Abstract
The temperature- and pH-sensitive poly(N-isopropylacrylamide-co-acrylic acid) hydrogels, poly(NIPAM-co-AA), were synthesized by radical polymerization. The characterizations of hydrogels based on N-isopropylacrylamide (NIPAM) and acrylic acid (AA) before and after adsorption of heavy metal ions was performed by Fourier transform infrared spectroscopy and scanning electron microscopy. Heavy metal ions (Cr, Mn, Pb) adsorbed onto poly(NIPAM-co-AA) hydrogels were identified using the energy-dispersive X-ray spectroscopy. The mechanism of the water transport within the matrix of synthesized poly(NIPAM-co-AA) hydrogels at pH 4.5 is Super Case II diffusion, and at pH 6.8 corresponds to the non-Fickian diffusion mechanism. The effect of pH, temperature, contact time, and the initial concentration of heavy metals on the adsorption process of Cr(VI), Mn(II), and Pb(II) ions from aqueous solutions onto poly(NIPAM-co-AA) hydrogels were investigated. The kinetic and equilibrium data were best fitted by the pseudo-second-order model and Langmuir adsorption isotherm. Thermodynamic results indicate that the removal process of heavy metal ions from aqueous solutions by poly(NIPAM-co-AA) hydrogels was spontaneous and exothermic in nature. Maximum adsorption capacities of poly(NIPAM-co-AA) hydrogels for heavy metal ions decrease in the following order: Pb(II) > Cr(VI) > Mn(II).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, water pollution with heavy metals is a serious environmental problem, because these compounds are not degraded by natural processes; they are bioaccumulated and have toxic effect on living organisms [1,2,3]. The presence of heavy metals in wastewaters is due to the activity of chemical industry (smelters of non-ferrous metals, thermal power plants, and iron and steel works) and effluent discharge from households [4,5,6].
Chromium (Cr) exhibits two oxidation states, Cr(VI) and Cr(III), wherein Cr(VI) is more toxic and that in the long-term exposition can lead to lung and digestive tract cancer [7, 8]. Lead (Pb) poisoning in the human body causes serious dysfunctions in kidneys, liver, brain, nervous system, and reproductive system [9], while a high concentration of manganese (Mn) in brain is related to neurological disorders similar to Parkinson’s disease [10].
The sources of contamination of surface water by Cr(VI) ion are: dye works, leather tanneries, and the aluminum production [11]. The main sources of contamination of wastewater by lead and manganese are metallurgical processes, ore production, batteries, ammunition, ceramics, as well as pigments and dyes [12,13,14].
Many conventional techniques are used for the removal of heavy metals from water like chemical precipitation, filtration, ultrafiltration, oxidation, solvent extraction, electrolysis, reverse osmosis, and ion exchange [15, 16]. All these techniques are characterized by low efficiency, longer duration of the process, and high energy consumption [17]. Therefore, in recent times, the attention has been focused on adsorption processes and the application of polymer hydrogels as sorbents [18, 19]. Hydrogels are three-dimensional polymer networks able to absorb high amounts of liquids whereby they maintain dimensional stability [20, 21]. Polymer hydrogels are good adsorbents for the removal of heavy metals from water due to cost effectiveness, a high capacity of water absorption, easy use and reuse, and selectivity for certain heavy metals [22, 23]. Based on earlier investigation on the removal of metal ions using hydrogels done by other authors, a rough assumption is that the removal mechanism can be explained by physical adsorption, hydrogen bonds, chelating, and ion exchange [24]. Chelating polymers (polychelatogens) can create coordinate bonds with metals (Lewis acid), because in their structure they have one or more electron donor atoms (Lewis base) like N, S, O, and P [25]. Hydrogels having amino, amide, hydroxyl, carboxyl or sulfonyl group can bind heavy metal by chelating [26,27,28].
In this work, hydrogels based on N-isopropylacrylamide (NIPAM) and acrylic acid (AA) were synthesized and used as adsorbents for the removal of Cr(VI), Mn(II), and Pb(II) ions from aqueous solutions. Hydrogels sensitive to external stimuli respond to changes in pH, ionic strength, or temperature, whereby significant changes in the network structure occur: permeability or mechanical strength, swelling, and sol-to-gel transition [29]. One of the important “smart” polymers is the temperature-sensitive poly(N-isopropylacrylamide) hydrogel, poly(NIPAM), which has the lower critical solution temperature (LCST) at 32 °C in aqueous medium [30]. Introducing ionic hydrophilic comonomer AA in the structure of poly(NIPAM) increases LCST, hydrophilicity, a swelling degree, and copolymer sensitivity to pH change of the medium [31,32,33]. Other investigations show that the adsorption of Pb(II) and Cu(II) ions by hydrogels based on NIPAM and AA, probably occurs through complexation with carboxyl (COOH) or amide (CONH2) functional group [27, 28, 34]. The adsorption of Cr(VI), Mn(II), and Pb(II) ions from aqueous solutions by poly(N-isopropylacrylamide-co-acrylic acid) hydrogels, poly(NIPAM-co-AA), has not been reported in the literature available.
The poly(NIPAM-co-AA) hydrogels are also used in diagnostics and monitoring of tumors [35], and as carriers for the delivery of drugs [36] in addition to removing heavy metals from water [27].
Experimental
Materials
Monomer N-isopropylacrylamide (NIPAM; purity 99%) and the initiator 2,2′-azobis(2-methylpropionitrile) (AZDN; purity 99%) were bought from Acros organics (New Jersey, USA), while the comonomer acrylic acid (AA; purity 98%) and the cross-linker ethylene glycol dimethacrylate (EGDM; purity 97%) were bought from Fluka (AG Buchs SG; Chemical Corp., CH). Potassium dichromate, K2Cr2O7 (Zorka, Šabac, RS), manganese(II)chloride tetrahydrate, MnCl2·4H2O (Acros Organics, UK), and lead(II)acetate trihydrate, Pb(CH3COO)2·3H2O (Zdravlje, Leskovac, RS) were used without the previous purification, as the sources of heavy metals dissolved in redistilled water. All other solvents and chemicals used were of p.a. purity.
Synthesis of poly(NIPAM-co-AA) hydrogels
Copolymer hydrogels of poly(NIPAM-co-AA) were synthesized by radical polymerization of NIPAM and AA (5 mol%) monomers using EGDM (1, 1.5, 2, and 3 mol%) as a cross-linker. The polymerization reaction was initiated by adding 2.7 mol% of AZDN.
