Abstract
The aim of this work was the development and evaluation of controlled release formulations consisting of methacrylate derivatives and ethyl cellulose synthesized using oil-in-oil solvent evaporation method. Drug release studies were performed in different dissolution media. Maximum drug released was observed at pH 7.4. Fourier transform infrared spectroscopy spectra, SEM and thermal analysis showed compatibility between drug and polymers. Pharmacokinetic parameters were calculated by Phoenix WinNonlin® version 6.3 software. The average AUC0–t was found to be 2483.71 ± 13.173 ng/ml h, 5954.37 ± 12.110 ng/ml h, 6400.82 ± 19.131 ng/ml h and 7427.4 ± 49.322 ng/ml h for group 1–4, respectively. The maximum concentration (Cmax) of IBH for all groups predicted from pharmacokinetics data was 880.38 ng/ml, 718.43 ng/ml, 721.87 ng/ml and 805.11 ng/ml, respectively. Thus, in vitro and in vivo drug release studies of polymeric microspheres proved their controlled release behavior with preferential delivery for an extended period of time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oral conventional drug administration usually does not deliver rate-controlled release or target specificity. In many cases, conventional drug delivery provides sharp increases of drug concentration at potentially toxic levels. Today new methods of drug delivery are possible: desired drug release can be provided by rate-controlling membranes or by implanted biodegradable polymers containing dispersed medication. Over the last 30 years most of research has been focused on biodegradable polymeric microspheres for drug delivery. Administration of medications through such systems has advantageous because microspheres can be injected or ingested; they can be tailored for desired release [1]. Microspheres is a quickly developing technology for achieving controlled release formulations. It is a well-known method that is used to modify and delay drug release from pharmaceutical dosage form. A large number of techniques are available for the formation of sustained and controlled release drug delivery systems [2]. Controlled release polymeric-based system has become the most widespread topics in pharmaceutical technology [3]. Among numerous routes of delivery oral route is the most preferred route to the patient and the clinician alike. However, it presents some problems for a large number of drugs; the enzymes in the gastrointestinal fluid (GIT); pH conditions of GIT; and the enzymes linked to membranes of GIT are the main factors accountable for the bioavailability problems. The blood that drains the GIT transfers the drug directly to the liver leading to first-pass metabolism resulting in poor bioavailability [4,5,6]. These problems can be solved either by changing routes of administration or by modifying the formulation. Controlled drug delivery is an alternative method of drug administration orally in form of polymeric drug-loaded microspheres.
Ivabradine-HCl (IBH) is 3-[3-[[(7S)-3, 4-dimethoxy-7-bicyclo [4.2.0] octa-1, 3, 5-trienyl] methyl-ethylamino] propyl-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-onehydrochloride, shown in Fig. 1. Heart rate reduction (HRR) is a significant target for management of patients with stable angina. The drugs available for the management of HRR include calcium channels blockers (CCB) and beta blockers (BB) [7,8,9]. For the symptomatic management of stable angina pectoris, IBH a novel medication is used [8, 10]. IBH has different modes of action from CCB and BB. IBH is a cardiotonic agent, and it produced anti-anginal effect by reducing the heart rate via specific inhibition of the pacemaker current [11]. The plasma half-life of IBH is about 2 h with 40% bioavailability [12].
The present research focuses on developing polymeric microspheres for enhancing and improving the drug release in a controlled fashion and follows a logical approach in terms of pharmaceutical design using novel drug. Optimized formulations were used by using different methacrylate derivatives and EC. The purpose was to evaluate controlled drug delivery of various polymeric formulations prepared by solvent evaporation technique. Methacrylate derivatives Eudragit® L100-55-EC, Eudragit® FS30D-EC and Kollicoat® MAE 100P-EC were formulated and evaluated for in vitro and in vivo evaluation. In vitro dissolution studies were carried out at pH 1.2, 5.5 and 7.4 at 37 °C to determine the pH-dependent behavior of drug release. FTIR, scanning electron microscopy (SEM), X-ray diffractometry (XRD) and differential scanning calorimetry (DSC) were performed to study to evaluate the polymeric microspheres. On the basis of in vitro results pharmacokinetic analysis of IBH was performed in healthy albino rabbits after oral administration of drug solution and microspheres formulations containing IBH.
Experimental
Materials and methods
Materials
IBH was received as a gift sample from CCL Pharmaceuticals, Lahore. Ethyl cellulose (viscosity 300cP, 5% in toluene/ethanol 80:20, 48% ethoxyl), Span 80, Eudragit® L100-55, Eudragit® FS30D, Kollicoat® MAE100P, light liquid paraffin, n-hexane were purchased from Sigma-Aldrich (Evonik Krefeld, Germany). Ethanol, dichloromethane (DCM) and acetone were purchased from BDH, Pakistan. Sodium hydroxide (NaOH) was purchased from Merck, Darmstadt, Germany. Potassium dihydrogen phosphate (KH2PO4) was purchased from Merck, Darmstadt, Germany, and Hydrochloric acid was purchased from BDH, Pakistan. Potassium bromide (KBr) of FTIR grade was purchased from Fischer Scientific, Leicestershire, UK. All chemicals and solvents used were of analytical grades.
