Abstract
This research paper describes the development, optimization and in vitro characterization of chemically cross-linked pectin–polyvinyl alcohol-co-poly(2-Acrylamido-2-methylpropane sulfonic acid) semi-interpenetrating polymer network hydrogel [pectin–PVA-co-poly(AMPS) semi-IPN hydrogel] for controlled delivery of model drug tramadol HCl. Response surface methodology based on 32 factorial design was used for optimization and investigating the effect of independent factors: polymer-blend ratio (pectin:PVA = X 1) and monomer (AMPS = X 2) concentration on the dependent variables, swelling ratio (q 18th), percent drug release (R 18th, %), time required for 80 % drug release (t 80 %, h), drug encapsulation efficiency (DEE, %) and drug loaded contents (DLC, mg/g) in pectin-PVA-co-poly(AMPS) gels prepared by free radical polymerization. The optimized semi-IPN gel (FPP-10) showed controlled in vitro drug release (R 18th) of 56.34 % in 18 h, t 80 % of 30 h, and DEE of 23.40 %. These semi-IPN hydrogels were also characterized through SEM, FTIR, sol–gel analysis, swelling studies and drug release characteristics. Therefore, this newly synthesized polymeric network could be a potential polymeric system for controlled drug delivery of tramadol HCl for prolonged drug release.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interpenetrating polymer networks (IPN) hydrogels can be described as a class of polymer blends, being “alloys” of cross-linked polymers forming a blended network of two or more polymers in which at least one of the systems is synthesized in the presence of another polymer system [1–3]. These are formed through physical cross-linking by entanglement or penetration of one polymer in another, with no covalent bonds between them [4, 5]. Semi- or pseudo-interpenetrating polymer network is one of the subtypes of IPN based on a structure in which one of the polymeric components, i.e., synthetic or biopolymer exhibits linear instead of network or cross-linked structure being embedded in a matrix system. These semi-IPN gel systems can be synthesized through both simultaneous and sequential preparation techniques [1, 2].
Polymer combination must be able to produce an advanced multicomponent polymeric system exhibiting a new profile [6]. IPNs are found to exhibit superior performances and wide range of applications over the conventional individual polymeric systems because of their relatively dense, stiffer and tougher mechanical properties which provide tunable physical properties, and more efficient drug-loading capacity as compared to the conventional hydrogels [7]. Due to their advanced properties of stability, biocompatibility, non-toxicity, biodegradability and swelling capacity, IPN hydrogels have attracted considerable attention in pharmaceutical field, particularly in delivering bioactive molecules to the target site, and, thus, have emerged as novel carriers for controlled drug delivery [8].
IPN hydrogels are usually fabricated from naturally occurring polysaccharide polymers, biopolymers, synthetic polymers, and combination of natural and synthetic polymers; and protein-based IPN hydrogels are also being studied and prepared [2]. Blends of natural and synthetic polymers being termed as bioartificial or biosynthetic polymeric material have gained increased interest for biomedical application, since the last three decades, because of their improved mechanical and thermal properties and biocompatibility compared to those of a single component [9].
Pectins are polyanionic, non-starch and linear polysaccharides extracted from plant cell wall, predominantly comprising linear polymers of α-(1-4)- linked d-galacturonic acid and 1,2 d-rhamnose with d-galactose and d-arabinose side chains [10]. Pectins have been used as a gel former for many years; it is also used in the production of a wide range of specialty products including biodegradable and edible films, in adhesives, as paper substitutes, foams and plasticizers [11, 12]. Recently, there is an interest to use pectin gels in controlled delivery of drugs orally, nasally and through vagina [13–16] which are generally well tolerated and readily accepted by the patients [13, 14, 17] as biomedical implants, and as surface modifiers for medical devices [13, 14, 18].
Polyvinyl alcohol (PVA), a synthetic polymer, consists of hydroxyl groups synthesized by the polymerization of vinyl acetate to form polyvinyl acetate (PVAc), which are subsequently hydrolyzed to get PVA [19]. Due to its property of high swelling degree in water and its viscoelastic nature, PVA finds application in tissue engineering as it closely simulates and is readily accepted by natural body tissues and it serves as ideal candidate in the form of biomaterials for various biomedical and pharmaceutical applications. PVA-based hydrogels have been used for contact lenses, as artificial heart lining and for drug delivery applications mainly in topical pharmaceutical and ophthalmic formulations [20, 21].
Tramadol HCl, a racemic mixture chemically being (1RS,2RS)-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)-cyclohexanol hydrochloride, is a synthetic centrally acting analgesic being structurally related to opioid derivatives [22]. The racemate consisting of two enantiomers provides analgesic activity via two different mechanisms, i.e., (+)-tramadol and its metabolite (+)-O-desmethyl-tramadol (M1) are agonists at “mu” (μ) opioid receptor and inhibit serotonin reuptake, while (–)-tramadol prevents nor-epinephrine reuptake, thus contributing to the enhancement of inhibitory action on pain transmission in the spinal cord. This dual action has caused the US FDA classification of tramadol as nontraditional centrally acting analgesic [23].
In the present research work, semi-IPN hydrogels based on combination of natural (pectin) and synthetic (PVA) polymer have been fabricated incorporating tramadol HCl as the model drug for controlled drug delivery. Various formulations were prepared with varying polymer and monomer ratios to study the impact of independent factors on various dependent variables/responses. A total of nine formulations were prepared in accordance with three-level full factorial design in which the two studied independent factors (variables) were polymer ratio (pectin:PVA) and monomer concentration, while the dependent factors (responses) were swelling index at 18th hour (q 18 h), percent drug release at 18th hour (R 18 h), time required for 80 % drug release (t 80 %) drug entrapment efficiency (%DEE), and drug-loaded contents (mg/gm). Formulated IPN hydrogels were subjected to different in vitro evaluation tests to study all the relevant features.
