Abstract
The new tetraaryl substituted imidazole-based diamines were designed and synthesized through Debus–Radziszewski imidazole synthesis, and characterized by FT-IR, 1H NMR spectroscopy and MASS spectroscopy. A series of new fluorescent polyimides (PI) were prepared by polymerization of the tetra substituted imidazole diamines with tetracarboxylic dianhydrides, such as pyromellitic dianhydride, naphthalene tetra carboxylic dianhydride and perylene tetra carboxylic dianhydride. These polymers were readily soluble in a variety of organic solvents and they also possess good thermal stability. The glass transition temperature of these polymers was in the range of 398–453 °C. The ultraviolet–visible absorption spectra showed that all of the polymers had absorption maxima around 344–521 nm with a fluorescence emission maxima around 420–531 nm. The electrochemical band gaps of PI-1, PI-2 and PI-3 copolymers are estimated to be 2.37, 2.17 and 2 eV, respectively. Similarly, the optical band gap of PI-1, PI-2 and PI-3 copolymers was found to be 3.15, 3.05, and 2.15 eV, respectively. These polymer exhibits fluorescence quantum yield of 0.65, 0.46, and 0.3% for PI-1, PI-2 and PI-3, respectively. The PIs also exhibited good flame retardant behavior.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic polyimides are commercially important materials that are used extensively in a wide range of optoelectronic applications due to their excellent chemical, thermal, and dielectric properties [1–3]. Despite their outstanding properties, most of the conventional aromatic polyimides have high melting or glass-transition temperatures (T g) and limited solubility in most organic solvents because of their rigid backbones and strong interchain interactions. Thus, polyimide processing is generally carried out via poly(amic acid) precursor, and then converted to polyimide by vigorous thermal or chemical cyclodehydration [1, 4]. However, this process has inherent problems such as emission of volatile by-products and storage instability of poly(amic acid) solution. Recently, significant research has been focused on to design the structural features of polyimide to meet the easy processing and enhance thermal, and optoelectronic properties. Some of the approaches employed to modify the structure are introducing thermally stable moiety, such as rigid aryl groups in the side chain which decrease close-packing in the polymer backbone and lead to enhanced solubility of the polymer, flexible segments, large polar triaryl imidazole pendants and, disruption of symmetry and recurrence of regularity through copolymerization [5–11], and introduction of noncoplanar [12–14] and alicyclic units [15].
Among these approaches, introducing bulky pendent substituent and heteroaromatic rings into polyimide chains, namely imidazole compounds have attracted significant interest because of their widespread biological activities, such as herbicidal [16], fungicidal [17], and anti-inflammatory activities [18] and their use in synthetic chemistry [19]. In addition, they are applied in photography as photosensitive compounds [20]. Therefore, the synthesis of polyimides having heterocyclic rings has been attracted considerable interest.
Akutsu et al. [21] synthesized triaryl imidazole bearing polyimides having glass transition temperatures (T g) in the range of 279–353 °C, and weight residues at 600 °C were above 82%. The polymers also have good solubility in pyridine and m-cresol. Ghaemy et al. [22] synthesized polyimides from unsymmetrical diamine monomer containing triaryl imidazole as pendant group. PIs exhibited excellent solubility in aprotic polar solvents such as N,N-dimethylacetamide (DMAc), N-methyl-2-pyrrolidone (NMP), N,N-dimethylformamide (DMF), pyridine and methyl sulfoxide (DMSO). Those PIs showed high glass transition temperatures between 230 and 320 °C. Recently, Rafiee et al. [23] reported that the introduction of triaryl imidazole molecules as pendant in polyimide resulted in good solubility, with a glass transition temperature of 264 °C and having UV–Vis absorption maxima around 320 nm and fluorescence emission maxima around 388–407 nm. In the present work, we have synthesized new polyimides by introducing tetraaryl imidazoles in the polyimide backbone, as a maiden attempt, anticipating that the introduction of tetraaryl imidazole, particularly in the main chain will impart solubility, thermal as well as excellent electrochemical properties. The synthesized polyimides exhibited increased value of glass transition temperature, in the range of 398–453 °C. The ultraviolet–visible absorption spectra showed that all of the polymers had absorption maxima around 344–521 nm with a fluorescence emission maxima around 420–531 nm. The electrochemical band gaps of PI-1, PI-2 and PI-3 copolymers are estimated to be 2.37, 2.17 and 2 eV, respectively. This was designed for the low cost and for large-area employments in optoelectronic devices, such as ambipolar field effect transistors, solar cells, light-emitting devices and electrochromic devices.