Development of polymeric microspheres
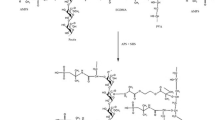
IBH-loaded Eudragit® L100-55 and EC microspheres were developed by O/O solvent evaporation method. Eudragit® L100-55, Eudragit® FS30D and Kollicoat® MAE100P were dissolved separately in ethanol using magnetic stirrer. EC was separately dissolved in DCM at 25 °C stirred at 300 rpm with a magnetic stirrer. Stirring was continued until a clear solution was obtained. Eudragit® L100-55 and EC are both polymers solution mixed to get a homogeneous solution. Similarly, Eudragit® FS30D-EC and Kollicoat® MAE100P-EC were mixed separately to prepare homogenous solution. Drug was dissolved separately in ethanol at 300 rpm with the help of magnetic stirrer at 25 °C. When clear solution of drug was achieved, it was added dropwise in the polymers solution at 300 rpm. Then, polymers and drug solution were stirred to obtain a homogenous solution. External phase was prepared by dissolving 1% span 80 in liquid paraffin at a stirring speed of 500 rpm. Homogenous solution of drug and polymers was added dropwise by using syringe into liquid paraffin comprising 1% span 80 at 40 °C, whereas stirring was continued at 800 rpm. These mixtures were stirred for 3 h till organic solvents were fully evaporated. After complete evaporation of DCM and ethanol, microspheres were filtered on a Whatman filter paper and collected. Then, developed polymeric microspheres were washed three times with n-hexane in order to remove excess of solvents adhering to the surface of microspheres. The washed microspheres were dried in oven at 45 °C for 24 h. The formulation plan of microspheres is shown in Table 1.
Characterization
Determination of percentage yield, drug loading and entrapment efficiency
Dried microspheres were weighed, and percentage yield w/w was measured by using formula as shown in Eq. 1 [13]. Drug loading was determined by dissolving 50 mg of microspheres in 100 ml of phosphate buffer pH 7.4 for 12 h at 37 °C. After filtration using 0.45-µm syringe filter an analysis of solution was carried out at 287 nm using UV–Vis spectrophotometer (PerkinElmer New York, USA). The absorbance of pure drug (100 mg) was also determined. Drug loading was determined by the following formula as shown in Eq. 2 [14].
Entrapment efficiency was calculated using the following formula.
Micromeritic properties of microspheres
The microspheres were characterized by their micromeritic properties such as angle of repose, bulk density, tapped density, compressibility index and Hausner’s ratio. These were measured by using the following equations [15].
Bulk density of prepared formulation was measured by using the following formula.
Tapped density was determined by the following formula
Compressibility index is also called as car index (Ci) and was calculated by this formula
Value of Ci < 15% designates good flow properties, while values of Ci > 25% characterize poor flow.
Hausner’s ratio was measured by the following equation.
A value of 1.2 represents free flow, and a ratio near to 1 indicates relatively good flow.
Particle size and morphology of microspheres
The mean particle size of microspheres was measured by optical microscope using pre-calibrated ocular micrometer and stage micrometer. About 100 particles of each formulation were observed [14]. SEM images were studied by scanning electron microscopy model (SEM, S-3400N, Hitachi, Japan) to determine the surface and morphology of loaded and unloaded microspheres at different magnifications.
Fourier transform infrared spectroscopy (FTIR)
FTIR spectra were taken to investigate any possible interaction between drug and polymers. The pure drug, polymers and loaded microspheres were subjected to FTIR studies using Shimadzu FTIR spectrophotometer, and sample was scanned at wavelength 4000 and 500 cm−1 [16].
Differential scanning calorimetry (DSC)
The nature of drug present in formulations was assessed by performing DSC of pure drug, EC, Eudragit® L100-55, Eudragit® FS30D, Kollicoat® MAE100P and IBH-loaded microspheres carried out simultaneously with STD Q600 DSC/TGA Analyzer, USA. An amount of 4–5 mg of crushed microspheres was placed in aluminum pans and sealed before to carry out test. Each sample was analyzed under stream of nitrogen gas of 100 ml/min and heated from 40 to 350 °C at the rate of 10 °C/min [17].
