl formamide (DMF) and Linde type 4 Å molecular sieves were purchased from Aldrich (Germany). β-CD was recrystalized from water on dried for 12 h in vacuo at 110 °C [20]. DMF was predried over molecular sieves, and then distilled under vacuum. The dried DMF was stored in a dark bottle over molecular sieves [20].
Synthesis of β-CD polymers crosslinked by maleic anhydride (MA)
The β-CD polymers crosslinked by MA were obtained as previously described [18, 21]. β-CD (0.001 mol) was dissolved in DMF (25 ml), and then solid NaH (0.001–0.007 mol) was slowly added into the solution with vigorous stirring. Stirring was continued for 24 h at room temperature. Then solid MA (0.001–0.011 mol) was slowly added to the solution of formed β-CD oxoanion or epoxide. The reaction mixture was continuously stirred in a sealed round bottom flask in an oil bath at a controlled temperature (25–130 °C) during 24 h. The reaction product was precipitated and washed with a large quantity of acetone, and finally dried in a vacuum desiccator at room temperature to obtain a white to yellow-brown powder.

Thermogravimetry (TG), differential thermogravimetry (DTG) and differential scanning calorimetry (DSC)
The thermal DSC–TG–DTG analysis was carried out with the NETZSCH STA-409 simultaneous thermal analyzer calibrated with standard indium, tin, zinc, and aluminium of 99.99% purity. Samples of approximately 0.020 g were heated in corundum crucibles with non-hermetic lids. Corundum (SINGLE *R) was the standard. The heating was performed under static conditions in the air in the range of 20–500 °C with the 5 K min−1 temperature rate. The measurements were duplicated. They provided the ±0.5 °C precision in reading of temperature. Recorded thermograms were analysed with the NETZSCH–TA–ANALYSIS and NETZSCH SEPARATION OF PEAKS programs.
Results and discussion
Six β-CD polymers P1–P6 were obtained at temperature 25–100 °C and R = ratio of CD:NaH:MA equal to 1:4:4 and 1:4:7. The above polymers may be divided into two groups: (1) polymers P1–P3 obtained at R = 1:4:4 and (2) polymers P4–P6 obtained at R = 1:4:7.
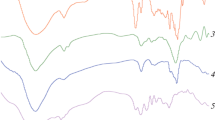
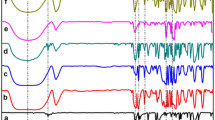
For all six polymers P1–P6 (Figs. 1–6), after the loss of water (12–14%) at tgα = 0.5–0.7 (the slope is defined as a mass loss percent (%) for 1 °C), accompanied by an endothermic effect at the low-temperature part of the DSC diagram (between 74–78 °C), on the TG line a plateau appears. Then the fast mass loss begins (tgα = 3.69–3.96). This point shows the start of polymer decomposition. Then the sharp, monotonous mass loss of polymer occurs, accompanied for all P1–P6 polymers by two endothermic effects at 260 and 305 °C. At ca 330 °C the fast mass loss is finished.
The TG line indicates the one-step mass loss of the sample. For P1–P6 the DTG peak is observed at ca. 300 °C. The DTG lines are symmetric and sharp, this fact confirms the one-step, fast polymer decomposition. Then the decomposition becomes slower and proceeds up to 500 °C, when 90% loss of the initial mass is observed.
Analysis of thermal properties of P1–P6, either of R = 1:4:4 or of R = 1:4:7 offers suggestions concerning their structure. The thermal properties of polymers P1–P3, obtained at R = 1:4:4 are presented in Table 1.
For polymers obtained at R = 1:4:4, i.e. P1–P3, the main peak of decomposition, accompanied by the exothermic effect, corresponding to oxidation processes of the system is observed at 340–370 °C region. According to Table 1, Figs. 1–3 and data concerning the molecular masses, the formation of the polymer shifts this exothermic peak toward higher temperatures. The above results show that for R = 1:4:4 the temperature strongly influences the formation of polymer. It was found that temperature equal to 100 °C is optimal for formation of polymers P1–P3.
The thermal properties of polymers P4–P6 obtained at R = 1:4:7 are presented in Table 2.
For polymers obtained at R = 1:4:7, i.e. P4–P6 it was found that temperature of the main peak of decomposition increases from 339 °C for P4, obtained at 25 °C, (Fig. 4) to 356 °C for P5 obtained at 60 °C (Fig. 5). For P6, obtained at 100 °C the temperature of this peak is equal to 339 °C. According to Table 2, Figs. 4–6 and literature data [18, 21] it may be concluded that temperature equal to 100 °C is optimal for formation of P4–P6 polymers.
Conclusion
β-Cyclodextrin (β-CD) polymers were prepared by crosslinking β-CD with maleic anhydride (SA) in anhydrous N,N-dimethyl formamide (DMF) in the presence of NaH. The thermal properties of the polymers were determined using thermogravimetric and calorimetric studies.