Experimental
Materials
Pectin (MW ≈ 30,000–100,000, Degree of esterification ≥74.0 %) was purchased from Sigma-Aldrich, United Kingdom. Polyvinyl alcohol (PVA) (MW ≈ 72,000) was obtained from AppliChem, Germany. 2-acrylamido-2-methylpropane sulfonic acid (AMPS) (MW ≈ 207.25), ammonium peroxo-disulfate (MW ≈ 228.19), sodium hydrogen sulfite (39 % solution in water), and ethylene glycol dimethacrylate (EGDMA) (MW ≈ 198.22) were purchased from Merck, Germany. Model drug tramadol HCl was received as a gift from Highnoon Laboratories (Pvt.) Ltd., Lahore, Pakistan.
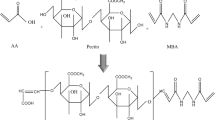
Preparation of pectin–PVA-co-poly(AMPS) semi-interpenetrating network (IPN) containing tramadol HCl
The composition for the synthesis of semi-IPN hydrogel cocktail is given in Table 1. Pectin–PVA-co-poly(AMPS) hydrogels were synthesized through the procedure as described by [24] with modifications using free radical polymerization technique. Briefly, an accurately weighed amount of pectin was dissolved in measured quantity of deionized distilled water in a reaction flask with continuous stirring until a uniform and viscous solution was obtained. The second polymer PVA was separately dispersed in aqueous media at 80–90 °C with stirring to obtain a clear, transparent and viscous solution, then cooled down to room temperature followed by slow dropwise addition to pectin solution. Similarly, measured quantity of AMPS and reaction mixture of initiator/co-initiator, i.e., ammonium peroxo-disulfate (APS) and sodium hydrogen sulfite (SHS), respectively, was accurately weighed, solubilized in distilled water and mixed with the mixture of polymers. To this mixture, sufficient quantity of distilled water was added to adjust the final volume of reaction mixture to 100 g. Finally, cross-linker EGDMA was added dropwise to the final reaction mixture of polymers, monomer and initiator with continuous stirring. To remove any dissolved oxygen from the reaction mixture, nitrogen stream was purged through the final polymeric reaction mixture for 30 min. This mixture was, then, immediately transferred to the glass test tubes and placed in water bath initially maintained at temperature of 55 °C for 2 h; after 2 h, the temperature of water bath was sequentially increased to 60 °C for 24 h. After this treatment, glass molds were cooled to room temperature, and solid, cylindrical and firm hydrogels were drawn out. These cylindrical hydrogels were cut into small disks having size of 8 mm, washed with ethanol to remove any extraneous matter such as impurity, unreacted monomer or catalyst. The disks were then dried in a vacuum oven at 40 °C for 1 week to obtain dried hydrogel disks.
All semi-IPN hydrogel formulations were loaded with tramadol HCl through diffusion process. Dried hydrogel disks of known weight were soaked in 1 % drug solution in 0.2 M phosphate buffer of pH 7.4 at room temperature. The disks were kept in drug solution for 48 h until the equilibrium was achieved (till constant weight), then removed from drug solution, washed and dried in vacuum oven at 40 °C to constant weight. Figure 1 represents the polymerization reaction for the formation of pectin–PVA-co-poly(AMPS) semi-IPN hydrogels.
Experimental design, statistical analysis and optimization
Designing controlled-release drug delivery formulations utilizing appropriate combination of process variables that will produce the product formulation having desired optimum properties with minimum number of trials is often very crucial for pharmaceutical scientists [25]. A full factorial experiment is more efficient in optimization of multi-factors compared with one-factor-at-a-time experiments, as it studies all factors in all possible combinations, thus analyzing the influence of individual formulation variables and their interactions using minimum experiments [26, 27]. Based on the factorial experimental designs, this RSM optimization technique encompasses the generation of model polynomial equations for investigating responses over the experimental domain, to determine the settings of factor values for achieving optimum formulation(s) [28].
In this study, a two-factor, three-level factorial design (32) was employed for formulation optimization of pectin–PVA-co-poly(AMPS) hydrogels containing tramadol HCl, in which the studied factors were pectin-to-PVA ratio (X 1, 1:4–3:4 or 0.2–0.6 % w/w) and amount of monomer (AMPS) (X 2, 24–40 % w/w) as independent variables (factors), each varied at three different levels: high (+1), medium (0), and low (−1) levels as mentioned in Table 2. The swelling index at 18th hour (Y 1 = q18 h), cumulative percent drug release at 18th h (Y 2 = % R 18 h), time required for 80 % drug release (Y 3 = t 80 %), drug entrapment efficiency (Y 4 = %DEE), and drug-loaded contents (Y 5 = mg/gm DLC), were selected as dependent variables (responses). Design-Expert® version 9.0.3 software (Stat-Ease Inc., USA) was employed for the generation and evaluation of the statistical factorial experimental design as summarized in Table 3. Polynomial models, including linear, interaction (2FI) and quadratic terms, for all the response variables were generated using the Design-Expert software. For optimization, the effects of independent variables upon the responses were modeled using polynomial quadratic mathematical equation [29]:
where Y is the response, b 0 is the intercept, and b 1, b 2, b 3, b 4, b 5 are regression coefficients. X 1 and X 2 are individual effects; X 21 and X 22 are quadratic effects; X 1 X 2 is the interaction effect.
Data analysis using Design-Expert software
The best fitting mathematical model was selected on the basis of the comparisons of several statistical parameters, as provided by Design-Expert® software including standard deviation (SD), multiple correlation coefficient (R 2), adjusted multiple correlation coefficient (adjusted R 2) and predicted multiple correlation coefficient (predicted R 2). Three-dimensional response surface plots and contour plots resulting from equations were generated by software to study the effect of independent factors on measured responses. Subsequently, the numerical and graphical optimization techniques using desirability approach and overlay plots, respectively, were used to generate optimum settings for the formulations with desired response.
