Abstract
Hydrogels are hydrophilic macromolecular systems that swell in water without dissolving. The nature of the solvent has a significant effect on the amount of water absorbed. Poly(acrylamide) hydrogel and its copolymer with diprotic maleic acid are prepared in this work by copolymerization and chemical crosslinking with methylene bisacrylamide; at different mass percentages of acrylamide (AAm, %) and maleic acid (MA, %), respectively: (100, 0%) and (80, 20%). The obtained results show the formation of copolymers with intermolecular hydrogen bonding between carboxylic groups and amide groups. The content of maleic acid within the crosslinked polymer (acid value) is determined. Two acid values are obtained; 14 and 57.2 mg g–1. Thermal stability of hydrogels is examined through TGA analysis. The thermal degradation of poly(acrylamide) occurs via two stage process; (204–330°C) and at temperature higher than 330°C. The thermal stability of the copolymer increases with incorporates of MA. The copolymer degrades in a multistage process; less than 230°C, (230–350°C) and above 350°C. Different percentages of crosslinker between 1–5 wt % and copolymer with maleic acid are used to study their swelling behavior in different aqueous solutions (NaCl, pH 9.18 and pH 2.2). The swelling is reduced with increasing degree of the cross-linking agent. The obtained hydrogels have shown substantial percent swelling in water and basic buffer and shrinking in saline solution and acidic buffer. The results show that MA20 hydrogel has pH and ionic sensitive characters. These sensitivities exhibit hydrogels an important utility in water treatments, especially as adsorbent of heavy metal ions. The study of oscillatory swelling of hydrogels at pH 4.01 and 9.18 as function of time are demonstrated in this work. The result shows that the synthesized hydrogels exhibit excellent reversible pH-sensibility character.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Hydrogels are water-swollen polymeric networks of chemically or physically cross-linked chains [1]. Therefore, the ability of hydrogels to swell in water is one of the most notable properties of these polymers, which has resulted in many applications in different fields (medical, pharmaceutical, agricultural, and others) [2, 3]. This ability is due to the hydrophilic nature of functional groups such as alcohol, carboxyl, and sulphonic acid on the polymeric backbone, while their opposition to dissolution arises from crosslink between network chains [4]. Ionic hydrogels are composed of polymeric backbones with ionic pendant groups [5]. In aqueous media of appropriate pH and ionic strength, the pendant groups ionize and develop fixed charges on the polymer network generating electrostatic repulsive forces responsible for pH-dependent swelling or de-swelling of the hydrogel [6]. Acrylamide and acrylic acid are widely used for pH-responsive hydrogels preparation [7]. Polyacrylamide hydrogels are mostly prepared by free-radical crosslinking copolymerization of acrylamide and methylene bisacrylamide. To increase their swelling capacity, an ionic comonomer such as acrylic acid is also included into the reaction mixture and copolymerization is a common method used to make such compounds [8]. In more recent years, pH-sensitive hydrogels were synthesized from copolymers of acrylamide and diprotic itaconic and maleic acid and showed that the use of even very small quantities of diprotic acid proved to impart remarkable properties to the hydrogels of starting monomers and/or homopolymers [9]. The hydrogel of poly(acrylamide) or poly(N-isopropylacrylamide) and diprotic acid synthesized by free radical copolymerization with high monomer ratio have not extensively studied. These hydrogels exhibit a very high capability to absorb water and possess good biocompatibility. The biocompatibility and non-toxicity of maleic acid have promoted its use in the synthesis of pH-sensitive hydrogels with acrylamide [10]. The advantage of maleic acid is a great hydrophilicity; they have two ionizable groups with different pKa, indicating a more pronounced pH-sensitivity in the system [11, 12]. Owing to this property and others, it may be used in the hydrogel preparations with interesting pH-sensitivity and swelling. Till now, anionic hydrogels are widely used as adsorbent of heavy metal ions in environmental applications [13, 14]. The adsorption of metal ions onto hydrogel is occurred by the diffusion mechanism and by the electrostatic interaction forces between metal and polymer. Where, the diffusion is governed by the swelling of network in water containing metal ions and by the flexibility of polymer chains.
