Abstract
To investigate the effects of polymeric excipients for dutasteride solid dispersion, experimental approaches together with physical interactions at molecular level were evaluated. The drug and various polymers (anionic, amphiphilic, and hydrophilic) were mixed physically into different ratios and their thermodynamic and physical properties were analyzed by differential scanning calorimetry and Fourier transform-infrared spectroscopy, respectively. The enhanced equilibrium solubility of dutasteride was also investigated. Dutasteride is non-ionic and showed low solubility in the tested pH ranges (lower than the detection limit of 20 ng/mL). Kollidon® MAE 100P, an anionic polymer, showed enhanced dutasteride solubility in aqueous solution followed by hydrophilic Kollidon® SR and the amphiphilic polymer, Soluplus®. Melting point (T m ) of dutasteride was 249.7 °C and was decreased to 229.84 °C when mixed evenly with Kollidon® MAE 100P. However, the melting point was not detected at a ratio of 1:4 since it fully dissolved or dispersed in the polymer. Glass transition temperature (T g ) of different compositions exhibited strong interaction of polymer and drug. The result was supported by spectra evidence that Kollidon® MAE 100P forms hydrogen bonds with dutasteride presenting strong physical interaction with the primary amine group of dutasteride. This study supports a convenient method that together with microscopic observation can perform polymer selection and characterize solid dispersions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To increase the targeting and selective properties of drug compounds, more compounds have been designed to be hydrophobic with higher molecular weight. Many novel compounds that have very poor solubility have been discovered. The solubility of a compound can be a key determinant for its development and oral bioavailability. Enhancement of solubility of poorly water-soluble drugs has been one of the challenges in developing drug formulations. Common methods to increase solubility is by reduction of particle size, modification of polymorphism, addition of salts, or using organic solvents (Chu et al. 2012; Kim and Sohn 2011; Serajuddin 1998). However, more efficient methods to increase oral bioavailability of poorly water-soluble drugs are still required (Serajuddin 1999).
One of the feasible approaches is to use amorphous solids in place of crystals in pharmaceutical formulations (Leuner and Dressman 2000; Craig 2002). This approach makes use of higher solubility of amorphous solids than their crystalline counterparts. After the administration of the amorphous drug, improved wettability of the polymer and prolonged in vivo supersaturation can synergistically enhance drug absorption (Joshi et al. 2004). To accomplish this approach, understating the stability of the amorphous state against crystallization is necessary, as crystallization may result in low solubility and bioavailability (Miyanishi et al. 2012 ; Zidan et al. 2012; Sinclair et al. 2011).
A crystalline drug dissolved or dispersed molecularly in polymeric matrices that adopts an amorphous form is a solid dispersion. A solid dispersion is generally produced by melting or solvent evaporation methods (Chiou and Riegelman 1971). Since a solid dispersion has a lower thermodynamic barrier of the amorphous drug, supersaturation may be formed kinetically in the diffusion layer, resulting in increased drug solubility (Hancock and Zografi 1997). However, the amorphous state is thermodynamically metastable and tends to return to a more stable solid state. To prevent the recrystallization process, higher amounts of polymers or solid dispersion formers can be added to stabilize or capture the amorphous state. This can be achieved with investigating the miscibility between drug and the solid dispersion polymer, which is the key factor to prepare amorphous/semi-amorphous solid dispersions (Al-Obaidi et al. 2013 ).
Miscibility mainly depends on the molecular interactions of the drug and the polymer. Strong interaction between the polymer and the crystalline drug is needed to keep the drug in the polymer. If the interaction is weak, the drug and the polymer will be separated and eventually fast recrystallization of the solid dispersion will occur (Zhu et al. 2010). In addition, a dosage form of development of a solid dispersion may face practical challenges including inadequate solubility and instability of drugs in carriers at elevated temperatures (Serajuddin 1999). A recent study demonstrated that the anionic hydrophilic polymer maintained griseofulvin in an amorphous state for more than 18 months, even when exposed to high temperatures and high humidity, due to the strong interaction of the polymer and the drug (Al-Obaidi and Buckton 2009). Therefore, a physical property might be dependent on the miscibility of polymer and drug. Recrystallization can be overcome by selecting an appropriate polymer that has strong interaction to the drug for the solid dispersion formulation.