Validation of experimental design and selection of optimized formulation
To validate 32 full factorial design, an extra check point formulation (FPP-10) was prepared. Optimized formulation (FPP-10) was selected based on the criteria of minimal percent drug release at 18th hour (R 18 h), maximum time required for 80 % drug release (t 80 %), and higher entrapment efficiency (%DEE) with good desirability.The predicted values for measured responses, i.e. % drug release at 18th h, t 80 % and %DEE for FPP-10 were determined on the basis of respective polynomial equations and quantitatively compared with the obtained experimental values; the relative error (%) was calculated using the following equation.
FTIR spectroscopy analyses
Attenuated total reflectance (ATR) technology along with software OPUS data collection was employed to encompass Fourier transform infrared (FTIR) spectra of the required sample employing Bruker FTIR (Tensor 27 series, Germany). Samples in solid/liquid form were individually placed on the pike miracle ATR cell covering ZnSe crystal surface, followed by rotation of assembly, thus forming a compact mass. Empty cell plate scan was carried out before analyzing any sample whose spectra were recorded by the above procedure and scanned in the range of 4000–650 cm−1.
Scanning electron microscopy analyses
Surface morphology of the synthesized optimized formulation of Pectin–PVA-co-poly(AMPS) semi-IPN hydrogel (FPP-10) sample was investigated using a scanning electron microscope (FEI Quanta 250) under high vacuum at an accelerating voltage of 10 kV. The samples for scanning electron microscopy (SEM) were prepared by sprinkling the vacuum oven-dried samples onto the aluminum stubs covered with double-coated carbon-conductive adhesive tabs. After sputter-coating with gold before microscopy, the hydrogel samples were examined and scanned at different magnifications.
Sol–gel fraction analyses
Dried thin slabs of hydrogel of 1.5 mm thickness were used to measure the sol–gel fraction. Soxhlet apparatus was used for determining the gel fraction gravimetrically by extracting sol fraction (uncross-linked polymer) from dried gel pieces using 100 ml of deionized distilled water at 90 °C for 4 h. The extracted gels were then removed and dried in vacuum oven at 55 °C for 1 week to constant weight. Following equations were applied to determine the sol fraction and gel fraction of Pectin–PVA-co-polymeric AMPS hydrogels.
where M i indicates initial weight of dry gel, and M e indicates weight of extracted gel after equilibrium drying.
Evaluation of swelling behavior
The dynamic swelling and equilibrium water content of semi-IPN hydrogels were determined by immersing dried hydrogel disks of known weight in 100 ml of 0.2- and 0.1-M buffer solutions of pH 7.4 and 1.2, respectively, at 37 °C to simulate the pH of gastrointestinal tract. The swollen gels were removed from buffer solution at predetermined time intervals, placed on bloating paper to remove superficial solvent and, then, weighed on an analytical balance to determine weight gain and, finally, replaced back into their respective buffer solutions. The swelling experiments were continued until hydrogel disks attained equilibrium weight.
The degree of swelling and equilibrium water content were determined using Eqs. 4 and 5, respectively [30];
where \( M_{\text{s}} \) indicates mass of swelling at predetermined time interval, \( M_{\text{d}} \) represents the weight of dried gel before starting of swelling experiments, and M eq represents the mass of gels after equilibrium weight is attained.
Determination of drug entrapment efficiency (DEE) and drug-loaded contents (DLC)
To evaluate drug entrapment efficiency and drug-loaded contents, drug-loaded disks of semi-IPN pectin–PVA-co-poly(AMPS) hydrogels were crushed thoroughly in a clean and dry pestle and mortar. A weighed amount of these powdered hydrogels was then placed in 500 ml phosphate buffer solution of pH 7.4 for 24 h with continuous stirring at 37 ± 0.5 °C and, then, centrifuged at 3000 rpm. The supernatant layer was separated, filtered through 0.45-µm filter paper and assayed for tramadol HCl using UV spectrophotometer at λ max 271 nm. The DEE (%) was calculated using the following formula [31], while drug-loaded contents (DLC) were determined as total drug amount loaded per gram of hydrogel.
In vitro drug release studies
In vitro drug release experiments were conducted to investigate drug delivery from fabricated semi-IPN hydrogels to assess the drug release as a function of pH. USP dissolution apparatus II (Curio; DL-0609) was used for carrying out dissolution study. For this purpose, drug-loaded disks of known weight were placed in 900 ml of buffer solutions of pH 1.2 (0.1 M HCl buffer) and pH 7.4 (0.2 M phosphate buffer) maintained at a temperature of 37 ± 0.5 °C, and the paddle speed was adjusted at 50 rpm to simulate gastrointestinal conditions. After specified time intervals, 5-ml volume of sample was withdrawn from the dissolution flasks; this withdrawn volume of dissolution medium was replaced by fresh buffer solutions. These aliquots of samples were diluted suitably using respective buffer solutions and were analyzed using UV–Visible spectrophotometer at λ max 271 nm.
Statistical analysis
ANOVA was used to establish the statistical validation of polynomial equations generated by Design-Expert® software (version 9.0.3, Stat-Ease), and in addition to this, independent sample t test using SPSS software (version 15.0) was used to statistically verify pH-independent characteristics of preformed hydrogels. All measured data are expressed as mean ± standard deviation (SD). The level of significance was considered to be set at p < 0.05.