Comparison of TG/DTG/DSC data (Figs. 3, 6) allows to conclude that optimal conditions for preparation of β-CD polymers with maleic anhydride is temperature of 100 °C and R equal to 1:4:4.
References
Larsen, K.L.: Large cyclodextrins. J. Incl. Phenom. Mol. Recognit. Chem 43, 1–13 (2002)
Bender, M.L., Komiyama, M.: Cyclodextrin Chemistry, pp. 2–8. Springer-Verlag, Berlin Heidelberg, New York (1978)
Szejtli, J.: Cyclodextrin and Their Inclusion Complexes, pp. 95–140. Akademiai Kiado, Budapest, Hungary (1982)
Szejtli, J.: Cyclodextrin Technology, pp. 45–58. Kluwer Academic Press, Dordrecht, The Netherlands (1988)
Astray, G., Gonzalez-Barreiro, C., Mejuto, J.C., Rial-Otero, R., Simal-Gandara, J.: A review on the use of cyclodextrins in foods. Food Hydrocoll. 23, 1631–1640 (2009)
Buschmann, H.-J., Schollmeyer, E.: Applications of cyclodextrins in cosmetic products: a review. J. Cosmet. Sci. 53, 185–191 (2002)
Fragoso, A., Almirall, E., Cao, R., Echegoyen, L., González-Jonte, R.,: A supramolecular approach to the selective detection of dopamine in the presence of ascorbate. Chem. Commun. 2230–2231 (2004)
Tang, B., Zhang, L., Zhang, J., Chen, Z.-Z., Wang, Y.: Synthesis of a novel host molecule of cross-linking-polymeric-β-cyclodextrin-o-vanillin furfuralhydrazone and spectrofluorimetric analysis of its identifying cadmium. Spectrochim Acta 60A, 2425 (2004)
Matsuzawa, H., Mikami, K.: Highly efficient discrimination of fluorous tags by β-cyclodextrin columns: new isolation method for fluorous mixture synthesis. Synlett. 1607–1612 (2002)
Shaw, P.E., Tatum, J.H., Wilson, C.W.: Improved flavor of navel orange and grapefruit juices by removal of bitter components with β-cyclodextrin polymer. J. Agric. Food Chem. 32, 832–836 (1984)
Shaw, P.E., Wilson, C.W.: Reduction of bitterness in grapefruit juice with β-cyclodextrin polymer in a continuous-flow process. J. Food Sci. 50, 1205–1207 (1985)
Harada, A., Furue, M., Nozakura, S.: Preparation of cyclodextrin-containing polymers and their catalysis in ester-hydrolysis. J. Polym. Sci. Polym. Lett. Edition 13, 357–360 (1975)
Harada, A., Furue, M., Nozakura, S.: Cyclodextrin-containing polymers. 1. Preparation of polymers. Macromolecules 9, 701–704 (1976)
Liu, Y., Chang, X., Hu, X., Guo, Y., Meng, S., Wang, F.: Highly selective determination of total mercury(II) sub microgram per liter by β-cyclodextrin polymer solid-phase spectrophotometry using 1, 3-di-(4-nitrodiazoamino)benzene. Anal. Chim. Acta 532, 121–128 (2005)
Asanuma, H., Hishiya, T., Komiyama, M.: Efficient separation of hydrophobic molecules by molecularly imprinted cyclodextrin polymers. J. Incl. Phenom. Macrocycl. Chem. 50, 51–55 (2004)
Rong, D., D’Souza, V.T.: A convenient method for functionalization of the 2-position of cyclodextrins. Tetrahedron Lett. 31, 4275–4278 (1990)
Khan, A.R., D’Souza, V.T.: Epoxides of the secondary side of cyclodextrins. J. Org. Chem. 61, 8301–8303 (1996)
Girek, T., Kozlowski, C.A., Koziol, J.J., Walkowiak, W., Korus, I.: Polymerisation of β-cyclodextrin with succinic anhydride—synthesis, characterization, and ion flotation of transition metals. Carbohydr. Polym. 59, 211–215 (2005)
Choi, J.-K., Girek, T., Shin, D.-H., Lim, S.-T.: Structural and physical characterization of octenylsuccinyl β-cyclodextrin. Carbohydr. Polym. 49, 289–296 (2002)
Armarego, W.L., Perrin, D.D.: Purification of Laboratory Chemicals, pp. 192–193. Butterworth-Heinemann, Oxford (1996)
Girek, T., Shin, D.-H., Lim, S.-T.: Polymerization of β-cyclodextrin with maleic anhydride and structural characterization of the polymers. Carbohydr. Polym. 42, 59–63 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Girek, T., Ciesielski, W. Polymerization of β-cyclodextrin with maleic anhydride along with thermogravimetric study of polymers. J Incl Phenom Macrocycl Chem 69, 445–451 (2011). https://doi.org/10.1007/s10847-010-9778-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9778-4