After homogenization and dissolution of the reactants in acetone (Centrohem, Belgrade, RS), the reaction mixture was injected into 5 mm diameter glass tubes. The glass tubes were sealed, and the polymerization reaction was thermally initiated as follows: 0.5 h at 75 °C, 2 h at 80 °C, and 0.5 h at 85 °C. To remove the unreacted amounts of reactants, the obtained hydrogels were immersed into 30 mL of methanol over the period of 72 h. After rinsing, the hydrogels were dried to constant weight in a drying oven for 3 h at 40 °C.
In sample designation, e.g., 95/5/1.5, the first number means mol% of NIPAM, the second number is mol% of AA in relation to NIPAM, and the third one is mol% of the cross-linker EGDM in relation to the total amount of comonomers NIPAM and AA.
Characterization
Fourier transform infrared spectroscopy (FTIR)
FTIR spectra of the synthesized hydrogels and hydrogels with adsorbed heavy metal ions (Cr, Mn, and Pb) were recorded by a technique of thin transparent potassium bromide pastilles. Before making the pastilles, the samples were ground to powder in an amalgamator (WIG-L-BVG, 31210-3A, USA). The pastilles were prepared with 150 mg of KBr (99%, Merck, Darmstadt, Germany) and 1 mg of the samples by vacuumization and pressing under the pressure of about 200 MPa. Recording of the sample spectra was conducted in the area of wave numbers of 4000–400 cm−1 on the Bomem Hartmann & Braun MB-series FTIR spectrophotometer, and the obtained spectra were analyzed using the Win-Bomem Easy software.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was used to examine the morphology of the synthesized hydrogels before and after the adsorption of heavy metals. Before recording, the samples were lyophilized in the swollen state on a Freeze Dryers Rotational-Vacuum Concentrator device (GAMMA 1-16 LSC, Germany). To prevent breakage and deformation, the lyophilized samples were immersed into nitrogen before cutting. After the nitrogen treatment, the samples were sprayed by an alloy of gold and palladium (85/15) under vacuum in a Fine Coat JEOL JFC-1100 Ion Sputter (JEOL Co., Japan). Metalized samples were scanned on a JEOL Scanning Electron Microscope JSM-5300 (JEOL Co., Japan).
Energy-dispersive X-ray (EDX) spectroscopy
The semi-quantitative analysis of metal contents in hydrogels was determined by EDX spectroscopy. The EDX spectra were recorded using a Link-Analytical QX-2000 microprobe, mounted on scanning electron microscope (SEM, JEOL JSM-5300), operating at 20 kV. The EDX spectra were analyzed using EDS analyzer v2© software.
Swelling behavior
Hydrogels in a dry state (xerogels) were immersed into solutions with pH values of 4.5 and 6.8, and the swelling process was monitored gravimetrically at room temperature (25 °C). The effect of temperature on swelling of synthesized hydrogels was investigated in the range of 20–80 °C in the solution of pH 6. The samples were taken out from the solutions and the surplus of the solution was removed from their surface. The sample mass was weighed after specified time periods until equilibrium was reached, i.e., constant mass. The swelling degree, α, was calculated according to Eq. (1):
where m0 is the mass of the dry hydrogel and m is the mass of the swollen hydrogel in the time interval t.
The solutions with specified pH were prepared by adding HCl (Zorka, Šabac, RS) or NaOH (Centrohem, Belgrade, RS) using a digital pH meter (HI9318-HI9219, HANNA, Portugal).
Adsorption of heavy metal ions
Xerogels (0.01 g) were immersed into 25 mL of aqueous solutions of Cr(VI), Mn(II), and Pb(II) ions specified pH values and temperature. The stock solutions of heavy metals with different concentrations were prepared from the following heavy metal salts: K2Cr2O7, MnCl2·4H2O, and Pb(CH3COO)2·3H2O. pH values of heavy metal solutions (2.2; 3.5; 4.5; 5.5; 6.8; 8; and 9.1) were adjusted by the addition of 0.1 M HCl and 0.1 M NaOH using a digital pH meter. The heavy metal adsorption by hydrogels was monitored for 72 h. The effect of pH and contact time on the adsorption ability of hydrogel was investigated at the heavy metal concentration of 500 mg/L and the temperature of 25 °C. A set of experiments for the determination of adsorption isotherms and thermodynamic parameters was performed in the concentration range of heavy metals from 100 to 500 mg/L, at temperatures 25, 35, and 45 °C under optimum pH values.
To determine the residual concentrations of heavy metal ions in aqueous solutions, the samples of 0.05 mL were taken from the solutions and dissolved in 4.95 mL distilled water of LC–MS purity. All the samples were filtrated on the cellulose membrane filter with the pore diameter of 0.45 μm (Econofilters, Agilent Technologies, Germany) and analyzed on ICP-OES (inductively coupled plasma-optical emission spectrometry, ARCOS FHE12 SPECTRO, Germany) instrument. As a carrier gas, Argon 5.0 (purity 99.999%) was used.
The amount of the metal adsorbed by the gel at the time interval t, Qt (mg/g), was calculated by the following Eq. (2):
where c1 and c2 are the initial concentration of metal ions in the solution and the concentration of metal ions in the solution at the time interval t (mg/L), respectively. V1 is the initial volume of the metal ion solution, and V2 is the volume of the metal ion solution at the time interval t (L), while W is the mass of the dry hydrogel (g).
Results and discussion
Characterization
FTIR analysis
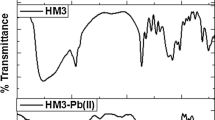
FTIR spectra of poly(NIPAM-co-AA) hydrogel with 5 mol% of AA and 1.5 mol% of EGDM, sample 95/5/1.5, before and after adsorption of Cr(VI), Mn(II), and Pb(II) ions, are shown in Fig. 1.
In FTIR spectrum of copolymer (Fig. 1a), there is a broad absorption band with two saddles, one at 3434 cm−1 assigned to OH valence vibrations, ν(OH), from AA monomer, and the other at 3316 cm−1 assigned to N–H valence vibrations, ν(N–H), from NIPAM monomer. This broad band in the range of wave numbers 3000–3700 cm−1 is the result of carboxyl and amide group bonded by hydrogen, COO–H–N(C=O)H [37]. The confirmation of AA presence in the poly(NIPAM-co-AA) copolymer structure is the absorption band with its maximum at 1715 cm−1 assigned to valence C=O vibrations of carboxyl group [27]. Characteristic absorption bands of NIPAM amide group in FTIR spectrum of poly(NIPAM-co-AA) hydrogel, Fig. 1a, appear with maximums at 1651 cm−1 (amide band I) and 1546 cm−1 (amide band II). The amide band I is the result of C=O valence vibrations, while the amide band II is the result of coupling of N–H deformation vibrations in plane, δ(N–H), and C–N bond valence vibrations, ν(C–N). Absorption bands of medium intensity with maximums at 1388 and 1368 cm−1 correspond to C–H bond deformation vibrations in plane, δ(C–H), from isopropyl group, –CH(CH3)2 [38].