X-ray diffractometry (XRD)
Crystallinity of IBH, polymers and drug-loaded microspheres was evaluated by using X-ray diffractometer (Bruker D8 Discover, Germany) using Ni-filtered CuK alpha radiation source. The tube voltage of 40 kV, tube current 35 mA and scanning rate 5°/min are over a range of 8°–80° of diffraction angle range [18].
In vitro drug release study
The in vitro drug release study of IBH-loaded microspheres was carried out using USP dissolution paddle apparatus (Pharma test, Germany) at speed of 100 rpm. Temperature was set at 37 ± 0.5 °C. Accurately weighed microspheres (50 mg) were taken in cellulose dialysis membrane and tied it to paddles. Phosphate buffer solution of pH 1.2, 5.5 and 7.4 was used as dissolution medium (500 ml). The samples were collected after fixed intervals of time, i.e., 0.5, 1, 2, 3, 4, 6, 8, 10 and 12 h from dissolution medium. Samples (5 ml) were analyzed via measuring the absorption of IBH at 287 nm by using UV–Vis spectrophotometer (PerkinElmer). Since ivabradine is in its salt form (IBH), there is no need of maintaining sink condition. Measurement of each sample was carried out in triplicate [19]. Drug concentrations were measured by standard calibration curve.
Drug release kinetics
The in vitro drug release data obtained are evaluated by different kinetics models including zero order (Ft = Kot) where Ft is drug release fraction in time t and Ko is rate constant (for zero-order release), first order (In (1 − F) = − K1t where F is fraction of drug release in time t and K1 is release constant (for first order), Higuchi (F = K2t1/2) where K2 is Higuchi’s constant and F is fraction of drug release at time t and Korsmeyer–Peppas model (Mt/M = K3tn) where K3 is Peppas constant, Mt is quantity of drug released in time t, M is infinity amount of drug release at time infinity, and n is diffusion constant. In microspheres, if n is less than 0.43, it represents Fickian (Case-I) and if value lies among 0.43–0.85, then it is non-Fickian (Case-II) zero-order drug release mechanism [20].
In vivo studies
The pH-dependent polymeric microspheres were successfully developed and characterized for the prevention of plasma drug fluctuation and to control the drug release pattern. For this purpose, methacrylate derivatives and EC-based IBH-loaded carries were synthesized. The major objective was to establish controlled release microsphere having ability to deliver drug at a predetermined rate for an extended period of time. The concentrations were determined using a validated HPLC method. Duffull et al. [21] develop a pharmacokinetic simulation model in healthy male volunteers. IBH pharmacokinetic studies were also reported in animals [22, 23].
Study design
Albino rabbits of weight about 2.0–2.5 kg were obtained from animal house of Pharmacology Laboratory, Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Punjab (Pakistan). The research protocols were studied and approved by the departmental animal ethics committee for use of experimental laboratory animals. All rabbits were housed in well-maintained separate chamber with proper temperature conditions (25 ± 1 °C). These animals are supplied with free access to food and water as a standard diet. Over-night fasted rabbits were used for the experimental studies. Experimental animals were then divided into four groups (1, 2, 3 and 4), 12 rabbits in each group. Animals were fasted at least for 12 h before starting the experiment. Before drug administration animals were allowed a free access to water. Rabbits were appropriately labeled and placed in wooden cages during the process of sampling. At the first stage, drug solution (IBH, 1 mg/kg) was administered to group 1 via feeding tube followed by 10–20 ml of water. This group was tagged as control group. In phase second, group 2 administered formulation F7 (microspheres having IBH equivalent to 1 mg/kg), F14 (microspheres having IBH comparable to 1 mg/kg) was given to group 3, and F21 (microspheres having IBH same to 1 mg/kg) was given to group 4.
Blood sampling protocol
0.5 ml sample were obtained from each experimental animal from jugular vein. The sampling was done at fixed time interval of 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12 and 24 h after oral administration. Blood samples were collected in citrated tubes. These sampling tubes were centrifuged at 5000 revolutions per minutes (rpm) for 10 min. After plasma isolation all samples are stored at − 20 °C till further study. Separation was attained by using a mobile phase (acetonitrile: buffer (pH 6.0), 40:60 V/V), with a flow rate of 1 ml/min at room temperature (22 + 2 °C). The mobile phase was filtered through a 0.45 µm and degassed by sonication before running in HPLC.