Despite its importance, there have been few standard techniques for measuring the drug/polymer solubility. Since the high viscosity of polymers and drug/polymer dispersions tend to be glasses or liquids, it is difficult to achieve equilibrium solubility. In this study, however, apparent solubility of the drug/polymer was defined by various analytical methods and also finds the most miscible polymer for the drug. One of the main goals of this work was to investigate drug/polymer solubility, miscibility, physical interactions, and thermodynamic characterizations, and find the most suitable polymer efficiently for the preparation of solid dispersion.
Dutasteride is a synthetic 4-azasteride derivative used for the treatment of prostate diseases, such as benign prostatic hyperplasia, prostate cancer, acne, male pattern baldness, and hirsutism (Page et al. 2011). It was selected as a model drug in this study since it is poorly water soluble with a high melting temperature (T m ). Three categorized polymers—anionic, amphiphilic, and hydrophilic—were selected with various drug–polymer ratios. By using differential scanning calorimetry (DSC), thermodynamic properties of physical mixtures [i.e. T m , glass transition temperature (T g ), and heat of fusion] were observed. Their physical interactions were also investigated at the molecular level by attenuated total reflection Fourier-transform infrared spectroscopy (ATR FT-IR). By using a diamond crystal ATR accessory, fast and accurate spectra could be obtained. In addition, all prepared samples were scanned by polarized microscopy to monitor recrystallization after the preparation of solid dispersions.
Materials and methods
Materials
Dutasteride was purchased from Cipla (Mumbai, India). Polyvinylpyrrolidones including Kollidon® 17PF, Kollidon® 25, Kollidon® 30, and Kollidon® VA64, Lutrol® F68 (Poloxamer 188, polyoxyethylene–polyoxypropylene glycol), Kollidon® SR (polyvinyl acetate), Soluplus®, and Kollicoat® MAE 100P (methacrylic acid ethylacrylate copolymer) were obtained from BASF (Ludwigshafen, Germany). All other reagents were of analytical or high-performance liquid chromatography (HPLC) grade and were used as received. The chemical structures of the polymers and the model drug are shown in Fig. 1.
Methods
pH solubility profiles and solubility enhancement in polymer solutions
Solutions of various pH values from 1 to 12 were prepared simply using hydrochloric acid (HCl) and sodium hydroxide (NaOH) to reduce the effects of ionic strength. Serial dilution was adopted to prepare each pH point and its value was measured by a model 827 lab pH meter (Metrohm, Zurich, Switzerland). An excess amount of the drug was placed in a glass vial containing 1 mL of the solution. The capped vials were shaken for 24 h and equilibrated at room temperature. Saturated solutions were centrifuged for 30 min at 12,000 rpm. The drug concentrations were determined using a 1100 Series HPLC system (Agilent, Santa Clara, CA, USA). All measurements were carried out three times.
Aqueous polymeric solutions (10 % w/v) were prepared with pH 6.8 phosphate buffer. An excess amount of the drug was also added to the prepared polymeric solutions and shaken for 72 h. All samples were centrifuged for 30 min at 12,000 rpm. The equilibrium solubility of the drug was analyzed by the aforementioned HPLC system.
HPLC analysis
Analytical experiments were carried out using the aforementioned HPLC system, in which the wavelength of the ultraviolet detector was set at 210 nm. A Symmetry® C18 reverse phase column (250 × 4.6 mm ID, particle size 5 μm; Waters, Ireland) was used and maintained at approximately 30 °C. The mobile phase was a mixture of acetonitrile and water at a v/v ratio of 60:40. The flow rate of the mobile phase was 1.0 mL/min. Each analytical sample was collected in Eppendorf tubes, centrifuged, and 50 μL of each supernatant was injected into the HPLC.
Preparation of physical mixtures
Dutasteride/polymer physical mixtures were prepared by lightly grinding accurately weighed quantities of crystalline or amorphous drug and a polymer in a mortar for 2 min at the required dutasteride/polymer level. The weight ratios of the drug and the polymer were 4:1, 1:1, and 1:4. Particles with the size of 25–250 μm were collected for further analysis.