The absence of absorption bands which originate from the valence vibrations of C=C bonds, ν(C=C) [39], and deformation vibrations in plane of AA and NIPAM vinyl groups, δ(=C–H), in FTIR spectrum of poly(NIPAM-co-AA) copolymer (Fig. 1a) indicates a successful polymerization by breaking double bonds, C=C. The absorption band with the maximum at 3077 cm−1 is the result of asymmetric valence vibrations of vinyl groups of cross-linker, νas (=CH). Dangling chains of unreacted EGDM were probably present in the structure of poly(NIPAM-co-AA) hydrogel.
By comparative analysis of FTIR spectra of pure poly(NIPAM-co-AA) copolymer and copolymer with adsorbed Cr(VI), Mn(II), and Pb(II) ions’ (Fig. 1) shifts of absorption bands which are the result of vibration of amide and carboxyl groups were observed. In FTIR spectra of copolymers with heavy metals (Fig. 1b–d), there are shifts of absorption bands assigned to valence vibrations of OH group of up to 40 units towards lower wave numbers, and of absorption bands of C=O group in carboxyl part of the structure amounting to 5 units towards lower wave numbers compared to the same bands in FTIR spectrum of pure copolymer (Fig. 1a). The above shifts of absorption band centroids suggest the possibility of complexation of Cr(VI), Mn(II), and Pb(II) ions with the carboxyl group of poly(NIPAM-co-AA) copolymer. The amide group also includes electron donor atoms N and O and there is a possibility of the heavy metal adsorption. In this case, it is confirmed by absorption bands’ shift which appears in FTIR spectrum of pure poly(NIPAM-co-AA) copolymer at 3316, 1651, and 1546 cm−1. Two new absorption bands of medium and strong intensity appear at 942 and 885 cm−1, respectively, in FTIR spectrum of the copolymer with adsorbed Cr(VI) ion (Fig. 1b). According to other authors’ investigations, the bands are probably the result of asymmetric and symmetric vibrations of CrO3 structure, i.e., Cr–O and Cr=O bonds [40, 41].
The results of FTIR analysis confirm the fact that carboxyl and amide groups are responsible for heavy metal adsorption, in this case Cr(VI), Mn(II), and Pb(II) ions, which is in accordance with other authors` investigations [27, 28].
SEM analysis
Morphologies of surface structures of swollen poly(NIPAM-co-AA) hydrogel, sample 95/5/1.5, before and after adsorption of Cr(VI), Mn(II), and Pb(II) ions are shown in Fig. 2.
In Fig. 2a, it is easy to observe the porous structure of poly(NIPAM-co-AA) hydrogel, which provides a better transport of heavy metal ions into the hydrogel. In the three-dimensional structure of poly(NIPAM-co-AA) hydrogels, there are different pore sizes, indicating that the process has not achieved a uniform cross-linking in the polymer structure. The pore size of the synthesized copolymer poly(NIPAM-co-AA) in the swollen state is up to 150 µm. Based on the pore size, the synthesized hydrogel (Fig. 2a) is classified as macroporous hydrogel [42, 43]. A larger pore size of poly(NIPAM-co-AA) hydrogels can be achieved by copolymerization of the monomer NIPAM and AA in an alkaline solution, because of the electrostatic repulsion between the carboxyl ions (COO−) of AA and the formation of the extended network [44]. On SEM micrographs of poly(NIPAM-co-AA) hydrogels with adsorbed Cr(VI) and Pb(II) ions (Fig. 2b, d), accumulations which probably originate from heavy metals are observed.
EDX analysis
The elemental analysis of hydrogels after adsorption of metal ions can be done using EDX spectroscopy. Figure 3 shows EDX spectra of poly(NIPAM-co-AA) hydrogels after adsorption of metal ions (Cr, Mn, and Pb) and indicates the presence of the following elements: O (Kα 0.525 keV), N (Kα 0.392 keV), C (Kα 0.277 keV), and metals Cr (Kα 5.411, Lα 0.573 keV; Fig. 3a), Mn (Kα 5.894, Lα 0.637 keV; Fig. 3b), and Pb (Lα 10.550, M 2.342 keV; Fig. 3c).
Swelling behavior
Molecules of the solvent penetrate inside the hydrogel polymer network during contact and, after some time, the meshes of the network in the rubbery phase start expanding thus enabling the penetration of other solvent molecules [45].
The equilibrium swelling degree is a parameter which describes the water amount inside hydrogels in the state of equilibrium and depends on hydrogel characteristics: network structure, cross-linking density, hydrophilicity, and ionization degree of functional groups [20, 46].Using Eq. (3), the nature of the diffusion process can be analyzed and determined [47, 48]:
where F is fractional sorption, Mt is the mass of the absorbed solvent at time interval t, Me is the mass of the absorbed solvent at equilibrium, k is a constant specific for the type of the polymer network (min1/n), and n is a diffusion exponent.
Equation (3) is valid for the condition Mt/Me ≤ 0.6, and by converting it to a logarithmic form, Eq. (4) is obtained:
The value of the diffusion exponent n is calculated from the slope and k constant from the intercept of the linear relationship between lnF and lnt. The water transport mechanism in the swollen hydrogel depends on the gel chemical structure, the equilibrium water content, a relative degree of the water diffusion, and the relaxation of macromolecular chains [47, 48].
The value of the diffusion exponent n determines the water diffusion mechanism and, if the value is ≤ 0.5 the transport of water corresponds to the Fickian diffusion mechanism; water diffusion in the polymer matrix controls the swelling process. In the case when the diffusion exponent value n is between 0.5 and 1, hydrogel swelling is controlled by both water diffusion in the matrix and the relaxation of polymer chains, corresponding to non-Fickian diffusion mechanism. If n = 1, the swelling process is controlled by the relaxation of polymer chains (type II, Case II). In Super Case II diffusion (type III, Case III), where n > 1, the increase of the solvent transport into the polymer matrix is faster than in the Case II diffusion, and hydrogel swelling is controlled by the polymer chain relaxation [48,49,50,51,52].