Determination of IBH concentration in plasma
Calibration curve was used for the determination of concentration of IBH from pure drug solution of known concentration in plasma. For plasma sample preparation 1 ml of plasma sample was spiked with 50 µl of internal standard (IS) working solution at 2 µg/ml. The plasma mixture was extracted with 4 ml of acetonitrile and mixed by vortex mixer (Seoulin Bioscience, Korea), for 1 min, and then centrifuged at 5000 rpm for 5 min. The organic layer was removed using a micropipette and evaporated under a stream of nitrogen gas in the thermostatically controlled water bath maintained at 40 °C until completely dry. The residue was dissolved in 100 µl mobile phase, vortex mixed for 3 min and centrifuged at 6000 rpm for 20 min. Twenty microliters of this supernatant was injected into column for analysis [24]. Analysis was performed using high-performance liquid chromatography (PerkinElmer, New York, USA) fitted out with column HSC-18 (25 cm × 4.6 mm, 5 µm) Supelco (Sigma-Aldrich). A sample of 20 µl was injected with a runtime of 10 min.
Pharmacokinetic parameters
Pharmacokinetic parameters were calculated by Phoenix WinNonlin® version 6.3 software; the linear trapezoidal method was used to calculate AUC from time versus plasma conc. Clearance was calculated by dividing given dose by AUClast. Non-compartmental analysis (NCA) was used to determine each pharmacokinetic profile. Pharmacokinetic parameters such as time of maximum drug conc. in plasma (Tmax), maximum plasma drug conc. (Cmax), area under plasma conc. curve (AUC), half-life (t1/2), rate of elimination (Ke), area under first moment curve (AUMC), mean residence time (MRT), distribution volume (Vd) and clearance (CL) were determined.
Statistical analysis
Statistical analysis was conducted using analysis of variance (ANOVA) for the purpose of calculating statistical significant as well as nonsignificant analysis at 95% CI, with P value > 0.05 considered to be a significant difference in results. Significance level was fixed at 5%. The value of P < 0.05 was considered to be significant, and P < 0.001 was measured extremely significant.
Results and discussion
Preparation and percentage yield of microspheres
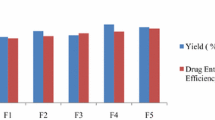
In the present study, IBH-loaded microspheres were prepared by using oil-in-oil (O/O) solvent evaporation method. This method was selected because drug and polymers were soluble in DCM and ethanol. Microspheres were successfully synthesized and evaluated. The maximum percentage entrapment efficiency and percentage yield for different formulations with various compositions of polymers are shown in Table 2.
Particle size and morphology of microspheres
SEM was used to determine the shape and surface morphology of microspheres. SEM images of unloaded microspheres were in spherical form mostly having smooth surfaces, while IBH-loaded microparticles showed comparatively rough surface as shown in Fig. 2. The desired spherical microspheres were obtained at stirring speed of 800 rpm. Internal morphology confirmed the presence of cavity which exhibited matrix responsible for controlled release of drug. Microspheres prepared with Kollicoat® MAE100P were spherical with smooth surfaces while in combination with EC irregular with smooth surface confirmed by SEM. The mean particle size of various formulations of microspheres was 60–80 µm and is given in Table 2. Particle size of microspheres increases gradually as the amount of EC increases, and coating layer around IBH became more and more thick. It was observed in most cases the larger the mean particle size, longer the diffusion path for drug releases, and consequently led to slow release of IBH.
Many studies have shown that the smaller the size of particle drug release will be more rapid due to increase in particle surface area. So release profile of drug from microspheres is predictable as being dependent on particle size [25].
Micromeritic properties microspheres
Micromeritics included bulk density, tapped density, Carr’s index (Ci), Hausner’s ratio (Hr) and angle of repose of all formulations were studied as shown in Table 3. Ci values lie within the range 11–21 which indicate an excellent flow of microspheres [26]. Hr values of all formulations were lower than 1.25 demonstrating good flow properties [27]. Values of angle of repose of all formulations were below 300 also representing free flow properties of microspheres that indicated that these can be handled easily.
FTIR
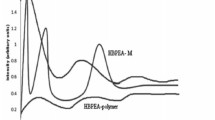
Figure 3 depicts the FTIR spectra of IBH, EC, EL100-55, Eudragit® FS30D, Kollicoat® MAE100P and IBH-loaded microspheres. IR spectra of IBH presented characteristics peaks such as C=C stretching bands at 1103 cm−1, C–C stretching bands at 1456 cm−1 and aliphatic C–N present stretching bands at 2451 cm−1 [28]. EC showed peaks at 2833 cm due to stretching vibration of OH group at carbon numbers 2, 3 and 6 [29]. The spectra of EL100-55 displayed several characteristic bands at 1701 cm−1 (CO carboxylic acid groups vibrations), 1736 cm−1 (esterified carboxyl groups vibrations), 1157 cm−1, 1184 cm−1 and 1261 cm−1 (ester vibrations), 1387 cm−1, 1479 cm−1 and 2979 cm−1 (CHX vibrations) and 3234 cm−1 (OH groups vibrations) [30]. Eudragit® FS30D showed band at 1732 cm−1 and 1603 cm−1 due to the MAA carboxylic acids C=O vibration [31]. The FTIR spectrum of Kollicoat® MAE100P showed a characteristic broad band at 3421 cm−1, which is assigned for OH stretching and a stretching vibration band of C=O at 1637 cm−1 as well as a C-H stretching vibration peak at 2915 cm−1 [32]. The peaks at 1723 and 1698 cm−1 represented the carbonyl group in Kollicoat® MAE100P [33]. The FTIR spectra of IBH-loaded microspheres indicated compatibility between IBH, EC and methacrylate derivatives. Therefore, the drug was chemically steady in microspheres.