Results and discussion
Analysis of data and optimization of design
According to computer-aided optimization technique using 32 full factorial design, nine trial formulations of pectin–PVA-co-poly(AMPS) semi-IPN hydrogels were prepared. Overview of the design matrix including two independent factors (i.e., pectin-to-PVA ratio, X 1 and concentration of AMPS as monomer, X 2) and the investigated responses (i.e., swelling index, percent drug release at 18th hour, time required for 80 % drug release, drug entrapment efficiency and drug loading) is presented in Table 3. The values of investigated responses measured for all the nine trial formulations were fitted in the 32 full factorial design using Design-Expert® version 9.0.3 software (Stat-Ease Inc., USA) to get suitable polynomial model equations for respective responses analyzed in this investigation, and these models were statistically evaluated using one-way ANOVA (p < 0.05) (Table 4).
Model polynomial equation relating q 18 h is as follows:
[R 2 = 0.8831; adjusted R 2 = 0.8246; predicted R 2 = 0.7245; SD = 1.48].
Model polynomial equation relating R 18 h is as follows:
[R 2 = 0.9294; adjusted R 2 = 0.8412; predicted R 2 = 0.2208; SD = 3.59].
Model polynomial equation relating t 80 % is as follows:
[R 2 = 0.8102; adjusted R 2 = 0.7153; predicted R 2 = 0.1401; SD = 1.96].
Model polynomial equation relating DEE (%) is as follows:
[R 2 = 0.8648; adjusted R 2 = 0.8262; predicted R 2 = 0.7196; SD = 1.56].
Model polynomial equation relating DLC is as follows:
[R 2 = 0.7102; adjusted R 2 = 0.6274; predicted R 2 = 0.4058; SD = 4.82].
The response surface methodology was used for investigating and analyzing the influence of independable factors on dependable responses (here, q 18 h, R 18 h, t 80 %, DEE and DLC). The two-dimensional contour graphs relating measured responses by giving visual representation of values and three-dimensional response surface graphs generated through the Design-Expert software indicated the main effects and mutual interactive behavior of factors (Fig. 2). The response surface graph and the corresponding contour graph relating q 18 h indicate a decrease in q 18 h with the increase of both pectin-to-PVA ratio (X 1) and concentration of AMPS as monomer (X 2). Similarly, an upward trend of the wire mesh for R 18 h was observed at lower level (−1), and the downward trend was at higher level (+1) with the increasing of both pectin-to-PVA ratio (X 1) and concentration of AMPS (X 2) as is indicated by the response surface graph and corresponding contour graph relating R 18 h, thereby concluding that the dependent variables (q 18 h and R 18 h) were inversely proportional to both the independent factors (pectin-to-PVA ratio and concentration of AMPS). The response surface graph and corresponding contour graph relating t 80 % andDEE (%) revealed that these dependent variables were increased significantly on increasing either pectin-to-PVA ratio (X 1) or AMPS concentration (X 2), i.e., t 80 % and DEE (%) values were found to be directly proportional to pectin and AMPS contents. However, an increase in DLC values with the increasing of pectin-to-PVA ratio (X 1) and a decrease in DLC values with the increasing concentration of AMPS (X 2) are indicated by the response surface graph and the corresponding contour graph relating DLC, thus showing that the DLC is directly proportional to pectin contents and inversely proportional to AMPS contents.
To develop new optimized formulation with optimum response value, numerical optimization technique based on the desirability approach was employed. The desirable ranges for the studied independent factors were restricted to 0.2 ≤ X 1 ≤ 0.6 % w/w, and 24 ≤ X 2 ≤ 40 % w/w; whereas the desirable ranges of dependent variables or responses were restricted to 50 ≤ R 18 h ≤ 60 %, 20 ≤ t 80 % ≤ 30 h, and 12 ≤ DEE ≤ 30 %. The Design-Expert 9.0.3 software based on the criterion of desirability was employed for determining optimal value of responses using numerical analysis technique. The desirability plot and overlay plot indicating the desirable regression ranges and the region of optimal process variable settings are presented in Fig. 3a, b, respectively.
For validating the optimization capability of the mathematical models generated according to the results of 32-level factorial design, optimized pectin–PVA-co-poly(AMPS) semi-IPN hydrogels containing tramadol HCl were prepared using one of the optimal process variable settings as proposed by the Design-Expert 9.0.3. software (R 2 = 1). The selected optimal process variable setting used for the fabrication of optimized formulation was X 1 = 0.6 % w/w and X 2 = 40 % w/w with desirability of 0.697; thus, the contents value optimized formulation as suggested by the Design-Expert was the same as for the FPP-9 formulation. Optimized pectin–PVA-co-poly(AMPS) semi-IPN hydrogels containing tramadol HCl (FPP-10) were evaluated for R 18 h (%), t 80 % (h), and DEE (%). They showed R 18 h (%) of 56.345, t 80 % (h) of 30 h, and DEE of 23.997 % with small percentage error values (2.25, 5.80, and 4.37, respectively).
The results of calculated small percentage error values revealed that the predicted response values as given by the mathematical models and observed response values are in good agreement with each other, thereby indicating the good fitting of the models obtained from the 32 factorial design.