To determine diffusion coefficient, D, Eq. (5) is applied:
where D is a diffusion coefficient (cm2/min) and l is the thickness of the dry hydrogel (cm).
By converting the exponential Eq. (5) to a logarithmic form, a linear relationship between ln(Mt/Me) and lnt (Eq. (6)) is obtained, and the diffusion coefficient D is calculated from the intercept of the graph:
The changes in the swelling degree, α, of poly(NIPAM-co-AA) hydrogels by time dependence in the solvents with pH values of 4.5 and 6.8 at 25 °C are shown in Fig. 4.
Among the synthesized poly(NIPAM-co-AA) hydrogels, the highest swelling degree in the solution of pH values of 4.5 and 6.8 is achieved by the hydrogel with 1.5 mol% of EGDM, α = 12.03 and α = 229.93, respectively (Fig. 4). The degree of cross-linking influences the swelling degree of poly(NIPAM-co-AA) hydrogels at both investigated pH values of the solution, except for hydrogels with 1 and 1.5 mol% of the cross-linking agents, and with the increase of the cross-linking degree, the swelling degree decreases (Fig. 4). The poly(NIPAM-co-AA) hydrogel with 1 mol% of EGDM has a smaller swelling degree than poly(NIPAM-co-AA) hydrogel with 1.5 mol% of EGDM, indicating a lack of cross-linking, i.e., a relatively low cross-linking density in the hydrogel with 1 mol% of EGDM.
The poly(NIPAM-co-AA) hydrogels have a significantly greater swelling degree in the solution at pH of 6.8 and the temperature of 25 °C. Based on these results, the conclusion is that the swelling degree increases with the increase of pH of the solution, which is the responsibility of the pH-sensitive component AA. The increase of pH value causes the increase in the number of ionized groups (COO−) and the expansion of the polymer network due to electrostatic repulsion between ionized carboxyl groups of the polymer chains [44].
After the repeated cycle of the swelling of poly(NIPAM-co-AA) hydrogel, sample 95/5/1.5, in the solution of pH 6.8 at room temperature, the swelling degree of a copolymer is slightly lower (1.93%), which is in accordance with the results obtained in the work of Cai et al. [53].
The swelling kinetic parameters, n, k, and D, for poly(NIPAM-co-AA) hydrogels at pH 4.5 and 6.8 and the temperature of 25 °C, determined by Eqs. (3)–(6) are given in Table 1.
The diffusion exponent for the series of poly(NIPAM-co-AA) hydrogels in the solution of the pH value of 4.5 has a value higher than 1 (Table 1), and the process of swelling is controlled by the relaxation of the polymer chains, Super Case II. Only the swelling of poly(NIPAM-co-AA) hydrogel with 3 mol% of EGDM in the solution of pH 4.5 is controlled by a diffusion process and the relaxation of the polymer chains, n = 0.720. The increase of the pH of the solution from 4.5 to 6.8 in poly(NIPAM-co-AA) hydrogel series leads to a decreases of the diffusion exponent value, and the increases of the diffusion coefficient value. The mechanism of the diffusion of the solvent for the copolymer series at a higher pH corresponds to the anomalous diffusion type, with the exception of the copolymer with 2 mol% of EGDM that corresponds to Fickian diffusion (Table 1). Higher values of the diffusion coefficient can be explained by the fact that the higher pH value leads to expansion of the polymer matrix and a faster diffusion of the solution within the hydrogel.
The sensitivity of poly(NIPAM-co-AA) hydrogels to the changes in temperature from 20 to 80 °C in the solution of pH 6 is given in Fig. 5.
Copolymer hydrogels of poly(NIPAM-co-AA) respond to the change of temperature in aqueous medium by forming, or by dissociation of hydrogen bonds with water molecules. Hydrophilic functional groups of hydrogels, carbonyl and carboxylic, are involved in the building of hydrogen bonds [27]. In Fig. 5, it is noted that the temperature has an antagonistic effect on the swelling degree of the poly(NIPAM-co-AA) hydrogel. Copolymers showed a significant reduction in the swelling degree with the increase of the temperature above 30 °C (Fig. 5), and they are classified as negative temperature-sensitive. By increasing the temperature above the LCST, the hydrophobic interactions between polymer network groups become dominant, and therefore, the copolymer contraction occurs [44].
Adsorption studies
Effect of adsorbate solution pH
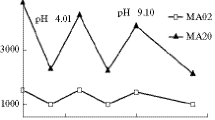
Adsorption of Cr(VI), Mn(II), and Pb(II) ions by poly(NIPAM-co-AA) hydrogel, sample 95/5/1.5, was investigated in the pH range from 2.2 to 9.1, as shown in Fig. 6.
A larger increase in the adsorption capacity of poly(NIPAM-co-AA) hydrogel for Mn(II) and Pb(II) ions occurs with increasing pH of the solution from 2.2 to 4.5, while for Cr(VI) ions, the increase of the adsorption capacity occurs up to the pH value of 6.8 (Fig. 6). At low pH values, amino and carboxyl groups of poly(NIPAM-co-AA) hydrogel are protonated (pKa of AA is 4.3), which leads to the electrostatic repulsion with metal ions and decreases the adsorption capacity of the metal by the complexation process [54, 55].
All metal ions at higher pH, particularly pH > 8, form insoluble metal hydroxides [18]. In this study, the adsorption of Pb(II) ions from aqueous solutions onto the poly(NIPAM-co-AA) hydrogel was monitored up to the pH value of 5.5, because at higher pH values, the turbidity of the solution, i.e., the precipitation of Pb(OH)2 occured. The selected pH value for further investigation of the adsorption of Mn(II) and Pb(II) ions onto poly(NIPAM-co-AA) copolymer was 4.5; and for the adsorption of Cr(VI) ions, pH value was 6.8.
Effect of the contact time and adsorption kinetics
The dependence of the adsorption capacity of poly(NIPAM-co-AA) hydrogel series for Cr(VI), Mn(II), and Pb(II) ions by time is shown in Fig. 7.
The adsorption process of Cr(VI), Mn(II), and Pb(II) ions from aqueous solutions of a certain pH value onto poly(NIPAM-co-AA) hydrogels occurs intensively for up to 5 h for Cr(VI) ions, 24 h for Mn(II) ions, and 48 h for Pb(II) ions (Fig. 7).