DSC
DSC was conducted to explore the melting characteristics of drug and polymers. DSC showed endothermic peak near 193◦C which is the indication of melting point of IBH, while drug-loaded microspheres showed no such peaks shown in Fig. 4. Similarly, endothermic peaks of EC were observed at glass transition temperature 132 °C and EL100-55 showed peaks at 97 °C. It suggested that the drug particles were uniformly distributed in polymer matrix. Figure 4 presents DSC pattern of IBH, EC, EL100-55, Eudragit® FS30D, Kollicoat® MAE100P and IBH-loaded microspheres.
XRD
XRD analysis was used to investigate the crystallinity of drug in prepared microspheres. Ivabradine was shown the characteristics intense peaks at 2θ of 11°, 15°, 20° and 25° due to its crystalline nature. The size of the crystals was not measured as we have only determined the nature of the particles that whether they are amorphous or crystalline. Furthermore, in formulation form the drug was completely captured by the polymeric matrix and now drug may be in the form of solid solution loss of crystallinity as XRD graphs have shown no identifiable or sharp peaks describing the conversion of drug into amorphous form. XRD patterns of pure drug (a) IBH, (b) EC, (c) EL 100-55, (d) Eudragit® FS30D, (e) Kollicoat® MAE100P, (f) IBH-loaded microspheres F7, (g) IBH-loaded F14 formulation, (h) IBH-loaded F21 formulation shown in Fig. 5 clearly designate that drug particles were distributed at molecular level in the polymeric matrix [34].
In vitro studies
The in vitro drug release of microspheres depends on polymer network characteristics including the chemical structure, network structure and release conditions of polymers. IBH was selected as model drug to study the release kinetics in prepared microspheres having a varying amount of both polymers. % Cumulative drug release from all formulations pH 7.4 is shown in Fig. 6a–c. Figure shows the cumulative release % at buffer solution of pH 7.4 indicating that drug release was higher than other pH 5.5 and 1.2. The in vitro drug release study indicated that the combination of polymers and their changed ratio changed the release rate of drug from microspheres [35]. Drug release of formulations was very slow at pH 1.2 because Eudragit derivatives are insoluble in an acid environment, and it should prevent dissolution of IBH as shown in Fig. 7a–c. At pH 5.5 FI showed sustained release rate because Eudragit® EL 100-55, Eudragit® FS30D and Kollicoat® MAE100P are soluble at pH 5.5, and dissolution process takes relatively longer because it involves the process of absorption, swelling and disentanglement, before drug release. Figure 8a–c represents the drug release pattern of all polymeric microspheres. All formulations showed maximum sustained release at pH 7.4 containing equal amount of methacrylate derivatives and EC. Due to EC the microparticles become impermeable to water and give very slow release of drug as observed at pH 7.4. An increase in polymer solution viscosity has produced microspheres with decreased porosity due to thickening of polymer wall. It is clearly known that higher concentration of EC results in a longer diffusional path length, so drug release is extended. The thick polymeric barrier slowed the entry of surrounding dissolution medium into the microspheres and hence less quantity of drug release out from the polymer matrices of the microspheres exhibiting sustained release [36]. It indicates that drug release mechanism gradually transfers from diffusion mechanism to erosion at dissolution medium of pH 7.4. Maximum drug release from all formulations showed almost more than 90% at pH 7.4. Results showed the cumulative release % at pH 7.4 indicating that drug release was higher at pH 7.4 demonstrating pH-dependent behavior. IBH was found to be soluble in pH 1.2, 5.5 and 7.4 phosphate buffer, and no significant effect of pH on solubility was observed [37]. Similar solubility was observed in different pH-responsive systems. Dissolution studies were repeated three times (n = 3).
Drug release kinetics
In vitro drug release mechanism was analyzed by means of applying various kinetic models including zero-order, first-order, Higuchi and Korsmeyer–Peppas models. However, on the basis of regression coefficient (R2), model best fitted to the release data was selected. The values of R2 indicated that drug release follows first-order model. The result proposed that first order was best fitted to the data and followed by drug release. By applying Korsmeyer–Peppas model the value of (n) for release of drug was calculated. The value of (n) was found to be between 0.408 and 0.585 which indicates that diffusion mechanism was non-Fickian [38]. Drug release from above formulation was likely controlled by a combination of diffusion and erosion mechanisms.