Experimental
Materials
4-Nitrobenzaldehyde (99%) and 4-nitroaniline (purchased from Spectrochem, Benzil, hydrazine HCl, palladium–carbon, N-methylpyrolidone (NMP) (99%) from SRL, acetic acid, ammonium acetate, tetrhydrofuron (THF) (99%), ethanol and methanol (99.8%) were received from Fisher scientific. 1H NMR (400 MHz) spectra were recorded on a Bruker instrument. Liquid chromatography–mass spectroscopy (LCMS) was measured in an Agilent instrument. FT-IR was recorded in a Perkin Elmer Spectrum RX1 instrument using KBr pellet technique. Thermo gravimetric analysis (TGA) and differential scanning calorimetry (DSC) analysis methods were used to explain the thermal behaviour of the polyimides. TGA was carried out by Hitachi-STA 7200 instrument and DSC carried out by Hitachi-DSC7020 instrument. The optical properties of polyamides were analyzed by UV–Vis and photoluminescence (PL) spectroscopy in THF solution and are presented in Fig. 6. UV–Vis absorption spectra were recorded in a Shimadzu spectrophotometer. PL spectra were carried out by Agilent spectrophotometer. All the polymers were taken about 0.001 mg/mL concentration in THF solvent for UV–Vis and photoluminescence analysis. The cyclic voltammograms of the polymers were done using CH-660D instrument.
Synthesis of 1,2-bis (4-nitrophenyl)-4,5-diphenyl-1H-imidazole
In a 100 mL, two-necked, round-bottomed flask equipped with a reflux condenser, a magnetic stirrer and nitrogen gas inlet tube, benzil (1.4 g, 6.6 mmol), 4-nitrobenzaldehyde (1.0 g, 6.6 mmol), 4-nitroaniline (0.9 g, 6.6 mmol) and ammonium acetate (2.3 g, 30 mmol) in 10–15 mL acetic acid were mixed and heated at 120 °C. The progress of reaction was monitored by thin layer chromatography (TLC) in 75:25 hexane ethyl acetate eluting solvent. After completion of reaction, the reaction mixture was cooled to room temperature. Then the product was filtered, washed with water and recrystallized from ethanol to afford 2.2 g (73%) of 1,2-bis (4-nitrophenyl)-4,5-diphenyl-1H-imidazole as yellow solid. FT-IR (KBr, cm−1): 3052 (aromatic C–H); 1599 (C=N); 1517, 1345 (NO2); 1H NMR (400 MHz, DMSO-d 6, ppm) = 6.85 (d, 2H), 7.4–7.6 (m, 10H), 7.81 (d, 2H), 8.02 (d, 2H), 8.26 (d, 2H) (Scheme 1).
Synthesis of 4,4′(4,5-diphenyl-1H-imidazol-1,2-diyl)dianiline
To a 50 mL two-necked flask equipped with a dropping funnel and a reflux condenser (1.2 g, 2.5 mmol) of 1,2-bis(4-nitrophenyl)-4,5-diphenyl-1H-imidazole and 50 mg of palladium on activated carbon (Pd/C, 10%) were dispersed in 10 mL of ethanol. The suspension solution was heated to reflux and 4 mL of hydrazine monohydrate was added slowly to the mixture. After refluxing for 5 h THF was added to the mixture and filtered hot to remove Pd/C, and the filtrate was cooled to precipitate orange powder. The product was collected by filtration, recrystallized from ethanol and dried in vacuum at 80 °C [22]. The yield obtained was 67% (0.66 g). FT-IR (KBr, cm−1): 3455, 3352, 3030, 1616, 1506, 1274, 769,714 cm−1. 1H NMR (400 MHz, DMSO-d 6), The 1H NMR of imidazole based amine shows signals of protons of N-substituted benzene ring at 5.25 (s, 2H), 5.35 (s, 2H), 6.42 (d, 2H), 6.65 (d, 2H), 6.81(d, 2H), 7.1–7.6 (m, 10H), 8.25(d, 2H). ES-MASS (m/z)—401.8.