DSC
Thermal properties of the test samples were evaluated using a TA DSC Q-2000 (TA Instrument, DE, USA). Small amount of physical mixtures (5–10 mg) was packed into aluminum pans. DSC was performed against a reference pan at temperatures between 0 and 280 °C at a 20 °C/min scanning rate under a nitrogen flow of 50 mL/min. The endpoint temperature was kept for 5 min, cooled to 0 °C, and reheated to 280 °C at a rate of 20 °C/min. The glass transition temperature (T g ) and melting temperatures (T m ) were evaluated using the Universal Analysis 2000 software provided with the equipment.
ATR FT-IR
ATR FT-IR spectra (4,000–600 cm−1) were collected at 4 cm−1 resolution by a Nicolet 6700 FT-IR spectrophotometer (Thermo Fisher, Waltham, MA, USA) with the golden gate accessory (diamond crystal). The powder samples were dropped on the diamond crystals and scanned immediately after background scanning. Spectra of individual components were calculated using the OMNIC software provided with the equipment.
Determination of solubilization capacity with polarized microscopy
Solubilization capacity of dutasteride in the polymers was determined by film casting experiments. The physical mixtures of dutasteride and polymer were dissolved in methanol. The solution was casted on a glass plate making a thin film. The film was stored in vacuum drying cabinet (50 °C, 10 mbar) for 1 h to ensure complete drying of the methanol. Visual inspection of the films was performed through polarized microscopy using a model 3000 microscope (Leica, Nussloch, Germany) after 7 days open storage at room temperature.
Powder X-ray diffraction (PXRD)
Powder X-ray diffraction (PXRD) was utilized to monitor the amorphous and crystalline states of the samples. The PXRD patterns were measured at room temperature using a Bruker D8 Advance diffractometer (Bruker AXS GmbH) with Cu as the anode material operated at a voltage of 40 kV and current of 40 mA. The samples were loaded onto a sample holder and smoothed with a spatula. The scan range was from 4° to 40° 2θ at a scanning rate of 4°/min.
Results and discussion
Solubility enhancement of dutasteride by polymeric effect
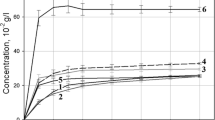
The pH solubility profile of the model drug suggested that it was non-ionic as it did not provide any particular increase in the solubility in an acidic or basic environment (Fig. 2). Dutasteride had very poor solubility in the tested pH ranges. The average solubility was almost 0.025 ± 0.004 μg/mL, which was close to the detection limit of 20 ng/mL. However, the solubility of the drug increased significantly in the various polymeric solutions (10 % w/v) (Fig. 3). As a reference, phosphate buffer at pH 6.8 was used and showed a solubility that was lower than the detection limit. The apparent solubility of dutasteride in solutions of Kollicoat® MAE 100P, Kollidon® SR, Soluplus®, Kollidon® 30, Lutrol® F68, Kollidon® 25, Kollidon® 17PF, Kollidon® VA64 were 576.50, 85.57, 62.64, 49.42, 44.32, 26.64, 19.11, and 17.98 μg/mL, respectively. All polymers increased drug solubility, with Kollicoat® MAE 100P (an anionic hydrophilic polymer) being the highest. In addition, more additives were evaluated. Kollidon® 12, Kollicoat® IR, Kollicoat® protect, citric acid monohydrate, lactose anhydrous, starch 1500, and polyethylene glycol (PEG) 6000 resulted in no HPLC detection (lower than detection limit) of dutasteride. The first eight polymers, which enhanced the solubility, were selected for further investigation.
Thermal properties of the samples
The prepared physical mixtures were thermally scanned by DSC, which also induced solid dispersion within the pan during the heating and cooling processes. Figure 4 shows the thermograms with different dutasteride/polymer ratios. Each figure has five thermograms: the top one is the thermogram of dutasteride (melting temperature; T m 248.93 °C) for reference. The following three thermograms are the dutasteride/polymer with increasing polymer contents and the last one is the thermogram of the polymer. The polymers were categorized tentatively into three groups based on their molecular structures—ionic, amphiphilic, and hydrophilic polymers. The resulting thermal properties of the mixtures are listed in Table 1.