The highest adsorption capacity of Cr(VI) ions is shown by poly(NIPAM-co-AA) hydrogel, sample 95/5/1.5, (Q = 443.89 mg/g), which is nearly ten times higher adsorption capacity compared to the synthesized hydrogel, poly(acrylic acid), poly(AA), in the work of Roy et al. (Q = 41.10 mg/g) [18]. The poly(NIPAM-co-AA) hydrogels adsorbed less Mn(II) ions from the aqueous solution, 35.04–232.30 mg/g (Fig. 7b), than Cr(VI) ions, from 106.10 to 443.89 mg/g (Fig. 7a). Removal capacities of Mn(II) ions obtained for synthesized hydrogels based on NIPAM and AA (Fig. 7b) were significantly higher compared to the investigation results by Ali et al. for poly(vinylpyrrolidone-co-acrylic acid) hydrogel, poly(VP-co-AA), which showed the removal capacity of 14 mg/g [56]. The highest adsorption capacity for Pb(II) ions from aqueous solutions has been achieved by poly(NIPAM-co-AA) hydrogel with 1.5 mol% of EGDM, 507.63 mg/g. The synthesized poly(NIPAM) hydrogel in the work of Ju et al. showed almost four times lower adsorption capacity for Pb(II)ions, about 120 mg/g [28].
A cross-linking degree has some effect on the heavy metal adsorption from aqueous solutions of certain pH values onto poly(NIPAM-co-AA) hydrogels and a better penetration of metal ions is observed with hydrogels with lower cross-linking density (Fig. 7).
Synthesized hydrogels based on NIPAM and AA showed the highest adsorption capacities for Cr(VI), Mn(II), and Pb(II) ions in comparison to the adsorption capacities of similar hydrogels reported in the available literature [18, 28, 56].
The evaluation of adsorption kinetics allows the obtainment of important data on the adsorption mechanism, and also the characteristics of the adsorbent for the removal of heavy metals from water. To evaluate the adsorption mechanism of heavy metals onto poly(NIPAM-co-AA) hydrogels, the models of pseudo-first, pseudo-second-order, and intra-particle diffusion are applied [17, 57]. The models of pseudo-first, pseudo-second-order, and intra-particle diffusion are shown in Eqs. (7), (8), and (9), respectively:
where Qe and Qt are adsorption capacities of hydrogels for Cr(VI), Mn(II), and Pb(II) ions at equilibrium and at time interval t, respectively. k1 is the pseudo-first-order reaction rate constant (1/min), k2 is the pseudo-second order reaction rate constant (g/mg min), and kid is the intra-particle diffusion rate constant (mg/g min1/2). Constants k1 and kid are obtained from the slope of the linear relationship log(Qe − Qt) vs. t, and the linear relationship Qt vs. t1/2, respectively. The values of k2 constant are calculated from the intercept of the graph of the linear relationship t/Qt vs. t.
The results of fitting the experimental data obtained by models of pseudo-first-order, pseudo-second-order, and intra-particle diffusion (constants, equilibrium adsorption capacities, and correlation coefficients) are shown in Table 2.
On the basis of high correlation coefficients (R2) and matching of experimental with calculated adsorption capacities (Qe,exp and Qe,cal), Table 2, it can be concluded that the adsorption of Cr(VI), Mn(II), and Pb(II) ions from aqueous solutions onto poly(NIPAM-co-AA) hydrogels is best described by the model of pseudo-second-order. Adsorption rate of Cr(VI), Mn(II), and Pb(II) ions is probably controlled by a chemical process, i.e., chemisorption is the adsorption control mechanism. If the dependence of Qt vs. t1/2 is linear and if the line passes through the origin, then the intra-particle diffusion is the only limiting step in the pollutant adsorption process [17], which is not the case for the removal of heavy metals from aqueous solutions by poly(NIPAM-co-AA) hydrogels. The plot of Qt vs. t1/2 shows multi-linearity (Fig. 8) and indicates that the adsorption of heavy metal ions onto poly(NIPAM-co-AA) hydrogel takes place in two steps.
The first straight line shown in Fig. 8 is the external surface adsorption of metal ions onto hydrogels, the transport of the metal ion through the diffusion boundary layer to the external surface of the adsorbent. The second straight line indicates the metal ion adsorption step where the intra-particle diffusion is a factor that controls the rate of the process [34]. It can be concluded that the chemisorption and the intra-particle diffusion affect the adsorption process of Cr(VI), Mn(II), and Pb(II) ions from aqueous solutions onto poly(NIPAM-co-AA) hydrogels. On the SEM micrograph of the poly(NIPAM-co-AA) hydrogel after the adsorption of Mn(II) ions, surface accumulations is not observed (Fig. 2c), and by EDX analysis, the presence of this heavy metal in the copolymer has been proven (Fig. 3b). These results show that in the case of the removal of Mn(II) ions from aqueous solutions by hydrogel, the intra-particle diffusion is mainly responsible.
Effect of the initial concentration and adsorption isotherms
The initial concentration of the heavy metal plays a significant role in the adsorption process of this pollutant onto poly(NIPAM-co-AA) hydrogels. The adsorption ability of the hydrogel was investigated in the concentration range of 100–500 mg/L at different temperatures (25, 35, and 45 °C), and the results are shown in Fig. 9.
The adsorption capacity of poly(NIPAM-co-AA) hydrogel, sample 95/5/2, depends on the initial concentration of heavy metal and the type of adsorbate (Fig. 9). With the increase of the concentration of heavy metals up to 300 or 400 mg/L, the adsorption capacity of poly(NIPAM-co-AA) hydrogel increases, which can be attributed to the availability of adsorption sites onto the hydrogel [58]. At concentrations greater than 400 mg/L, the removal degree of heavy metal ions by hydrogel was slightly changed, probably because of saturation of the copolymer adsorption sites. The influence of the temperature on the adsorption of heavy metal ions is also evident, and the highest equilibrium capacities were obtained at 25 °C (Fig. 9). Maximum adsorption capacities of hydrogels based on NIPAM and AA for heavy metal ions followed the following order: Pb(II) > Cr(VI) > Mn(II).
For the determination of adsorption isotherms, two models were applied: Langmuir and Freundlich. The Langmuir model assumes the monolayer adsorption with negligible interactions between the adsorbent particles. The binding of adsorbates does not depend on the degree occupancy of active adsorbent centers [59]. Unlike Langmuir model, the Freundlich model assumes the existence of interactions between adsorbed particles, as well as multilayer adsorption which takes place on the energy heterogeneous surface of the adsorbent [60]. Linear forms of Langmuir and Freundlich isotherms are given by Eqs. (10) and (11).