In vivo studies
On the basis of preliminary investigations, these three formulations (FA7, FA14 and F21) with maximum in vitro cumulative drug release were selected for in vivo evaluation. Single pharmacokinetic study was conducted in animals for in vivo assessment. Mean ± SD conc. of IBH determined in plasma of rabbits after oral administration of drug solution (group 1) and controlled release IBH-loaded microspheres (groups 2, 3 and 4) is shown in Table 4. The mean ± SD of plasma conc. versus time profile of IBH in groups 1, 2, 3 and 4 is illustrated in Fig. 9. Drug was detected quickly in rabbit plasma after oral provision of IBH solution, while from controlled release polymeric microspheres formulations it acquired nearly 2 h to detect measureable extent of drug in rabbit plasma. Lag time of nearby 2 h showed the extended release of experimental drug from microspheres. It revealed that drug might remain intact in stomach and most possibly released at higher intestinal pH. Various pharmacokinetics parameters of all groups (1–4) were assessed and are presented in Tables 5 and 6, respectively. ANOVA test was applied for statistics evaluation of pk. parameters at 5% significance level as given in Table 6. Results indicated that all parameters showed P values were < 0.0001 representing a significant difference among all assessed factors.
The mean plasma concentration (Cmax) of IBH for all groups (1–4) predicted from pk. data were 880.38 ng/ml, 718.43 ng/ml, 721.87 ng/ml and 805.11 ng/ml, respectively, achieved at Tmax of 2.01, 4.01, 4.0 and 4.56 h, respectively. As compared to other groups Cmax was significantly higher for group 1, while Tmax of groups 2–4 was significantly higher than control group. This prolonged Tmax revealed controlled release microspheres formulations that represent slow release of drug that resulted controlled released in vivo drug delivery. Our results showed good similarity to drug-loaded hydrogels presented with prolonged Tmax and lower Cmax as compared to conventional dosage form [10], reported similar finding of pk. parameters after oral administration of IBH with coadministration of puerarin.
AUC is very important tool for the assessment of bioavailability. In the current research AUC0–t for oral IBH solution (group 1); F7 (group 2); F14 (group 3); and F21 microspheres (group 4) was found to be 2483.71 ± 13.173 ng/ml h, 5954.37 ± 12.110 ng/ml h, 6400.82 ± 19.131 ng/ml h and 7427.4 ± 49.322 ng/ml h, respectively. AUC0–∞ for oral IBH solution (group 1); F7 (group 2); F214 (group 3); and F21 microspheres (group 4) was found to be 2558.95 ± 62.112 ng/ml h, 6422.72 ± 253.06 ng/ml h, 6680.98 ± 123.01 ng/ml h and 8020.44 ± 134.12 ng/ml h, respectively.
Results indicated clearly that AUC0–t and AUC0–∞ for the microspheres were higher significantly than oral IBH soln., thus signifying enhanced bioavailability in rabbits. Increased AUC could be correlated to increase the bioavailability [39]. Similar results were reported for improving bioavailability of IBH solid lipid microparticles [40]. Lodhi et al. [41] developed controlled release buccal film of IBH. AUC of pH-sensitive hydrogel formulations was greater comparable to conventional dosage form [42,43,44,45].
The controlled drug release features of polymeric microspheres also revealed in MRT values. The MRT values were significantly greater for the microspheres than for the IBH solution. Furthermore, microspheres have meaningfully prolonged elimination t1/2. This specifies IBH containing microspheres has effectively controlled release drug delivery.
Conclusion
The concepts for fabricating copolymeric microspheres of IBH offer an appropriate, sensible approach to accomplish a lingering therapeutic outcome by continuously releasing the drug over extended period of time. The results indicated that the present HPLC method is very simple and applicable to pharmacokinetic and bioavailability studies of IBH. Collectively, these in vivo results manifested that pH-dependent microspheres had a reasonable controlled release, with better drug delivery at higher pH environment. Results indicated that polymeric network protecting drug from acidic pH of stomach hence improved in vivo retention and decreased plasma drug concentration variation. It would be faster and more cost-effective in modifying imperative properties of the existing drugs than developing new drug entities; hence, this formulation will be windfall to novel drug dosage forms.