Therefore, the hydrogels of acrylamide and its copolymers with different diprotic acids exhibit a very high capability to absorb water and possess a specific property of adsorption.
Due to the importance of acrylamide-based hydrogels in different fields, it is necessary to study their notable properties that can be affected by the nature and the concentration of crosslinking agent, including an ionic comonomer in the reaction mixture and by the polymerization conditions. On this basis, we focus on how the concentration of crosslinker and the anionic comonomer acidity affect the ionic sensitive and the swelling of the acrylamide-based hydrogels. For this propose, poly(acrylamide) hydrogels at various concentrations of crosslinker and their copolymer with diprotic maleic acid are prepared by free radical solution copolymerization. Methylene bisacrylamide is used as crosslinker. All the hydrogels synthesized are characterized by thermal analysis. The maximum degree of swelling of obtained hydrogels as a function of crosslinking degree and pH is studied. The reversibility of swelling in buffered solutions of obtained hydrogels are investigated. The importance of obtained hydrogels as adsorbent of heavy metal ions is also studied.
MATERIALS AND METHODS
Materials
Acrylamide (AAm) and maleic acid (MA) are supplied by Panreac Chemicals and are used as monomers. N,N'-methylene-bis-acrylamide (NBAAm), potassium persulfate (KPS) and N,N,N',N'-tetramethyl ethylene diamine (TEMED) are supplied by Aldrich Chemical Co and are used as received.
Synthesis of Copolymer Hydrogels
Hydrogels based on poly(AAm) and its copolymer with MA are prepared by free radical crosslinking copolymerization procedure. Aqueous solutions of monomers (AAm and MA) and crosslinking agent (NBAAm) are prepared in 10 mL of distilled water at different mass percentages of Aam % and MA %, respectively: (100, 0%) and (80, 20%). The concentration of the crosslinking agent NBisAAm is typically varied beteween 1.0 and 5.0 wt %. The quantities of KPS (0.6 wt %) and TEMED (0.4 mL) are added to solution mixture (Table 1). The solution mixture is prepared under N2 atmosphere. The reaction is kept out for 24 h in the glass tubes at 25°C. After the reaction, crosslinked copolymers are cut into portions of about 5 mm length, then washed continually with distilled water for 3 days and finally dried in a vacuum oven at 35°C.
Determination of the Acid Value of Hydrogels (AVm)
To determine the content of MA within the crosslinked polymer, a classic potentiometric titration with NaOH solution is used. By this method, we can determinate the hydrogel acid values which are the quantity (mg) of carboxylic acids per one gram (g) of the polymer. Principally, we titrate the swollen samples by a basic solution (0.1 N of NaOH), in a presence of phenolphthalein.
Thermal Characterization
For thermogravimetric analysis, a NETZSCH 204 F1 Phoenix thermal apparatus is used with nitrogen flow (25 mL min–1). The polymer samples are heated in an aluminum holder from room temperature to 600°C at rate of 10°C min–1 on dry samples of 10 ± 0.5 mg. The weight loss (TG curve) is recorded simultaneously as a function of temperature.
Swelling Studies
The obtained hydrogels are immersed in distilled water to swell for equilibrium degree at room temperature. Weighing the hydrogels after and before immersing in distilled water tested the equilibrium swelling degree. The mass swelling percentage is calculated from the following equation [15, 16]
M0 and Me are the weights of the dry gel at time 0 and at equilibrium time, respectively. All the experiments are carried out in triplicate and the average values have been reported in the data.
The ionic strength and pH effects on swelling behavior are studied by measuring the EDS (%) of hydrogels in different media (NaCl, CaCl2, AlCl3 (0.9%)) and buffer solutions (pH 2.2 and pH 9.18), respectively.