Synthesis of polyimides
Polyimide was synthesised by two-step thermal imidization reaction. The synthesized diamine (0.1 mol) and dianhydrides (0.1 mol) are dissolved in NMP solvent and the mixture was stirred at room temperature overnight to obtain polyamic acids. The resulting polyamic acids was then cast on a glass substrate and thermally treated at 80 °C for 8 h, 200 °C for 2 h, and 300 °C for 2 h to obtain the respective cured PIs (Scheme 2).
Results and discussion
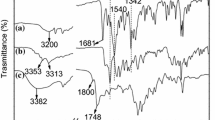
A new diamine monomer, 4,4′(4,5-diphenyl-1H-imidazol-1,2-diyl)dianiline, was synthesized by a two-step procedure according to the synthetic route shown in Scheme 1. Tetraaryl imidazole based amine was synthesised thorough Debus–Radziszewski imidazole synthesis [24–26] which involves the condensation of benzil with aromatic aldehyde in the presence of ammonium acetate. The structures of 1,2-bis(4-nitrophenyl)-4,5-diphenyl-1H-imidazole and 4,4′(4,5-diphenyl-1H-imidazol-1,2-diyl)dianiline were characterized by FT-IR, 1H NMR and MASS analyses, the results are presented in Figs. 1, 2 and 3, respectively. Spectral data from the FT-IR and 1H NMR and MASS analysis show the effective formation of the respective imidazole-based nitro and corresponding amine group. The synthesised amine functionality has been confirmed by IR spectrum. Figure 1 shows the FT-IR spectrum of imidazole based diamine. Diamine functional group has sharp absorption bands at 3455 and 3352 cm−1. The 1H NMR spectrum shown in Fig. 2. The synthesized imidazole compound has two aniline groups in different environment. One is substituted in Nitrogen atom of imidazole (first position of imidazole) and another one is substituted in carbon atom of imidazole (second position of imidazole). Ha, Hc and He protons belongs to aniline group in first position of imidazole. Similarly Hb, Hd and Hg belongs to aniline group in second position of imidazole. The integral areas of Ha, Hc and He is relatively smaller than that of Hb, Hd and Hg, because of more electronegative and down field desheilding protons resulting from first position of imidazole. The newly synthesised diamine thermally polymerised with three different types of dianhydrides such as pyromellitic dianhydride, naphthalene tetra carboxylic dianhydride and perylene tetra carboxylic dianhydride. The synthesised polyimides were confirmed by IR spectra. Figure 4 shows the FT-IR spectra of PI-1, PI-2 and PI-3. The absorption band at 1768 cm−1 confirms the presence of asymmetric stretching vibration of carbonyl –C=O in the imide groups. The absorption band at around 1720 cm−1 was due to the symmetrical stretching vibration of imide carbonyl –C=O functionality and the band at 1370 cm−1 confirms the presence of imide C–N stretching.
Solubility studies
The solubility of imidazole-based polyimides were tested with different common organic solvents such as 1-methyl-2-pyrrolidone (NMP), dimethyl formamide (DMF) N,N-dimethylacetamide (DMAc), dimethyl sulfoxide (DMSO), tetrahydrofuran (THF) and chloroform (CHCl3). Table 1 shows the solubility behavior of PIs. The results show that PI-1, PI-2 and PI-3 exhibit good solubility in organic solvents, due to the presence of non-planar imidazole moiety present in the polyimide. It was also found that NMP is the best solvent for polyimides synthesized in the present work.