Kollicoat® MAE 100P is a copolymer consisting of methacrylic acid and ethylacrylate in a ratio of 1:1. It is an anionic copolymer mainly used as a film former in enteric coatings for solid dosage forms. The DSC thermogram of Kollicoat® MAE 100P showed an endothermic peak near 210 °C but not as sharp as that of the drug. Heat of fusion (J/g) of dutasteride in the polymer decreased from 74.13 to 2.82 J/g when mixed evenly and became negligible as more polymers were added. In addition, the melting temperature (T m ) decreased with increasing amount of the polymer. This may suggest that more drugs might be dissolved as the amount of polymer increased. Each polymer may have its own dissolving capacity and, hence, the ratio between the drug and polymer may affect the overall solubility and thermal properties.
Soluplus® is a polyvinyl caprolactam–polyvinyl acetate–PEG graft copolymer and it is an amphiphilic polymer. Kollidon® SR is a spray-formulated mixture of polyvinyl acetate and polyvinylpyrrolidone (povidone), which is hydrophilic and used as sustained release matrix. Kollidon® VA64 consists of N-vinylpyrrolidone and vinyl acetate. Vinylpyrrolidone is a hydrophilic water-soluble monomer, whereas vinyl acetate is lipophilic (water-insoluble). These three polymers decreased T m as well as heat of fusion of dutasteride as their contents increased (Fig. 4). However, Lutrol® F68, which is a synthetic copolymer of ethylene oxide and propylene oxide having amphiphilic properties, could not decrease T m of the drug compared with the other polymers. In its thermograms, a sharp endothermic peak was observed around 50 °C, which was the T m of Lutrol® F68. These observations may indicate that Lutrol® F68 is a good solubilizer of the drug instead of a polymeric solvent in the solid state, since it melted before the T m of the drug and also decreased heat of fusion depending on its contents. However, recrystallization was observed during the cooling process within the sample pan as confirmed by the DSC evaluation.
Kollidon® 17PF, Kollidon® 25, and Kollidon® 30 are hydrophilic polymers consisting of polyvinylpyrrolidone with different nominal K values (viscosity index relating to molecular weight and is calculated by a formula with relative viscosity which is measured by capillary viscometer at 25 °C). These hydrophilic polymers exhibited endothermic peaks near 110 °C but they were broader than Kollicoat® MAE 100P. All hydrophilic polymers decreased T m and heat of fusion of dutasteride. Especially, Kollidon® 17PF decreased the T m of dutasteride to 205.51 °C and also decreased the heat of fusion to 3.649 J/g at the ratio of 1:4, suggesting a good polymeric solvent for dutasteride. However, Kollidon® 17PF had lowest glass transition temperature (T g ) out of the three polymers (Fig. 5).
Figure 5 depicts the secondary heat thermograms of dutasteride/polymer after cooling the primary heated mixtures down to 0 °C. The T g (temperature where the reversible glass transition occurs from a hard and relatively brittle state into a molten or rubber-like state) marked in each thermogram represents the transition from the glassy to the rubbery state of the solid dispersion prepared by the heat-quenching method. The result indicates that the T g of prepared solid dispersions shifted towards the T g of the polymer component. T g can be an indicator of interaction between polymer and drug, since T g is dependent on the amounts of polymer added and increases if strong interaction exists between polymer and drug. T g of dutasteride with Kollicoat® MAE 100P, Kollidon® 17PF, Kollidon® 25, and Kollidon® 30 was elevated as polymer contents increased. However, Kollidon® VA64 and Kollidon® SR had two significant split T g , which may indicate poor miscibility of drug and polymer. The T g of the model drug (second T g in Kollidon® SR) was increased to 154.74 °C, since it contained polyvinylpyrrolidone (i.e. Kollidon®s). On the other hand, Soluplus® and Lutrol® F68 seemed not suitable for solid dispersion, since the T g of the solid dispersion prepared by Soluplus® was lower than dutasteride.
Nevertheless, dutasteride/Kollicoat® MAE 100P at a ratio of 1:4 had a T g of 127.83 °C, which was significantly higher than Kollicoat® MAE 100P itself (119.56 °C). This explains why T m of dutasteride was not observed at the primary heating process. Since the strong physical interactions are involved between the drug and polymer, drug had fully dissolved in Kollicoat® MAE 100P. Moreover, this explains why the polymer markedly increased the solubility of dutasteride (Fig. 3). On the other hand, the PXRD showed that the model drug existed in amorphous state as shown in Fig. 6. Peaks that represent crystallinity of the drug were not observed from the diffraction patterns. Further investigation was performed to elucidate the physical interactions between the drug and polymer.