Oscillatory Swelling (Cycle ON/Off)
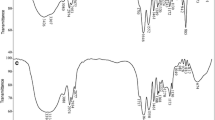
MA00 and MA20 hydrogels are initially immersed in basic solution of pH 9.18 to swell for 24 h. Then, the swollen hydrogels are taken out and are placed in an acidic solution of pH 4.01 for 24 h. The results obtained of reversible Swelling-deswelling of hydrogels under various pH values as function of time are illustrated in Fig. 1.
RESULTS AND DISCUSSIONS
Synthesis of Copolymers
Copolymers are prepared with two ratios of acrylamide and maleic acid. The copolymerization in water leads to the formation of copolymers with intermolecular hydrogen bonding between carboxylic groups and amide groups. Amide groups are very compatible with carboxylic groups and the growing comonomer radical of AAm also prefers to combine with MA [17].
Determination of the Acid Value of Hydrogels (AVm)
The acid values of obtained hydrogels are calculated. For pure poly(AAm) or MA00 is about of 14 mg g–1 and for poly(AAm-co-MA) is about of 57.2 mg g–1. These values indicate that the incorporation of maleic acid within the matrix hydrogels is successfully carried out. The AVm of MA00 hydrogel, which does not contain maleic acid, indicates the presence of carboxylic groups in its structure. This is possibly due to the hydrolysis of amide groups of acrylamide monomer during the polymerization. Hasine Kaşgöz et al. reported the same results about hydrolysis of acrylamide during the reaction [18].
Thermal Characterization
Thermogravimetric analysis provides a method for determination of mass changes in the polymer as a function of time and temperature, this technique reflects reactions which occur at the molecular level of the materials. The TG curves of the copolymer and pure poly(AAm) are shown in Fig. 2. The thermal degradation of poly(AAm) occurs via two stage process. The first step from 204 to 330°C corresponds to the loss of ammonia and water by imidization and dehydration, respectively. The second process, at temperature higher than 330°C, is attributed to processes accompanying main chain scission [19]. Mostly, thanks to the hydrogen bonds developed between carboxylic and amides groups, thermal stability of the copolymer increases with incorporate of maleic acid. The copolymer of AAm with MA degrades in a multistage process. After the breakage of most hydrogen bonds, the degradation of hydrogels components starts. The first stage, less than 230°C, corresponds to anhydride formation. The second process (230–350°C) is attributed to the loss of ammonia and water by imidization and dehydration, respectively [20]. The main chain scission process occurs in the third stage above 350°C.
Swelling Behavior in Distilled Water
The swelling behavior of poly(AAm) and poly(AAm-co-MA) hydrogels is 5013 and 16 271 g g–1, respectively. It is clear that the EDS % of poly(AAm-co-MA) copolymer hydrogel is higher than the EDS % of pure poly(acrylamide). This difference in swelling degree is attributed to the difference in the hydrophilicity of acrylamide and diprotic acid. The swelling degree increases in the following order:
Hydrogel (–COOH) > hydrogel (–CONH2) and Hydrogel [(–COOH) + (–CONH2)] \( \gg \) hydrogel (‒CONH2)
The pure poly(AAm) hydrogel, which have non ionic character, show unexcepted high swelling in distilled water compared to the one reported by Kaşgöz et al. (about 2200%) [18]. This propably due to the hydrolysis of amide groups during the reaction and to the high room temperature in which we have studied (T = 30°C). However, the pure poly(acrylamide) hydrogel is classed as temperature sensitive hydrogel which experience increased swelling with increasing temperature [21].
Effect of Crosslinking Degree on the Swelling of Poly(AAm) Hydrogel
The EDS % depends on many factors; one of these factors is the concentration of crosslinking agent [22]. In this work, we study the effect of crosslinking degree on the swelling of pure poly(AAm) hydrogel. In Fig. 3, it can be seen that the value of EDS % of hydrogels also depends very much on the crosslinking degree of the hydrogel. An increase in this last decreases the swelling degree.