Here, Qe and Qmax represent equilibrium and the maximum adsorption capacity for the given adsorbate (mg/g), respectively. ce is the concentration of adsorbate in the equilibrium state (mg/L). KL is the Langmuir constant (L/mg) relative to the adsorption energy, and KF Freundlich constant [(mg/g) (L/mg)1/n] relative to the adsorption capacity. Constant n in the Freundlich model indicates the favorability of the adsorption process.
The characteristic of Langmuir model is also a dimensionless constant called the separation factor (RL) which is calculated from the following equation:
where c0 corresponds to the initial concentration of the adsorbate (mg/L). The nature of adsorption can be determined based on the values of the separation factor: unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), and irreversible adsorption (RL = 0) [61].
The parameters of isotherms for the adsorption of Cr(VI), Mn(II), and Pb(II) ions onto the poly(NIPAM-co-AA) hydrogel, sample 95/5/2, were determined from the slopes and intercepts of plots of ce/Qe vs. ce for Langmuir isotherm and plots of logQe vs. logce for Freundlich isotherm (Table 3).
The higher correlation coefficients were obtained for the Langmuir model in relation to the Freundlich model at all temperatures (Table 3), so that Cr(VI), Mn(II), and Pb(II) ions are probably adsorbed monolayer onto the energetically equivalent sites of poly(NIPAM-co-AA) hydrogel [54, 62]. Based on the separation factor value, 0 < RL < 1, it is concluded that the adsorption of heavy metal ions onto hydrogel is a favorable process. The values of the constant n from the Freundlich model (Table 3) also indicate the favorability of the adsorption [17].
Adsorption thermodynamics
Spontaneity, nature, and type of the adsorption process are determined based on thermodynamic parameters: Gibbs free energy change (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°). The Gibbs free energy change is expressed by the following equation:
where ΔG° is Gibbs free energy change (J/mol), R is the universal gas constant (8.314 J/mol K), and T absolute temperature (K). K is the adsorption equilibrium constant which is obtained from Langmuir isotherm.
The relationship between thermodynamic parameters, ΔG°, ΔH°, and ΔS°, is given by Eq. (14):
By substituting Eq. (14) in Eq. (13), the dependence of the adsorption equilibrium constant K on the temperature is obtained:
where ΔH° (J/mol) and ΔS° (J/mol K) are calculated from the slope and intercept of the plot of lnK vs. 1/T (Fig. 10), respectively [22, 34]. The obtained values of thermodynamic parameters are given in Table 4.
The negative sign of ΔG° shows that the adsorption process of heavy metals ions onto poly(NIPAM-co-AA) hydrogel is spontaneous. With a temperature rise from 25 to 45 °C, the adsorption of the metal is more favorable and spontaneous, since the ΔG° values are decreased (Table 4) [22]. The removal of heavy metals ions by poly(NIPAM-co-AA) from aqueous solutions is exothermic process (negative value ΔH°), which is in accordance with the results obtained in the work of Antić et al. [63]. During the adsorption of Cr(VI), Mn(II), and Pb(II) ions, there is an increase in the randomness at the solid–solution interface (positive value ΔS°, Table 4).
Conclusions
Poly(NIPAM-co-AA) hydrogels are successfully synthesized by free radical polymerization from monomers NIPAM and AA, breaking double bonds, C=C. FTIR analysis showed that synthesized hydrogels probably chelate Cr(VI), Mn(II), and Pb(II) ions with carboxyl or amide groups. Using the SEM analysis accumulations on the surface of macroporous poly(NIPAM-co-AA) copolymers after adsorption of Cr(VI) and Pb(II) ions from aqueous solutions were identified. By EDX spectroscopy, the presence of heavy metal ions (Cr, Mn, and Pb) in the structure of hydrogels was proven after the adsorption process. The swelling of poly(NIPAM-co-AA) hydrogels is greatly influenced by the pH and temperature of the environment, and the highest swelling degrees were obtained at the solution pH of 6.8, at 25 °C. The swelling of poly(NIPAM-co-AA) copolymer series at pH 4.5 is controlled by the polymer chains relaxation, Super Case II, and at pH 6.8, the swelling is controlled both by the diffusion process and by the polymer chains relaxation—non-Fickian diffusion mechanism. Pseudo-second-order model better describes the adsorption of heavy metal ions than other kinetic models, while from the isotherms, Langmuir model is more suitable. The removal rate of heavy metal ions from aqueous solutions by poly(NIPAM-co-AA) hydrogels depends on the external surface chemisorption and intra-particle diffusion. Adsorption of cationic pollutants onto poly(NIPAM-co-AA) is exothermic and spontaneous process characterized by high efficiency in a temperature range from 25 to 45 °C.
References
Kelter PB, Grundman J, Hage DS, Carr JD, Castro-Acuña CM (1997) A discussion of water pollution in the United States and Mexico; with high school laboratory activities for the analysis of lead, atrazine, and nitrate. J Chem Educ 74:1413–1421. https://doi.org/10.1021/ed074p1413
Júnior OK, Gurgel LVA, de Freitas RP, Gil LF (2009) Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA dianhydride (EDTAD). Carbohydr Polym 77:643–650. https://doi.org/10.1016/j.carbpol.2009.02.016
Singh KP, Malik A, Sinha S (2005) Water quality assessment and apportionment of pollution sources of Gomti river (India) using multivariate statistical techniques-a case study. Anal Chim Acta 538:355–374. https://doi.org/10.1016/j.aca.2005.02.006
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139. https://doi.org/10.1038/333134a0
Honnen W, Rath K, Schlegel T, Schwinger A, Frahne D (2001) Chemical analyses of water, sediment and biota in two small streams in southwest Germany. J Aquat Ecosyst Stress Recovery 8:195–213. https://doi.org/10.1023/A:1012945427446