References
Freiberg S, Zhu X (2004) Polymer microspheres for controlled drug release. Int J Pharm 282:1–18
Kim B, Hwang S, Park J, Park HJ (2002) Preparation and characterization of drug-loaded polymethacrylate microspheres by an emulsion solvent evaporation method. J Microencapsul 19:811–822
Aydin O, Aydin B, Tezcaner A, Keskin D (2015) Study on physiochemical structure and in vitro release behaviors of doxycycline-loaded PCL microspheres. J Appl Polym Sci 132:1–13
Mutalik S, Udupa N (2005) Formulation development, in vitro and in vivo evaluation of membrane controlled transdermal systems of glibenclamide. J Pharm Pharm Sci 8:26–38
Verma S, Kaul M, Rawat A, Saini S (2011) An overview on buccal drug delivery system. IJPSR 2:1303–1321
Alagusundaram M, Chengaiah B, Ramkanth S, Parameswari SA, Madhu C, Chetty S, Dhachinamoorthi D (2009) Formulation and evaluation of mucoadhesive buccal films of ranitidine. Int J PharmTech Res 1:557–563
Difrancesco D, Camm JA (2004) Heart rate lowering by specific and selective If current inhibition with ivabradine. Drugs 64:1757–1765
Nawarskas JJ, Bowman BN, Anderson J (2015) Ivabradine: a unique and intriguing medication for treating cardiovascular disease. Cardiol Rev 23:201–211
Nar G, Inci S, Aksan G, Demirelli S, Soylu K, Yuksel S, Gulel O, Icli A (2015) Ivabradine on aortic stiffness in patients with heart failure. J Investig Med 63:620–625
Zhang Y, Sun W, Pan Y, Li T, Yang X, Xu R, Qiu X (2016) Pharmacokinetics changes of ivabradine and N-desmethyl ivabradine after oral administration with puerarin in rats. Int J Clin Exp Med. 9:8369–8374
Prasad UK, Gray D, Purcell H (2009) Review of the If selective channel inhibitor ivabradine in the treatment of chronic stable angina. Adv Ther 26:127–137
Guo Y-J, Liang D-L, Xu Z-S, Ye Q (2014) In vivo inhibitory effects of puerarin on selected rat cytochrome P450 isoenzymes. Die Pharm An IJPS 69:367–370
Kılıçarslan M, Baykara T (2003) The effect of the drug/polymer ratio on the properties of the verapamil HCl loaded microspheres. Int J Pharm 252:99–109
Dupinder K, Seema S (2014) Development and characterization of lovastatin loaded microspheres. IJPDT 4:71–78
Jain SK, Awasthi A, Jain N, Agrawal G (2005) Calcium silicate based microspheres of repaglinide for gastroretentive floating drug delivery: Preparation and in vitro characterization. J Control Release 107:300–309
Mundargi RC, Srirangarajan S, Agnihotri SA, Patil SA, Ravindra S, Setty SB, Aminabhavi TM (2007) Development and evaluation of novel biodegradable microspheres based on poly (d, l-lactide-co-glycolide) and poly (ε-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: in vitro and in vivo studies. J Control Release 119:59–68
Goldmann AS, Barner L, Kaupp M, Vogt AP, Barner-Kowollik C (2012) Orthogonal ligation to spherical polymeric microparticles: modular approaches for surface tailoring. Prog Polym Sci 37:975–984
Joshi GV, Kevadiya BD, Bajaj HC (2010) Design and evaluation of controlled drug delivery system of buspirone using inorganic layered clay mineral. Microporous Mesoporous Mater 132:526–530
Lim S, Martin GP, Berry D, Brown M (2000) Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel microspheres of hyaluronic acid and chitosan. J Control Release 66:281–292
Pérez-Martínez JI, Morillo E, Maqueda C, Gines JM (2001) Ethyl cellulose polymer microspheres for controlled release of norfluazon. Pest Manag Sci 57:688–694
Duffull SB, Chabaud S, Nony P, Laveille C, Girard P, Aarons L (2000) A pharmacokinetic simulation model for ivabradine in healthy volunteers. Eur J Pharm Sci 10:285–294
Riesen S, Ni W, Carnes C, Lindsey K, Phelps M, Schober K (2011) Pharmacokinetics of oral ivabradine in healthy cats. J Vet Pharmacol Ther 34:469–475
Westerhout J, Ploeger B, Smeets J, Danhof M, De Lange EC (2012) Physiologically based pharmacokinetic modeling to investigate regional brain distribution kinetics in rats. AAPS J 14:543–553
Lu C, Jia Y, Yang J, Jin X, Song Y, Liu W, Ding Y, Sun X, Wen A (2012) Simultaneous determination of ivabradine and N-desmethylivabradine in human plasma and urine using a LC-MS/MS method: application to a pharmacokinetic study. APSB 2:205–212
Arabi H, Hashemi S, Fooladi M (1996) Microencapsulation of allopurinol by solvent evaporation and controlled release investigation of drugs. J Microencapsul 13:527–535