Effects of Medium Solutions and Copolymerization on Swelling of Poly(AAm) Hydrogel
MA02 (poly(acrylamide) with 5% of NBAAm) and MA20 hydrogels are chosen to determine the effect of saline solution (NaCl 0.9%) and buffered solutions (pH 9.18 and pH 2.2) in their swelling behavior (Table 2).
In Table 2, all the values of EDS % of copolymer hydrogel (MA20) are higher than those of homo-polymer hydrogel MA02. This is due to the important hydrophilicity of copolymer hydrogel (ionic character).
The swelling degree of the copolymer hydrogels in saline solution (NaCl 0.9%) is appreciably decreased comparing to the values measured in distilled water. This is due to the presence of (Na+) in external solution which decreases the osmotic pressure within hydrogel resulting on few water absorption. The counter ions Na+ take up places next the carboxylate sites and limit the formation of hydrogen bonding between (–COO–) and water molecules. This limitation results in a decrease in repulsive forces among (–COO–) groups along polymeric segments, which reduce the osmotic pressure and hence the swollen hydrogels shrink dramatically. These results demonstrate the ionic and pH sensibilities of obtained hydrogels. Then, the swelling degree of copolymer hydrogel increases with pH values. The copolymer of poly(AAm-co-MA) hydrogel contain number of carboxylate groups in their networks and their group dissociation (pka values) is dependent on the pH of the external solution. Because the two values of pKa of carboxylic acid containing in the polymer is about 1.89 and 6.23 corresponding the first and the second dissociation, respectively. The carboxyl groups of hydrogel tend to dissociate at a pH > 6.23, the osmotic pressure inside the hydrogels increases and the swelling ratio increases also. In the pH value of 2.2 that is lower than pK2 of pure diprotic acid, the EDS % of copolymer is low. It is due to the protonation of carboxylic groups (–COOH) (nonionic character).
Ionic Sensitivity (Effect of Ionic Strength on Swelling Behavior)
It is well known that the swelling behavior of anionic hydrogels in various salt solutions is significantly decreased comparing to the swelling values in distilled water. This decrease differs from one hydrogel to another, according to its sensitivity. To achieve a comparative measure of sensitivity of the hydrogels towards the kind of external solution, a dimensionless swelling factor, f is defined as following equation [23]:
MA02 and MA20 hydrogels are used to determine the effect of salt solutions (NaCl, CaCl2 and AlCl3) on their swelling. The polymer hydrogels are placed in the salt solutions of 0.9% concentration, to reach equilibrium swelling. The results are shown in Table 3 and Fig. 4.
The salinity of ionic solutions and their concentration can be expressed by the term “ionic strength”. The ionic strength is determined by the following equation:
I, Ci and Zi are the ionic strength (mol-ion dm–3), ionic concentration and ionic charge of ionic solution respectively.
According to results presented in Fig. 4 and Table 3, swelling degree decreases with ionic strength of external solution and the factor of ionic sensitivity increases too, for all hydrogels. However, this force depends on both the nature of counter-ions presented in the external solution and their charge or valence. In case of aluminum cations (Al3+) which are multivalent cations the swelling is weaker (Fig. 4).
It is well known that the swelling of anionic hydrogels in salt solutions is decreased comparing to the swelling values in distilled water as seen for MA20 hydrogel. The swelling of poly(acrylamide) in the salt solutions shows a little reduction, once it is decreased in NaCl (compared to distilled water values); it keeps this reduction in the other solutions. These results we confirm that poly(acrylamide) hydrogel is non-ionic.
The reduction in swelling values is often attributed to a “charge screening effect” of the additional cations. This effect causes a non-perfect anion-anion electrostatic repulsion and a reduction of osmotic pressure between gel and external solution [24]. In other word, when the hydrogels are placed into salt solution, carboxylate groups of hydrogel neutralized by the cations of external solution resulting in the formation of hydrophobic complex, decreasing the swelling degree. A few amounts of trivalent cations (Al3+) are able to neutralize several anion sites (–COO–) at the same time. This ability of neutralization decreases with the valence of cation (+2 to +1). The swelling degree decreases in the following order:
In addition, increasing of f with ionic strength indicates that hydrogels have a higher absorbency-loss in salt solutions due to “charge screening effect” of additional cations. As seen in Table 3, MA20 has a highest factor which increases with ionic strength. These results show that MA20 hydrogel has highly ionic sensitivity and can be used for the excellent detection of Na+, Ca2+ and Al3+.