ATR FT-IR evaluation of the solid dispersions
The presence of non-covalent interactions between the drug and polymers may affect the physicochemical stability of the solid dispersion. This was examined using ATR FT-IR spectroscopy focusing on the solid dispersion and physical mixtures of dutasteride and Kollicoat® MAE 100P. Figure 7 shows the FT-IR spectra obtained from the physical mixtures (Fig. 7a) and corresponding solid dispersions (Fig. 7b) at ratios of 1:4, 1:1, and 4:1. The peaks at 1,723 and 1,698 cm−1 represented the carbonyl group in Kollicoat® MAE 100P while the peaks at 1,713 and 1,677 cm−1 represented the stretching of the two carbonyl groups in dutasteride (Fig. 1). As can be seen from the FT-IR spectra in Fig. 7b, when Kollicoat® MAE 100P was added beyond the ratio of 1:1 in the solid dispersion, there was a broadening in the carbonyl group peak at 1,713 cm−1 attributed to the presence of dutasteride, which was not observed in the corresponding physical mixtures. In addition, the peak at 1,677 cm−1 was also broadened and shifted to 1,668 cm−1 when more polymers were added. The broadening of the carbonyl groups of dutasteride was followed by the disappearance of the aromatic C=C stretching from dutasteride 1590, 1538, and 3469 cm−1, possibly indicating the transformation of dutasteride into amorphous form, reflecting the disordered nature of the solid dispersion.
Figure 7c shows the FT-IR spectra obtained for the solid dispersion and physical mixtures where the wavenumber was from 3,600 to 3,200 cm−1. The peak at 3,469 cm−1 represented the amide group of dutasteride. It disappeared when prepared as solid dispersion. This might be evidence indicating a breakdown in the crystalline structure of dutasteride leading to the formation of the amorphous state. Furthermore, the changes in the shape of the peaks at 1,713 and 1,677 cm−1, which were particularly evident at Kollicoat® MAE 100P contents above the ratio 1:1, most likely indicated a breakdown in the crystalline structure. However, due to the qualitative manner of FT-IR, it was not easy to quantitatively investigate the changes described.
On the other hand, when the results obtained from the physical mixtures were compared with those from the corresponding solid dispersions, the carbonyl peaks at 1,713 and 1,677 cm−1 attributable to dutasteride remained distinct and sharp, and did not shift upon mixing with Kollicoat® MAE 100P. This may suggest that the changes in the shape and position of the peaks in the solid dispersions resulted from hydrogen bond formation between dutasteride and Kollicoat® MAE 100P.
Polarized microscopic view
Figure 8 shows the microscopic observation of dutasteride solid dispersion with various polymers after 7 days storage at room temperature. To evaluate the solubilization capacity of the polymers, the incorporated amounts of drug increased for the film casting process (25, 33, and 50 % w/w). It was assumed that the higher the clarity of the samples, the higher the solubilization capacity would be. Kollicoat® MAE 100P and Soluplus® showed higher solubilization capacity, followed by Kollidon® SR. Kollidon® series of lower molecular weights dissolved only smaller amounts of the drug. In addition, Lutrol® F68 exhibited very rapid recrystallization of the drug, even in lower concentrations, since the polymer had poor miscibility with the drug based on the DSC data. The solubilization capacity of polymers for dutasteride is listed in Table 2. From the polarized microscopic view, drug in polymer with decent miscibility exhibited a key factor, suppressing recrystallization of the drug for long-term storage. Therefore, film-casing with microscopic observation might be one of the efficient tools to select excipients or polymers for solid dispersion preparations.
Conclusions
Selecting suitable vehicles or polymers for solid dispersion can be an important stage to facilitate product development, as there are many excipients in the market for this purpose. This study presents data supporting a fast and convenient method for polymer selection and simple characterization of solid dispersion by thermal and spectroscopic analyses. Miscibility of the model drug and polymers was evaluated and was found to exhibit a critical limit depending on the polymer ratio in the solid dispersion, since thermal stability of solid dispersion changed with polymer ratio. Kollicoat® MAE 100P, an anionic polymer, was suitable for maintaining the amorphous state of dutasteride, since the glass transition temperature of the amorphous drug in the solid dispersion indicated very high molecular interactions and high miscibility. High interactions showed good suppression of recrystallization. These observations together with the microscopy data support an efficient strategy to select excipients or polymers for solid dispersion development.