Sahoo SK, Mallick AA, Barik B, Senapati PC (2005) Formulation and in vitro evaluation of Eudragit® microspheres of stavudine. Trop J Pharm Res 4:369–375
Soppimath KS, Kulkarni AR, Aminabhavi TM (2001) Development of hollow microspheres as floating controlled-release systems for cardiovascular drugs: preparation and release characteristics. Drug Dev Ind Pharm 27:507–515
Wadood A, Riaz M, Jamal SB, Shah M, Lodhi MA (2013) Molecular docking study of P4-Benzoxaborolesubstituted ligands as inhibitors of HCV NS3/4A protease. Bioinformation 9:309–314
Naeem M, Mahmood A, Khan S, Shahiq Z (2010) Development and evaluation of controlled-release bilayer tablets containing microencapsulated tramadol and acetaminophen. Trop J Pharm Res 9:347–354
Hao S, Wang B, Wang Y, Zhu L, Wang B, Guo T (2013) Preparation of Eudragit L 100-55 enteric nanoparticles by a novel emulsion diffusion method. Colloids Surf B 108:127–133
Moustafine RI, Bodrov AV, Kemenova VA, Rombaut P, Van Den Mooter G (2012) Drug release modification by interpolymer interaction between countercharged types of Eudragit® RL 30D and FS 30D in double-layer films. Int J Pharm 439:17–21
Ibrahim MA, Shazly GA, El-Badry M (2015) Albendazole microparticles prepared by spray drying technique: improvement of drug dissolution. Trop J Pharm Res 13:1963–1970
Kim NA, Choi DH, Lim JY, Kim KH, Lim DG, Lee E, Park E-S, Jeong SH (2014) Investigation of polymeric excipients for dutasteride solid dispersion and its physicochemical characterization. Arch Pharm Res 37:214–224
Ramesh Babu V, Krishna Rao K, Sairam M, Naidu B, Hosamani KM, Aminabhavi TM (2006) pH sensitive interpenetrating network microgels of sodium alginate-acrylic acid for the controlled release of ibuprofen. J Appl Polym Sci 99:2671–2678
Dang T, Cui Y, Chen Y, Meng X, Tang B, Wu J (2015) Preparation and characterization of colon-specific microspheres of diclofenac for colorectal cancer. Trop J Pharm Res 14:1541–1547
Chella N, Yada KK, Vempati R (2010) Preparation and evaluation of ethyl cellulose microspheres containing diclofenac sodium by novel w/o/o emulsion method. J Pharm Sci Res 2:884–888
Selva Kumar P, Pandiyan K, Rajagopal K (2014) Development and validation of rapid RP-HPLC method for dissolution release of ivabradine hydrochloride in solid oral dosage form. WJPPS 3:1877–1888
Khan F, Razzak MSMI, Khan MZR, Azam KR, Sadat SMA, Reza MS (2008) Preparation and in vitro evaluation of theophylline loaded gastroretentive floating tablets of METHOCEL K4M. Dhaka Univ J Pharm Sci 7:65–70
Feng MR, Lou X, Brown RR, Hutchaleelaha A (2000) Allometric pharmacokinetic scaling: towards the prediction of human oral pharmacokinetics. Pharm Res 17:410–418
Khan H, Hanif M (2017) Ivabradine loaded solid lipid microparticles: formulation, characterization and optimization by central composite rotatable design. Acta Pol Pharm 74:211–226
Lodhi M, Dubey A, Narayan R, Prabhu P, Priya S (2013) Formulation and evaluation of buccal film of Ivabradine hydrochloride for the treatment of stable angina pectoris. Int J Pharm Investig 3:47–53
Tulain UR, Ahmad M, Rashid A, Malik MZ, Iqbal FM (2018) Fabrication of pH-responsive hydrogel and its in vitro and in vivo evaluation. Adv Polym Technol 37:290–304
Ramteke KH, Nath L (2012) Formulation, evaluation and optimization of controlled release hydrogel microspheres for colon targeted drug delivery. J Pharm Sci Res 4:1739–1747
Oprea A-M, Nistor M-T, Profire L, Popa MI, Lupusoru CE, Vasile C (2013) Evaluation of the controlled release ability of theophylline from xanthan/chondroitin sulfate hydrogels. JBNB 4:123–131
Ranjha NM, Madni A, Bakar AA, Talib N, Ahmad S, Ahmad H (2014) Preparation and characterization of isosorbide mononitrate hydrogels obtained by free-radical polymerization for site-specific delivery. Trop J Pharm Res 13:1979–1985
Acknowledgements
The authors are very thankful to Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan, for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Majeed, A., Ranjha, N.M., Hussain, M. et al. Fabrication and evaluation of pH-dependent polymeric microspheres of ivabradine and their in vitro and in vivo studies. Polym. Bull. 76, 3127–3151 (2019). https://doi.org/10.1007/s00289-018-2546-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2546-0