Gallo M, Trento A, Alvarez A, Beldoménico H, Campagnoli D (2006) Dissolved and particulate heavy metals in the Salado river (Santa Fe, Argentina). Water Air Soil Pollut 174:367–384. https://doi.org/10.1007/s11270-006-9128-8
Smith RG (1972) Five of potential significance. In: Lec DHK (ed) Metallic contaminants and human health. Academic Press, New York, pp 139–162
Kaufaman DB, DiNicola W, McIntosh R (1970) Acute potassium dichromate poisoning. Treated by peritoneal dialysis. Am J Dis Child 119:374–376. https://doi.org/10.1001/archpedi.1970.02100050376021
Naiya TK, Bhattacharya AK, Mandal S, Das SK (2009) The sorption of lead(II) ions on rice husk ash. J Hazard Mater 163:1254–1264. https://doi.org/10.1016/j.jhazmat.2008.07.119
Takeda A (2003) Manganese action in brain function. Brain Res Rev 41:79–87. https://doi.org/10.1016/S0165-0173(02)00234-5
Mohanty K, Jha M, Meikap BC, Biswas MN (2005) Removal of chromium (VI) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride. Chem Eng Sci 60:3049–3059. https://doi.org/10.1016/j.ces.2004.12.049
Jamali MK, Kazi TG, Arain MB, Afridi HI, Jalbani N, Memon AR (2007) Heavy metal contents of vegetables grown in soil, irrigated with mixtures of wastewater and sewage sludge in Pakistan, using ultrasonic assisted pseudo-digestion. J Agron Crop Sci 193:218–228. https://doi.org/10.1111/j.1439-037X.2007.00261.x
Ghaedi M, Asadpour E, Vafaie A (2006) Simultaneous preconcentration and determination of copper, nickel, cobalt, lead, and iron content using a surfactant-coated alumina. Bull Chem Soc Jpn 79:432–436. https://doi.org/10.1246/bcsj.79.432
Nassar MM (2006) Adsorption of Fe3+ and Mn2+ from ground water onto maize cobs using batch adsorber and fixed bed column. Sep Sci Technol 41:943–959. https://doi.org/10.1080/01496390600588796
Fei C, Huang D, Feng S (2012) Adsorption behavior of amphoteric double-network hydrogel based on poly(acrylic acid) and silica gel. J Polym Res 19:9929–9935. https://doi.org/10.1007/s10965-012-9929-y
Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59:203–216. https://doi.org/10.1016/S0304-386X(00)00160-2
Al-qudah YHF, Mahmoud GA, Abdel Khalek MA (2014) Radiation crosslinked poly (vinyl alcohol)/acrylic acid copolymer for removal of heavy metal ions from aqueous solutions. J Radiat Res Appl Sci 7:135–145. https://doi.org/10.1016/j.jrras.2013.12.008
Roy PK, Swami V, Kumar D, Rajagopal C (2011) Removal of toxic metals using superabsorbent polyelectrolytic hydrogels. J Appl Polym Sci 122:2415–2423. https://doi.org/10.1002/app.34384
Yetimoğlu EK, Kahraman MV, Ercan Ö, Akdemir ZS, Apohan NK (2007) N-Vinylpyrrolidone/acrylic acid/2-acrylamido-2-methylpropane sulfonic acid based hydrogels: synthesis, characterization and their application in the removal of heavy metals. React Funct Polym 67:451–460. https://doi.org/10.1016/j.reactfunctpolym.2007.02.007
Peppas NA, Bures P, Leobandung W, Ichikawa H (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50:27–46. https://doi.org/10.1016/S0939-6411(00)00090-4
Ebara M, Kotsuchibashi Y, Narain R, Idota N, Kim YJ, Hoffman JM, Uto K, Aoyagi T (2014) Smart Hydrogels. In: Ohashi N, Ohmura T, Tateyama Y, Taniguchi T, Terabe K, Naito M, Hanagata N, Miyano K (eds) Smart biomaterials. Springer Japan, Tokyo, pp 9–65
Souda P, Sreejith L (2015) Magnetic hydrogel for better adsorption of heavy metals from aqueous solutions. J Environ Chem Eng 3:1882–1891. https://doi.org/10.1016/j.jece.2015.03.007
Mahmoud GA, Mohamed SF (2012) Removal of lead ions from aqueous solution using (sodium alginate/itaconic acid) hydrogel prepared by gamma radiation. Aust J Basic Appl Sci 6:262–273
Lo IMC, Yin K, Tang SCN (2011) Combining material characterization with single and multi-oxyanion adsorption for mechanistic study of chromate removal by cationic hydrogel. J Environ Sci 23:1004–1010. https://doi.org/10.1016/S1001-0742(10)60507-4
Warshawasky A (1987) Chelating ion exchangers. In: Streat M, Naden D (eds) Ion exchange and sorption processes in hydrometallurgy, critical reports on applied chemistry, vol 15. Wiley, New York, pp 166–225
Chauhan GS, Kumar S, Kumari A, Sharma R (2003) Study on the synthesis, characterization, and sorption of some metal ions on gelatin- and acrylamide-based hydrogels. J Appl Polym Sci 90:3856–3871. https://doi.org/10.1002/app.13086
Chen JJ, Ahmad AL, Ooi BS (2014) Thermo-responsive properties of poly(N-isopropylacrylamide-co-acrylic acid) hydrogel and its effect on copper ion removal and fouling of polymer-enhanced ultrafiltration. J Memb Sci 469:73–79. https://doi.org/10.1016/j.memsci.2014.05.062
Ju XJ, Zhang SB, Zhou MY, Xie R, Yang L, Chu LY (2009) Novel heavy-metal adsorption material: ion-recognition p(NIPAM-co-BCAm) hydrogels for removal of lead(II) ions. J Hazard Mater 167:114–118. https://doi.org/10.1016/j.jhazmat.2008.12.089
Peppas NA (1991) Physiological responsive gels. J Bioact Compat Polym 6:241–246. https://doi.org/10.1177/088391159100600303
Heskins M, Guillet JE (1968) Solution properties of poly(N-isopropylacrylamide). J Macromol Sci Chem A 2:1441–1455. https://doi.org/10.1080/10601326808051910
Velada JL, Liu Y, Hugh MB (1998) Effect of pH on the swelling behaviour of hydrogels based on N-isopropylacrylamide with acidic comonomers. Macromol Chem Phys 199:1127–1134. https://doi.org/10.1002/(sici)1521-3935(19980601)199:6<1127::aid-macp1127>3.0.co;2-9
Lin CL, Chiu WY, Lee CF (2006) Preparation, morphology, and thermoresponsive properties of poly(N-isopropylacrylamide)-based copolymer microgels. J Polym Sci A Polym Chem 44:356–370. https://doi.org/10.1002/pola.21134
Chen H, Fang Y (2013) Synthesis and characterization of temperature and pH responsive poly(N-isopropylacrylamide) copolymer. 2013 AIChE annual meeting global challenges for engineering a sustainable future, San Francisco, California, USA, pp 123
Chen JJ, Ahmad AL, Ooi BS (2013) Poly(N-isopropylacrylamide-co-acrylic acid) hydrogels for copper ion adsorption: equilibrium isotherms, kinetic and thermodynamic studies. J Environ Chem Eng 1:339–348. https://doi.org/10.1016/j.jece.2013.05.012