Thermal behaviour
Thermo gravimetric analysis (TGA) and differential scanning calorimetry (DSC) analysis methods were used to explain the thermal behaviour of the polyimides. TGA was carried out by Hitachi-STA 7200 instrument and DSC carried out by Hitachi-DSC7020 instrument. DSC and TGA thermograms of the polyimides are shown in Fig. 5a, b, respectively. Synthesised polyimide samples were dried for 2 h at 150 °C. Completely dried samples were used for TGA and DSC analysis. The thermal analysis data from the TGA and DSC thermograms of the polyimides are summarized in the Table 2. The T g values of the polyimides measured by DSC at a heating rate of 10 °C min−1 under nitrogen atmosphere. The T g value of the polyimide PI-1, PI-2 and PI-3 were obtained at the range of 398, 419 and 453 °C, respectively. In this DSC thermogram no endothermic peaks associated with melting were observed in the DSC traces. The T g of polyimide PI-3 (453 °C) is higher than T g of the polyimide PI-1 (398) and PI-2 (419) because of the increment of aryl groups. The thermal stability of the polymers was determined by thermogravimetric analysis (TGA) at a heating rate of 20 °C min−1 under nitrogen atmosphere (Fig. 5). Thermal stability of the polymers was calculated in terms of the temperature at 10% weight loss, maximum degradation temperature (T max), char yield at 750 °C as listed in Table 2. As observed from Fig. 5a, 60% residual weight at 750 °C of PI derived from perylenetetracarboxylic dianhydride had the highest values in comparison with other PI-1 and PI-2 at 56, 58%, because of the very rigid dianhydride monomer and aggregation of the polymer chain. The high char yields of these polymers could be attributed to their high aromatic content. The T max of the polymers was measured at 544, 558 and 575 °C. Limiting oxygen index (LOI) was estimated according to the Van Krevelen and Hoftyzer equation [27–29]:
where CR is the percentage of polymer remaining at 750 °C. All polymers have LOI more than 35 and such polymers can act as flame-retardant materials. The LOI of PI, PI-2 and PI-3 estimated at 40 and 41 and 42, respectively.
Optical behaviour
The optical properties of polyamides were analyzed by UV–Vis and photoluminescence (PL) spectroscopy in THF solution and are presented in Fig. 6. All the polymer samples are prepared by taking about 0.001 mg/mL in THF solvent for UV–Vis and photoluminescence analysis. The absorption maxima for PI-1, PI-2 and PI-3 are 344, 364, and 521 nm, respectively. The broader absorption peak of PI-3 at 521 nm obtained due to presence of perylenetetracarboxylic dianhydride structure in the backbone of these polymers. The longer absorption bands for the polyimide copolymer systems may be explained due to the intramolecular charge transfer between donors and acceptor moieties in the polymer network. The PI-1 polyimide copolymer of pyromellitic dianhydride and tetrasubstituted imidazole absorbs at 344 nm and emits at 429, 405, 452, 539 nm. Similarly, PI-2 polyimide based on naphthalene and tetrasubstituted imidazole amine synthesised showed multiple absorption bands at 355, 364 nm and emission bands at 436, 415, 460, 500, 537 nm. PI-3 polyimide also showed multiple absorption bands at 457, 485, 521 and emission bands at 531, 566, 610 nm. These multi band absorption and emission peaks may be due to the presence of many aryl substituent. In general naphthalene- and perylene-based systems will show strong multi absorption band due to conjugation of poly aryl molecule π–π* transition, which corresponding polyimide also had strong multi absorption and red shifted due to aryl substituted imiazole influence. These polymers have small absorption n–π* transition for carbonyl (C=O) and imine (N=C) group. Iqbal et al. [30] reported the optical behavior of polyimides with noncoplanar carbazole–triphenylamine unit and pyromellitic dianhydride has an absorption of 264 and 353 nm and emission at 447 and 490 nm. Similar results of multi band absorption and emission has been reported for polyimide having naphthalene and perylene units.[31, 32] photoluminescence quantum yield and band gap of PI-1, PI-2 and PI-3 were calculated by UV–Vis and photoluminescence spectroscopy. [33–39] The calculated band gaps (E g) for PI-1, PI-2, and PI-3 were 3.15, 3.05, and 2.15 eV, respectively, since polymer with extended conjugation and stronger acceptor moiety PI-3 found to have low band gap. Single phenylene ring-based pyromellitic dianhydride act as strong acceptor. Similarly two phenyl ring fused naphthalene tetra carboxylic dianhydride is strong electron acceptor moiety then single ring dianhydride and also perylene tetra carboxylic dianhydride act as very strong acceptor than other two anhydrides because it has more bulky dianhydride moiety. During the improvement of the electron delocalisation or electron donor acceptor segments band gap energy of the polymeric material may get lower. Here electron acceptor property improved from PI-1 to PI-3. From these results perylene-based PI-3 have lower band gap than other PIs.