Swelling index at 18th hour (q 18 h)
Swelling index was used as an indicator of swelling characteristics; swelling index at 18th hour (q 18 h) for semi-IPN hydrogel formulations FPP-1 to FPP-9 ranged from 15.533 to 26.170. FPP-1 exhibited highest swelling index of 26.170, while the FPP-3 formulation showed lowest swelling index value of 15.533. The results of study revealed that increase in polymer as well as monomer contents caused a significant decrease in swelling index (p < 0.05). This overall decrease in swelling ratio on increasing pectin-to-PVA ratio would be attributed to more complex interaction between pectin and PVA, such as hydrogen bonding, hydrophobic interactions or physical cross-linking as polymer entanglements, thereby hindering mobility and relaxation of polymeric chains, which, in turn, caused increase in resistance to water absorption [32, 33], whereas reduction in swelling index on increasing monomer concentration may be attributed to: (i) AMPS counterions inside swollen gel do not contribute to the swelling process, (ii) hydrophobic interactions between alkyl groups of AMPS units induce aggregate formation, and the counterions within these aggregates may condense leading to decrease in osmotic pressure and, thus, restricts swelling, (iii) increased viscosity of medium which restricts the movement of monomer molecules, and (iv) an increase in sol fraction of hydrogels [34–36]. Similar results of decreased swelling index with increased pectin contents have been reported in superabsorbent hydrogels based on pectin and poly(sodium acrylate) [33]. In a study based on development of poly(2-acrylamido-2-methylpropane sulfonic acid)/chitosan hydrogels which have been employed for wastewater treatment, these hydrogels exhibited similar results of decreasing swelling ratio on increasing AMPS concentration in hydrogels formulation [37].
Percent drug release at 18th hour (R 18 h)
The developed semi-IPN gels exhibited pH-independent release characteristics, i.e., p value (p > 0.05) computed from independent sample t test using SPSS software (version 15.0) revealed that there was statistically insignificant difference in drug release at both pH 1.2 and pH 7.4 buffer medium. Percent drug release at 18th hour for FPP-1 to FPP-9 ranged from 55.521 to 77.125 %. FPP-2 exhibited highest percent drug release at 18th hour, while FPP-8 showed lowest percent drug release. In vitro drug release studies revealed that percent drug release at 18th hour was reduced significantly on increasing either pectin-to-PVA ratio (X 1) or AMPS concentration (X 2). The decrease in percent drug release on increasing pectin and monomer contents may be attributed to: (i) at high polymeric contents, the more hydrophilic property of polymers could probably lead to better binding with water, forming viscous gel structure which might block pores on the gel surface, thus delaying drug release [38], (ii) formation of a diffusion control layer of pectin led to the formation of a stringent barrier due to absorption of water, thus leading to the development of a highly viscous gelatinous diffusion control layer to water penetration, drug diffusion and, hence, drug release from hydrogels [39], (iii) increased amount of monomer provides more reactive functional groups to cross-link, both physically and chemically, with polymer, forming a compact and dense polymer structure, thus decreasing the porosity of polymeric network. In a study, similar results of delayed drug release on increasing pectin contents were observed in calcium pectinate–fenugreek seed mucilage mucoadhesive beads developed for controlled delivery of metformin HCl [38]. Similar results of decreasing percent drug release were obtained on increasing AMPS contents in polymer hydrogel containing polyvinyl alcohol, 2-acrylamide-2-methylpropane sulfonic acid and acrylamide [40].
Time required for 80 % drug release (t 80 %)
Time required for 80 % drug release for FPP-1 to FPP-9 ranged from 20 to 30 h. FPP-2 required less time for 80 % drug release, while for FPP-9, the time required for 80 % drug release was the longest. In vitro drug release studies revealed that t 80 % was increased significantly on increasing either pectin-to-PVA ratio (X 1) or AMPS concentration (X 2), i.e., t 80 % value was found to be directly proportional to pectin and AMPS contents. The possible explanation for this increase in time requirement for 80 % drug release may be attributed to the fact that increasing pectin contents lead to the formation of highly viscous gelatinous diffusion control layer which restricts water penetration, drug diffusion and, hence, drug release from hydrogels, thereby providing delayed drug release from these semi-IPN hydrogels. There is also formation of more dense polymeric network as well as decrease in porosity of gels which can further contribute to delayed drug release. Similar results have been found in a study that an increase in amount of Zn-pectinate in microparticles leads to increase in time requirement for 50 % drug release [41]. In a study, similar finding of decrease in percent drug release on increasing AMPS contents was reported which showed that time required for drug release is delayed [42].
Drug entrapment efficiency (%DEE) and drug-loading contents (DLC)
Drug entrapment efficiency for FPP-1 to FPP-9 ranged from 12.05 to 24.27 %. Studies revealed that DEE (%) was increased significantly on increasing either pectin-to-PVA ratio (X 1) or AMPS concentration (X 2), i.e., drug entrapment efficiency value was found to be directly proportional to pectin and AMPS contents. This increase in DEE with the increasing amount of pectin and monomer in these newly developed semi-IPN hydrogels could be attributed to the increasing viscosity of the polymer-blend solutions with the increasing amount of polymer addition which might have prevented drug leaching from the prepared gels to the solution. Also, these high polymer contents in semi-IPN hydrogels might have increased the capacity for uptaking more drug molecules into the gel network due to more availability of polymer to encapsulate drug [38, 43]. Similar results of increasing drug entrapment efficiency on increasing polymer contents have been observed in blends of jackfruit seed starch–pectin mucoadhesive beads containing metformin HCl [44]. Another study reported similar finding of increasing DEE on increasing AMPS contents in surfactant-modified poly(acrylamide-co-acrylamido propane sulfonic acid) hydrogels [45].
Amount of drug-loaded content for FPP-1 to FPP-9 ranged from 141.31 to 165.33 mg/gm. For FPP-3, drug loaded in semi-IPN gels was the lowest, while maximum amount of drug was loaded in FPP-4. Studies revealed that drug-loading efficiency was not increased significantly on increasing pectin-to-PVA ratio (X 1), but on increasing AMPS concentration (X 2), a significant decrease in drug-loading efficiency was observed. This slight insignificant increase in drug-loaded content on increasing pectin contents may be attributed to the more availability of polymer to uptake the drug. Similar results of increasing drug-loaded contents on increasing pectin contents have been reported in ethyl cellulose-coated pectin microspheres incorporating 5-FU for colon targeting [46].