Chen H, Zhang J, Qian Z, Liu F, Chen X, Hu Y, Gu Y (2008) In vivo non-invasive optical imaging of temperature-sensitive co-polymeric nanohydrogel. Nanotechnology 19:185707–185716. https://doi.org/10.1088/0957-4484/19/18/185707
Abu Samah NH, Heard CM (2013) Enhanced in vitro transdermal delivery of caffeine using a temperature- and pH-sensitive nanogel, poly(NIPAM-co-AAc). Int J Pharm 453:630–640. https://doi.org/10.1016/j.ijpharm.2013.05.042
Venegas-Sanchez JA, Kusunoki T, Yamamoto M, Kobayash T (2013) Sono-respond on thermosensitive polymer microgels based on cross-linked poly(N-isopropylacrylamide-co-acrylic acid). Ultrason Sonochem 20:1271–1275. https://doi.org/10.1016/j.ultsonch.2013.02.003
Hu X, Hao X, Wu Y, Zhang J, Zhang X, Wang PC, Zou G, Liang XJ (2013) Multifunctional hybrid silica nanoparticles for controlled doxorubicin loading and release with thermal and pH dual response. J Mater Chem B 1:1109–1118. https://doi.org/10.1039/C2TB00223J
Farooqi ZH, Khan HU, Shah SM, Siddiq M (2017) Stability of poly(N-isopropylacrylamide-co-acrylic acid) polymer microgels under various conditions of temperature, pH and salt concentration. Arab J Chem 10:329–335. https://doi.org/10.1016/j.arabjc.2013.07.031
Palopoli CM, Etcheverry SB, Baran EJ (1988) Vibrational and thermal behaviour of nicotinium dichromate. Thermochim Acta 131:273–277. https://doi.org/10.1016/0040-6031(88)80080-7
Bates JB, Toth LM, Quist AS, Boyd GE (1973) Vibrational spectra of crystalline, molten and aqueous potassium dichromate. Spectrochim Acta A 29:1585–1600. https://doi.org/10.1016/0584-8539(73)80109-6
Ratner BD, Hoffman AS (1976) Synthetic hydrogels for biomedical applications. In: Andrade JD (ed) Hydrogels for medical and related applications, vol 31. ACS symposium series, Washington DC, pp 1–36
Park K, Chen J, Park H (2001) Hydrogel composites and superporous hydrogel composites having fast swelling, high mechanical strength, and superabsorbent properties. US6271278 B1, USA
Zhang XZ, Yang YY, Wang FJ, Chung TS (2002) Thermosensitive poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with expanded network structures and improved oscillating swelling-deswelling properties. Langmuir 18:2013–2018. https://doi.org/10.1021/la011325b
Ganji F, Vasheghani-Farahani S, Vasheghani-Farahani E (2010) Theoretical description of hydrogel swelling: A review. Iran Polym J 19:375–398
Bajpai AK, Sharma M (2006) Preparation and characterization of novel pH-sensitive binary grafted polymeric blends of gelatin and poly(vinyl alcohol): Water sorption and blood compatibility study. J Appl Polym Sci 100:599–617. https://doi.org/10.1002/app.23370
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42. https://doi.org/10.1016/0168-3659(87)90035-6
Bajpai SK (2001) Swelling–deswelling behavior of poly(acrylamide-co-maleic acid) hydrogels. J Appl Polym Sci 80:2782–2789. https://doi.org/10.1002/app.1394
Khare AR, Peppas NA (1995) Swelling/deswelling of anionic copolymer gels. Biomaterials 16:559–567. https://doi.org/10.1016/0142-9612(95)91130-Q
Mercea P (2008) Models for diffusion in polymers. In: Piringer OG, Baner AL (eds) Plastic packaging: interactions with food and pharmaceuticals. Wiley-VCH, Weinheim, pp 123–162
Crank J (1975) The mathematics of diffusion. Clarendon Press, Oxford
Hansen CM (2010) The significance of the surface condition in solutions to the diffusion equation: explaining “anomalous” sigmoidal, Case II, and Super Case II absorption behavior. Eur Polym J 46:651–662. https://doi.org/10.1016/j.eurpolymj.2009.12.008
Cai W, Anderson EC, Gupta RB (2001) Separation of lignin from aqueous mixtures by ionic and nonionic temperature-sensitive hydrogels. Ind Eng Chem Res 40:2283–2288. https://doi.org/10.1021/ie0009435
Lu Q, Yu J, Gao J, Yang W, Li Y (2012) A promising absorbent of acrylic acid/poly(ethylene glycol) hydrogel prepared by glow-discharge electrolysis plasma. Cent Eur J Chem 10:1349–1359. https://doi.org/10.2478/s11532-012-0055-9
Chen CY, Lin MS, Hsu KR (2008) Recovery of Cu(II) and Cd(II) by a chelating resin containing aspartate groups. J Hazard Mater 152:986–993. https://doi.org/10.1016/j.jhazmat.2007.07.074
El Hag Ali A, Shawky HA, Abd El Rehim HA, Hegazy EA (2003) Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution. Eur Polym J 39:2337–2344. https://doi.org/10.1016/S0014-3057(03)00150-2
Ho YS, McKay G (1998) Comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76:332–340. https://doi.org/10.1205/095758298529696
Abdel-Halim ES, Al-Deyab SS (2014) Preparation of poly(acrylic acid)/starch hydrogel and its application for cadmium ion removal from aqueous solutions. React Funct Polym 75:1–8. https://doi.org/10.1016/j.reactfunctpolym.2013.12.003
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5:212–223. https://doi.org/10.1021/i160018a011
Li Z, Wang Y, Wu N, Chen Q, Wu K (2013) Removal of heavy metal ions from wastewater by a novel HEA/AMPS copolymer hydrogel: preparation, characterization, and mechanism. Environ Sci Pollut Res 20:1511–1525. https://doi.org/10.1007/s11356-012-0973-2
Antić KM, Babić MM, Vuković JS, Onjia AE, Filipović JM, Tomić SLj (2016) Removal of Pb2+ from aqueous solution by P(HEA/IA) hydrogels. Hem Ind 70:695–705. https://doi.org/10.2298/HEMIND151225006A
Acknowledgements
This work is part of the project MNTR TR-34012 financed by the Ministry of Education, Science and Technological Development of the Republic of Serbia. The authors are grateful for the support provided by the Ministry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zdravković, A., Nikolić, L., Ilić-Stojanović, S. et al. The removal of heavy metal ions from aqueous solutions by hydrogels based on N-isopropylacrylamide and acrylic acid. Polym. Bull. 75, 4797–4821 (2018). https://doi.org/10.1007/s00289-018-2295-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2295-0