References
Al-Obaidi, H., and G. Buckton. 2009. Evaluation of griseofulvin binary and ternary solid dispersions with HPMCAS. AAPS PharmSciTech 10: 1172–11777.
Al-Obaidi, H., M.J. Lawrence, N. Al-Saden, and P. Ke. 2013. Investigation of griseofulvin and hydroxypropylmethyl cellulose acetate succinate miscibility in ball milled solid dispersions. The International Journal of Pharmaceutics 443(1–2): 95–102.
Chiou, W.L., and S. Riegelman. 1971. Pharmaceutical applications of solid dispersion systems. Journal of Pharmaceutical Sciences 60: 1281–1302.
Chu, K.R., E. Lee, S.H. Jeong, and E.S. Park. 2012. Effect of particle size on the dissolution behaviors of poorly water-soluble drugs. Archives of Pharmacal Research 35: 1187–1195.
Craig, D.Q. 2002. The mechanisms of drug release from solid dispersions in water-soluble polymers. International Journal of Pharmaceutics 231: 131–144.
Hancock, B.C., and G. Zografi. 1997. Characteristics and significance of the amorphous state in pharmaceutical systems. Journal of Pharmaceutical Sciences 86: 1–12.
Joshi, H.N., R.W. Tejwani, M. Davidovich, V.P. Sahasrabudhe, M. Jemal, M.S. Bathala, S.A. Varia, and A.T. Serajuddin. 2004. Bioavailability enhancement of a poorly water-soluble drug by solid dispersion in polyethylene glycol–polysorbate 80 mixture. International Journal of Pharmaceutics 269: 251–258.
Kim, B.Y., and Y.T. Sohn. 2011. Solid state of CG-400549, a novel FabI inhibitor: Characterization, dissolution, transformation. Archives of Pharmacal Research 34: 775–779.
Leuner, C., and J. Dressman. 2000. Improving drug solubility for oral delivery using solid dispersions. European Journal of Pharmaceutics and Biopharmaceutics 50: 47–60.
Miyanishi, H., T. Nemoto, M. Mizuno, H. Mimura, S. Kitamura, Y. Iwao, S. Noguchi, and S. Itai. 2012. Evaluation of crystallization behavior on the surface of nifedipine solid dispersion powder using inverse gas chromatography. Pharmaceutical Research 30: 502–511.
Page, S.T., L. Hirano, J. Gilchriest, M. Dighe, J.K. Amory, B.T. Marck, and A.M. Matsumoto. 2011. Dutasteride reduces prostate size and prostate specific antigen in older hypogonadal men with benign prostatic hyperplasia undergoing testosterone replacement therapy. Journal of Urology 186: 191–197.
Serajuddin, A.T. 1998. Education in pharmaceutical sciences needs a brand new direction to meet the challenges of drug research and development. Pharmaceutical Research 15: 8–10.
Serajuddin, A.T. 1999. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. Journal of Pharmaceutical Sciences 88: 1058–1066.
Sinclair, W., M. Leane, G. Clarke, A. Dennis, M. Tobyn, and P. Timmins. 2011. Physical stability and recrystallization kinetics of amorphous ibipinabant drug product by Fourier transform Raman spectroscopy. Journal of Pharmaceutical Sciences 100: 4687–4699.
Zhu, Q., L.S. Taylor, and M.T. Harris. 2010. Evaluation of the microstructure of semicrystalline solid dispersions. Molecular Pharmaceutics 7: 1291–1300.
Zidan, A.S., Z. Rahman, V. Sayeed, A. Raw, L. Yu, and M.A. Khan. 2012. Crystallinity evaluation of tacrolimus solid dispersions by chemometric analysis. International Journal of Pharmaceutics 423: 341–350.
Acknowledgments
This work was supported by a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A092018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, N.A., Choi, D.H., Lim, J.Y. et al. Investigation of polymeric excipients for dutasteride solid dispersion and its physicochemical characterization. Arch. Pharm. Res. 37, 214–224 (2014). https://doi.org/10.1007/s12272-013-0180-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0180-9