Poly(acrylamide) hydrogel shows a little increase of f with ionic strength (0.51–0.55) due to his ionic insensitivity as mentioned above.
Oscillatory Swelling (Cycle On/Off)
The equilibrium swelling (EDS %) of hydrogels is determined as described earlier for each cycle (Eq. (1)). The obtained results of oscillatory swelling of hydrogels at pH 4.01 and 9.18 as function of time are illustrated in Fig. 1. As given in this figure, the on-off cycles are periodically repeated without any change in the swelling capacities for all hydrogels. This result shows that the copolymeric hydrogel synthesized exhibit excellent reversible pH-sensibility character. For poly(acrylamide), the swelling- deswelling cycles are slightly appeared. This possibly due to the hydrolysis of amide groups under experimental condition, as described above [18].
CONCLUSIONS
In this work, we investigate a serie of hydrogel based on neutral acrylamide and maleic acid, with high concentration of maleic acid (20%), by free radical cross-linking/copolymerization in aqueous solution using NBAAm as the cross-linker. The EDS (%) values increase with incorporation of acid in neutral acrylamide hydrogel and decrese with crosslinking agent content.
However, the EDS (%) values in physiologic water are appreciably decreased comparing to those in distilled water. These values show that the obtained copolymeric hydrogels can be used as a super-absorbent material. Swelling of the polymeric networks is affected by the hydrogel composition and by pH of the external media. In addition, the sudden and sharp swelling-deswelling behavior at different pH values make the system to be highly pH-responsive. The obtained results show that hydrogel prepared in this work exhibits different sensitivities which make it important as mterial adsorbent of heavy metal ions from water.
REFERENCES
Omidian, H. and Park, K., Fundamentals and Applications of controlled Release Drug Delivery, New York: Springer-Verlag, 2012.
Peppas, N.A. and Khare, A.R., Preparation, structure and diffusional behavior of hydrogels in controlled release, Adv. Drug. Delivery Rev., 1993, vol. 11, nos. 1–2, pp. 1–35.
Sassolas, A., Blum, L.J., and Leca-Bouvier, B.D., Immobilization strategies to develop enzymatic biosensors, Biotechnol. Adv., 2012, vol. 30, no. 3, pp. 489–511.
Kost, J. and Langer, R., Responsive polymeric delivery systems, Adv. Drug. Delivery Rev., 2012, vol. 64, pp. 327–341.
Kim, S.W., Bae, Y.H., and Okano, T., Hydrogels: Swelling, drug loading, and release, Pharm. Res., 1992, vol. 9, no. 3, pp. 283–290.
Kopeček, J. and Yang, J., Hydrogels as smart biomaterials, Polym. Int., 2007, vol. 56, no. 9, pp. 1078–1098.
Wang, Y., Byrne, J.D., Napier, M.E., and De-Simone, J.M., Engineering nanomedicines using stimuli-responsive biomaterials, Adv. Drug. Delivery Rev., 2012, vol. 64, no. 11, pp. 1021–1030.
Şolpan, D., Duran, S., Saraydin, D., and Güven, O., Adsorption of methyl violet in aqueous solutions by poly (acrylamide-co-acrylic acid) hydrogels, Radiat. Phys. Chem., 2003, vol. 66, no. 2, pp. 117–127.
Saraydin, D., Karadaǧ, E., Caldiran, Y., and Güven, O., Nicotine-selective radiation-induced poly (acrylamide/maleic acid) hydrogels, Radiat. Phys. Chem., 2001, vol. 60, no. 3, pp. 203–210.
Saraydin, D., Karadaǧ, E., and Güven, O., Use of superswelling acrylamide/maleic acid hydrogels for monovalent cationic dye adsorption, J. Appl. Polym. Sci., 2001, vol. 79, no. 10, pp. 1809–1815.