Similar results of decreasing drug-loading efficiency in microgels on increasing AMPS contents in the poly(acrylic acid–co-2-hydroxyethyl methacrylate-co-2-acrylamido-2-methyl-1-propanesulfonic acid) microgels encapsulating two different drugs, i.e., lidocaine HCl and methylene blue have been reported [47].
Sol–gel fraction
The sol–gel fraction of all pectin–PVA-co-poly(AMPS) semi-IPN gels was determined to assess the extent of consumption of reactants during free radical polymerization to form a stable product. The sol–gel fraction study revealed that there was an increase in gel fraction of interpenetrating network on increasing either the pectin or AMPS contents in the polymeric hydrogel formulation as shown in Table 5. This increase in gel fraction can be attributed to the fact that by increasing amount of reactant (pectin or AMPS) of formulation reaction mixture, there is an increase in possible reactive sites for free radical polymerization reaction.
Similar findings of increasing gel fraction on increasing pectin contents were reported in pH-sensitive pectin/acrylic acid hydrogels for controlled delivery of verapamil [48]. Another study based on development of hybrid copolymer polyvinyl alcohol hydrogels incorporating antidepressant drug for controlled drug delivery reported similar results of increasing gel fraction on increasing AMPS contents in the formulation [49].
FTIR analyses
The spectrum of pure pectin gives characteristic broad peak due to –OH stretching at 3368 cm−1, while the spectral peak at 1737 cm−1 is indicative of strong stretching absorbance due to the presence of ester carbonyl (>C=O) groups; those at 1605 and 1227 cm−1 correspond to C=C and C–O stretching vibrations, respectively. PVA spectrum shows strong, broad absorbance band at 3300 cm−1 linked to the –OH stretching due to inter- and intra-molecular hydrogen bonding; peak at 2934 cm−1 indicates the presence of >C–H broad alkyl stretching band. A peak at 1089 cm−1 suggests the presence of carboxyl (>C–O) stretching band attributed to crystallinity of PVA. AMPS spectrum reveals peaks at 2986 cm−1 representing the C–H stretching frequency of –CH2, while two bands around 1666 and 1612 cm−1 are due to C=O stretching (amide I band) and N–H2 bending (amide II band), respectively. Two peaks observed at 1372 and 1126 cm−1 indicate asymmetric and symmetric stretching due to >SO2 group. Tramadol HCl gives characteristic strong peak of alcoholic –OH stretching at 3301 cm−1, another peaks at 2930 and 2862 cm−1 show stretching vibration due to aliphatic >C–H group. Bands observed at 2601 and 2483 cm−1 are attributed to ammonium N+–H stretching, while those displayed at 1607 and 1479 cm−1 indicate the aromatic ring skeleton stretching vibrations Fig. 4a.
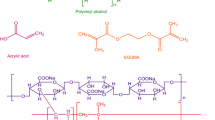
The FTIR spectrum of pectin–PVA-co-poly(AMPS) semi-IPN hydrogels without tramadol HCl exhibited a quite different peak pattern from the spectra of pure components, thus indicating the formation of a new polymeric IPN network showing all characteristic peaks of pectin, PVA and AMPS, without any significant interaction. FTIR spectrum of semi-IPN hydrogels gives –OH stretching peak with lowered intensity at 3295 cm−1, thus confirming the formation of hydrogen bond between pectin and PVA [32, 50]. Similarly, the peak of esterified carboxyl group in pure pectin at 1737 cm−1 is shifted to lower frequency of 1639 cm−1 which is due to chemical bonding between pectin, PVA and AMPS, while asymmetric and symmetric stretching due to >SO2 group at 1372 and 1126 cm−1 in AMPS is also shifted to higher frequency of 1391 and 1147 cm−1 indicating grafting of monomer onto polymeric backbone and possible cross-linking between polymers and the monomer. In the FTIR spectrum of pectin–PVA-co-poly(AMPS) hydrogels containing tramadol HCl, various characteristic peaks of pectin, PVA, AMPS and tramadol HCl appeared without any significant shifting, therefore suggesting no possible interaction between drug (tramadol HCl) and polymers (pectin and PVA) in the semi-IPN hydrogels Fig. 4b.
SEM analysis
The surface morphological analysis of the optimized pectin–PVA-co-poly(AMPS) semi-IPN hydrogels was visualized by SEM and is presented in Fig. 5. The SEM photograph of these hydrogels revealed non-porous and rough surface. The surface of these hydrogels exhibited extremely rough surface with characteristic rough ridges, large wrinkles and cracks. These observed cracks and wrinkles on the surface may be caused as a result of partial collapsing of the polymeric gel network of hydrogel during drying process. Along with this, the polymeric derbies were also seen on the gel surface which could be attributed to the employed method of preparation (i.e., simultaneous gel preparation on mixing and formation of the polymer-blend matrix of semi-IPN hydrogel).
Swelling behavior
Swelling properties of newly formulated pectin–PVA-co-poly(AMPS) semi-IPN hydrogels were evaluated at pH 1.2 and pH 7.4 as a function of time. All developed semi-IPN hydrogels exhibited pH-independent swelling behavior due to the effective grafting of AMPS onto the polymeric backbone. Dynamic swelling index of optimized semi-IPN gel (FPP-10) at pH 1.2 and pH 7.4 is shown in Fig. 6. Swelling studies revealed that pectin–PVA-co-poly(AMPS) hydrogels exhibited same degree of swelling at both pH media as indicated by their swelling index. This pH-independent swelling behavior is attributed to the inherent strongly acidic property of AMPS (pKa = 1.9) due to the presence of sulfonate groups which causes it to completely dissociate over the entire pH range of 2–10 at constant ionic strength [34, 51, 52]. Thus, hydrogels grafted with AMPS as ionizable co-monomer exhibit pH-independent swelling characteristics.