To measure the photoluminescence quantum yields (Φ PL), [36–39] dilute polymer solutions in THF were used. Rhodamine 6G (Φ PL = 0.95) was used as a reference [33, 36–39]. The emission spectrum of polymer PI-1, PI-2 and PI-3 were recorded under excitation wavelength in the maximum of the absorption band, i.e., 430, 440 and 531 (563) nm, respectively. The emission wavelengths were observed at 344–521 nm. The quantum yield of polyimides summarized in Table 3 and the PL spectrum of all polymers are presented in Fig. 6b.
Electrochemical properties
It is known that energy-level is important parameter for optoelectronic device; the energy-level band gap of the polymers was calculated using cyclic voltammeter (CV). The CV cell consisted of a glassy carbon electrode, a Pt wire counter electrode, and an Ag/AgCl reference electrode. All measurements were performed using acetonitrile solution of 0.1 M Bu4N(ClO4) as a supporting electrolyte with a scan rate of 50 mV s−1. All the potentials were calibrated with ferrocene as an external standard. The voltagramms are shown in Fig. 7. The LUMO energy levels are estimated by the onset of reduction potential and the onset reduction potential were recorded to be −1.1, −0.99, and −0.95 V; thus, the corresponding LUMO energy levels of PI-1, PI-2, and PI-3 are −3.30, −3.41, and −3.45 eV, respectively. LUMO levels were calculated from onset of reduction with the following equation [33, 38–41]
The HOMO levels were calculated from LUMO and optical band gap. HOMO energy levels of PI-1, PI-2, and PI-3 were calculated at −6.45, −6.46, and −5.60 eV, respectively. Similar results were recorded and published by Chen et al., and Hsiao et al., Hu et al. [[9, 42, 43] it is evidenced that the introduction of tetra substituted imidazole group on polyimide has good impact on its HOMO–LUMO energy level as shown in Fig. 8. Structures of the dianhydrides reveal that pi electron delocalization is in the order of PI-1 < PI-2 < PI-3 which influence the energy separation values between the HOMO and LUMO. The aromatic rings increasing from PI-1 to PI-3. Hence Energy gap of HOMO/LUMO has decreases from PI-1 to PI-3. These results reveals that the synthesized polyimides are may be suitable to be used in ambipolar field effect transistors, solar cells, light-emitting devices and electrochromic devices.
From a materials point of view, the active semiconductors are required to have HOMO energy levels below −6.0 eV for stable hole transport, and the LUMO level needs to be close to or below −3.0 eV for stable electron transport. Therefore, the energy gap between the HOMO and LUMO levels should not be too large to avoid charge injection barriers. The development of novel strong electron-withdrawing building blocks plays an essential role for such applications.
Conclusion
The new tetrasubstituted imidazole diamine monomer was successfully synthesized with high purity and characterized. Aromatic polyimides PI-1, PI-2 and PI-3 were successfully synthesised through polycondensation reaction of tetra substituted imidazole-based diamines with three different dianhydrides. All the polymers were readily soluble in organic polar solvents, such as tetrahydrofuran, CHCl3, DMF, DMAC. The DSC and TGA analysis indicated that the synthesised PIs have high thermal stability with higher glass transition temperature than the reported imidazole containing PIs. Among the synthesised polymers, the polymer PI-1 has a low band gap value of 2.37 eV with a photoluminescence quantum yield of 65%.