Jagur-Grodzinski, J., Polymeric gels and hydrogels for biomedical and pharmaceutical applications, Polym. Adv. Technol., 2010, vol. 21, no. 1, pp. 27–47.
Krušić, M.K., Ilić, M., and Filipović, J., Swelling behavior and paracetamol release from poly (N-isopropylacrylamide-itaconic acid) hydrogels, Polym. Bull., 2009, vol. 63, no. 2, pp. 197–211.
Ali, A.E.H., Shawky, H.A., El Rehim, H.A., and Hegazy, E.A., Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution, Eur. Polym. J., 2003, vol. 39, no. 12, pp. 2337–2344.
Yildiz, U., Kemik, Ö.F., and Hazer, B., The removal of heavy metal ions from aqueous solutions by novel pH-sensitive hydrogels, J. Hazard. Mater., 2010, vol. 183, nos. 1–3, pp. 521–532.
Gudeman, L.F. and Peppas, N.A., Preparation and characterization of pH-sensitive, interpenetrating networks of poly(vinyl alcohol) and poly(acrylic acid), J. Appl. Polym. Sci., 1995, vol. 55, no. 6, pp. 919–928.
Saraydin, D., Karadaǧ, E., and Güven, O., Adsorptions of some heavy metal ions in aqueous solutions by acrylamide/maleic acid hydrogels, Sep. Sci. Technol., 1995, vol. 30, no. 17, pp. 3287–3298.
El-Hamshary, H., Synthesis and water sorption studies of pH sensitive poly(acrylamide-co-itaconic acid) hydrogels, Eur. Polym. J., 2007, vol. 43, no. 11, pp. 4830–4838.
Kaşgöz, H., Aydın, İ., and Kaşgöz, A., The effect of PEG(400)DA crosslinking agent on swelling behaviour of acrylamide-maleic acid hydrogels, Polym. Bull., 2005, vol. 54, no. 6, pp. 387–397.
van Dyke, J.D. and Kasperski, K.L., Thermogravimetric study of polyacrylamide with evolved gas analysis, J. Polym. Sci., Part A: Polym. Chem., 1993, vol. 31, no. 7, pp. 1807–1823.
Filipović, J.M., Katsikas, L., Popović, I.G., Velićković, S.J., Djakov, T.A., and Petrović-Djakov, D.M., The thermal degradation of some alkali metal salts of poly(itaconic acid), J. Therm. Anal., 1997, vol. 49, no. 1, pp. 335–341.
Neamtu, I., Chiriac, A.P., and Nita, L.E., Characterization of poly (acrylamide) as temperature-sensitive hydrogel, J. Optoelectron. Adv. Mater., 2006, vol. 8, no. 5, pp. 1939–1943.
Roy, S.G., Kumar, A., and De, P., Amino acid containing cross-linked co-polymer gels: pH, thermo and salt responsiveness, Polymer, 2016, vol. 85, pp. 1–9.
Zohuriaan-Mehr, M.J. and Pourjavadi, A., Superabsorbent hydrogels from starch-g-PAN: Effect of some reaction variables on swelling, J. Polym. Mater., 2003, vol. 20, no. 1, pp. 113.
Sadeghi, M. and Hosseinzadeh, H., Synthesis and swelling behavior of starch-poly (sodium acrylate-co-acrylamide) superabsorbent hydrogel, Turk. J. Chem., 2008, vol. 32, no. 3, pp. 375–388.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Nour-Elhouda Angar's ORCID is 0000-0002-5035-6553.
About this article
Cite this article
Nour-Elhouda Angar, Djamel Aliouche Effect of Ionic Strength, Crosslinking Degree and Copolymerization on Properties of Poly(Acrylamide) Hydrogel, Application as Adsorbent of Heavy Metal Ions. J. Water Chem. Technol. 43, 228–235 (2021). https://doi.org/10.3103/S1063455X21030036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X21030036