Conclusion
Novel and effective drug delivery system for controlled release of tramadol HCl was developed in the form of semi-IPN polymeric matrices using natural and synthetic biodegradable, biocompatible and non-immunogenic polymers. These chemically cross-linked polymeric semi-IPN pectin–PVA-co-poly(AMPS) hydrogels exhibited several promising and excellent characteristics as indicated by in vitro evaluation; thereby combining the unique features of the controlled drug delivery, pH-independent release and time-dependent release to provide drug release for prolonged span of time. These highly stable chemically cross-linked pectin–PVA-co-poly(AMPS)-based polymeric matrices showed maximum swelling, drug release and drug loading at pH 7.4. Tramadol HCl was released from this semi-IPN polymeric matrix for up to 48 h.
References
Bajpai A, Shukla SK, Bhanu S, Kankane S (2008) Responsive polymers in controlled drug delivery. Prog Polym Sci 33(11):1088–1118
Dragan ES (2014) Design and applications of interpenetrating polymer network hydrogels. A review. Chem Eng J 243:572–590
Prashantha K (2001) IPNs based on polyol modified castor oil polyurethane and poly (HEMA): synthesis, chemical, mechanical and thermal properties. Bull Mater Sci 24:535–538
Myung D, Waters D, Wiseman M, Duhamel PE, Noolandi J, Ta CN, Frank CW (2008) Progress in the development of interpenetrating polymer network hydrogels. Polym Advan Technol 19(6):647–657
Sperling LH (1991) Interpenetrating polymer networks: an overview. Interpenetrating polymer networks. American Chemical Society, Washington
Kim SJ, Yoon SG, Kim SI (2004) Synthesis and characteristics of interpenetrating polymer network hydrogels composed of alginate and poly (diallydimethylammonium chloride). J Appl Polym Sci 91(6):3705–3709
Mohamadnia Z, Zohuriaan-Mehr M, Kabiri K, Jamshidi A, Mobedi H (2007) pH-sensitive IPN hydrogel beads of carrageenan-alginate for controlled drug delivery. J Bioact Compat Pol 22(3):342–356
Lohani A, Singh G, Bhattacharya SS, Verma A (2014) Interpenetrating polymer networks as innovative drug delivery systems. J Drug Deliv 2014:11
Sionkowska A (2011) Current research on the blends of natural and synthetic polymers as new biomaterials: review. Prog Polym Sci 36(9):1254–1276
Mukhiddinov ZK, Khalikov DK, Abdusamiev FT, Avloev CC (2000) Isolation and structural characterization of a pectin homo and ramnogalacturonan. Talanta 53(1):171–176
Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11(3):266–277
Sriamornsak P (2003) Chemistry of pectin and its pharmaceutical uses: a review. Silpakorn Univ Int J 3(1–2):206–228
Liu L, Fishman ML, Hicks KB (2007) Pectin in controlled drug delivery—a review. Cellulose 14(1):15–24
Liu L, Fishman ML, Kost J, Hicks KB (2003) Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 24(19):3333–3343. doi:10.1016/S0142-9612(03)00213-8
Peppas N, Bures P, Leobandung W, Ichikawa H (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50(1):27–46
Sinha V, Kumria R (2001) Polysaccharides in colon-specific drug delivery. Int J Pharm 224(1):19–38
Yadav N, Morris G, Harding S, Ang S, Adams G (2009) Various non-injectable delivery systems for the treatment of diabetes mellitus. Endocr Metab Immune Disord Drug Targets (Former Curr Drug Targets Immune, Endocr Metab Disord) 9(1):1–13
Sungthongjeen S, Sriamornsak P, Pitaksuteepong T, Somsiri A, Puttipipatkhachorn S (2004) Effect of degree of esterification of pectin and calcium amount on drug release from pectin-based matrix tablets. Aaps Pharmscitech 5(1):50–57
Tubbs RK (1966) Sequence distribution of partially hydrolyzed poly (vinyl acetate). J Polym Sci A1 Polym Chem 4(3):623–629
Kadajji VG, Betageri GV (2011) Water soluble polymers for pharmaceutical applications. Polymers 3(4):1972–2009
Peppas N, Huang Y, Torres-Lugo M, Ward J, Zhang J (2000) Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng 2(1):9–29
Mattia C, Coluzzi F (2006) Once-daily tramadol in rheumatological pain. Expert Opin Pharmacother 7(13):1811–1823
Budd K (1999) The role of tramadol in acute pain management. Acute Pain 2(4):189–196
Minhas MU, Ahmad M, Ali L, Sohail M (2013) Synthesis of chemically cross-linked polyvinyl alcohol-co-poly (methacrylic acid) hydrogels by copolymerization; a potential graft-polymeric carrier for oral delivery of 5-fluorouracil. DARU J Pharm Sci 21(1):44. doi:10.1186/2008-2231-21-44
Hamed E, Sakr A (2001) Application of multiple response optimization technique to extended release formulations design. J Control Release 73(2):329–338
Lin YH, Liang HF, Chung CK, Chen MC, Sung HW (2005) Physically crosslinked alginate/N, O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. Biomaterials 26(14):2105–2113
Malakar J, Nayak AK (2012) Formulation and statistical optimization of multiple-unit ibuprofen-loaded buoyant system using 23-factorial design. Chem Eng Res Des 90(11):1834–1846
Guru PR, Nayak AK, Sahu RK (2013) Oil-entrapped sterculia gum–alginate buoyant systems of aceclofenac: development and in vitro evaluation. Colloids Surf B 104:268–275
Nayak AK, Pal D, Santra K (2014) Development of calcium pectinate-tamarind seed polysaccharide mucoadhesive beads containing metformin HCl. Carbohyd Polym 101:220–230
Peppas NA, Barr-Howell BD (1986) Characterization of the cross-linked structure of hydrogels. Hydrogels Med Pharm 1:27–56
Varaprasad K, Reddy NN, Ravindra S, Vimala K, Raju KM (2011) Synthesis and characterizations of macroporous poly (acrylamide-2-acrylamido-2-methyl-1-propanesulfonic acid) hydrogels for in vitro drug release of ranitidine hydrochloride. Int J Polym Mater 60(7):490–503
Kaczmarek H, Vuković-Kwiatkowska I (2011) Accelerated weathering of pectin/poly (vinyl alcohol) blends studied by spectroscopic methods. J Appl Polym Sci 122(3):1936–1945
Xiaoye M, Ruili W, Jinwei C, Jiping C, Juan Z (2011) Synthesis and characterization of pectin/poly (sodium acrylate) hydrogels. Carbohyd Polym 86(1):313–319
Durmaz S, Okay O (2000) Acrylamide/2-acrylamido-2-methylpropane sulfonic acid sodium salt-based hydrogels: synthesis and characterization. Polymer 41(10):3693–3704
Pourjavadi A, Barzegar S, Zeidabadi F (2007) Synthesis and properties of biodegradable hydrogels of κ-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React Funct Polym 67(7):644–654
Pourjavadi A, Hosseinzadeh H, Mazidi R (2005) Modified carrageenan. 4. Synthesis and swelling behavior of crosslinked κC-g-AMPS superabsorbent hydrogel with antisalt and pH-responsiveness properties. J Appl Polym Sci 98(1):255–263
Gad Y (2008) Preparation and characterization of poly (2-acrylamido-2-methylpropane-sulfonic acid)/Chitosan hydrogel using gamma irradiation and its application in wastewater treatment. Radiat Phys Chem 77(9):1101–1107
Nayak AK, Pal D, Das S (2013) Calcium pectinate-fenugreek seed mucilage mucoadhesive beads for controlled delivery of metformin HCl. Carbohyd Polym 96(1):349–357
Sriamornsak P, Thirawong N, Weerapol Y, Nunthanid J, Sungthongjeen S (2007) Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur J Pharm Biopharm 67(1):211–219
Devi N, Narzary A (2012) Release dynamics of brufen from a drug-loaded polymer hydrogel containing polyvinyl alcohol, 2-acrylamide-2-methylpropane sulfonic acid and acrylamide. Int J Polym Mater 61(11):821–833
El-Gibaly I (2002) Oral delayed-release system based on Zn-pectinate gel (ZPG) microparticles as an alternative carrier to calcium pectinate beads for colonic drug delivery. Int J Pharm 232(1):199–211
Bartlett RL, Medow MR, Panitch A, Seal B (2012) Hemocompatible poly (NIPAm-MBA-AMPS) colloidal nanoparticles as carriers of anti-inflammatory cell penetrating peptides. Biomacromolecules 13(4):1204–1211
Mahajan HS, Tatiya BV, Nerkar PP (2012) Ondansetron loaded pectin based microspheres for nasal administration: in vitro and in vivo studies. Powder Technol 221:168–176. doi:10.1016/j.powtec.2011.12.063
Nayak AK, Pal D (2013) Blends of jackfruit seed starch–pectin in the development of mucoadhesive beads containing metformin HCl. Int J Biol Macromol 62:137–145. doi:10.1016/j.ijbiomac.2013.08.020
Ravindra S, Mohan YM, Varaprasad K, Reddy NN, Vimala K, Raju KM (2009) Surfactant-modified poly (acrylamide-co-acrylamido propane sulphonic acid) hydrogels. Int J Biol Macromol 58(5):278–296
Mazumder R, Allamneni Y, Firdous S, Parya H, Chowdhury A (2013) Formulation, development and in vitro release effects of ethyl cellulose coated pectin microspheres for colon targeting. Asian J Pharm Clin Res 6(5):134–144
Nart Z, Kayaman-Apohan N (2011) Preparation, characterization and drug release behavior of poly (acrylic acid–co-2-hydroxyethyl methacrylate-co-2-acrylamido-2-methyl-1-propanesulfonic acid) microgels. J Polym Res 18(5):869–874
Ranjha NM, Mudassir J, Sheikh ZZ (2011) Synthesis and characterization of pH-sensitive pectin/acrylic acid hydrogels for verapamil release study. Iran Polym J 20(2):147–159
Ali L, Ahmad M, Usman M, Yousuf M (2014) Controlled release of highly water-soluble antidepressant from hybrid copolymer poly vinyl alcohol hydrogels. Polym Bull 71(1):31–46
Kowalonek J, Kaczmarek H, Dąbrowska A (2010) Air plasma or UV-irradiation applied to surface modification of pectin/poly (vinyl alcohol) blends. Appl Surf Sci 257(1):325–331
Jeong KS, Kee LC, KS I (2004) Electrical/pH responsive properties of poly (2-acrylamido-2-methylpropane sulfonic acid)/hyaluronic acid hydrogels. J Appl Polym Sci 92(3):1731–1736
Zhang C, Easteal AJ (2003) Study of poly (acrylamide-co-2-acrylamido-2-methylpropane sulfonic acid) hydrogels made using gamma radiation initiation. J Appl Polym Sci 89(5):1322–1330
Acknowledgments
Authors are thankful to Higher Education Commission of Pakistan and the Islamia University of Bahawalpur for financing the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehmani, S., Ahmad, M., Minhas, M.U. et al. Development of natural and synthetic polymer-based semi-interpenetrating polymer network for controlled drug delivery: optimization and in vitro evaluation studies. Polym. Bull. 74, 737–761 (2017). https://doi.org/10.1007/s00289-016-1743-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1743-y