References
Hu Z, Zhang K, Huang F, Cao Y (2015) Water/alcohol soluble conjugated polymers for the interface engineering of highly efficient polymer light-emitting diodes and polymer solar cells. Chem Commun 51:5572–5585
Gong X, Tong MH, Park SH, Liu M, Jen A, Heeger AJ (2010) Semiconducting polymer photodetectors with electron and hole blocking layers: high detectivity in the near-infrared. Sensors 10:6488–6496
Li L, Zhang F, Wang W, An Q, Wang J, Sun Q, Zhang M (2015) Improved performance of photomultiplication polymer photodetectors by adjustment of P3HT molecular arrangement. ACS Appl Mater Interfaces 7:5890–5897
Li C, Mao Z, Chen H, Zheng L, Huang J, Zhao B, Tan S, Yu G (2015) Synthesis, characterization, and field-effect transistors properties of novel copolymers incorporating nonplanar biindeno[2,1-b]thiophenylidene building blocks. Macromolecules 48:2444–2453
Zhao J, Peng L, Zhu YL, Song YJ, Wang LJ, Shen YZ (2016) Synthesis and memory characteristics of novel soluble polyimides based on asymmetrical diamines containing carbazole. Polymer 91:118–127
Rafiee Z, Golriz L (2014) Synthesis and properties of thermally stable polyimides bearing pendent fluorene moieties. Polym Adv Technol 25:1523–1529
Chern YT, Tsai JY (2008) Low dielectric constant and high organosolubility of novel polyimide derived from unsymmetric 1,4-bis(4-aminophenoxy)-2,6-di-tert-butylbenzene. Macromolecules 41:9556–9564
Rafiee Z, Khalili S (2013) Synthesis and characterization of highly soluble and thermally stable new polyimides based on 3,5-diamino benzoyl amino phenyl-14H-dibenzoxanthene. Polym Bull 70:2423–2435
Hsiao SH, Wang HM, Chen WJ, Lee TM, Leu CM (2011) Synthesis and properties of novel triptycene-based polyimides. J Polym Sci A Polym Chem 49:3109–3120
Chen JC, Wu JA, Li SW, Chou SC (2014) Highly phenylated polyimides containing 4,4′-diphenylether moiety. React Funct Polym 78:23–31
Yang Z, Chen Y, Wang Q, Wang T (2016) High performance multiple-shape memory behaviors of poly(benzoxazole-co-imide)s. Polymer 88:19–28
Liu C, Wang J, Lin E, Zong L, Jian X (2012) Synthesis and properties of phthalonitrile-terminated oligomeric poly(ether imide)s containing phthalazinone moiety. Polym Degrad Stab 97:460–468
Liu C, Pei X, Huang X, Wei C, Sun X (2015) Novel non-coplanar and tertbutyl-substituted polyimides: solubility, optical, thermal and dielectric properties. Chin J Chem 33:277–284
Liaw DJ, Chang FC, Leung MK, Muellen K (2005) High thermal stability and rigid rod of novel organosoluble polyimides and polyamides based on bulky and noncoplanar naphthalene–biphenyldiamine. Macromolecules 38:4024–4029
Yamada M, Kusama M, Matsumoto T, Kurosaki T (1993) Soluble polyimides with polyalicyclic structure. 2. Polyimides from bicyclo[2.2.1]heptane-2-exo-3-exo-5-exo-6-exo-tetracarboxylic 2,3:5,6-dianhydride. Macromolecules 26:4961–4963
Liebl R, Randte R, Mildenberger H, Bauer K, Bieringer H (1987) Preparation and characterization of polyimides containing triaryl imidazole side groups. Ger Offen DE, 3604 042, Aug. 13 (Chem. Abstr. 108, 1988, 6018 g)
Pozherskii AF, Soldatenkov AT, Katritzky AY (1997) Heterocycles in life and society. Wiley, New York
Lambardino JG, Wiseman EH (1974) Preparation and antiinflammatory activity of some nonacidic trisubstituted imidazoles. J Med Chem 17:1182–1188
Heravi MM, Bakhtiari K, Oskooie HA, Taheri S (2007) Synthesis of 2,4,5-triaryl-imidazoles catalyzed by NiCl2·6H2O under heterogeneous system. J Mol Catal A Chem 263:279–281
Yang WH, Xiao GM (2002) Efficient improved synthesis of 2-aryl-4,5-diphenylimidazole by heating. Chin Fine Chem 19:155–157
Akutsu F, Inoki M, Sawano M, Kasashima Y, Naruchi K, Miura M (1998) Preparation and characterization of novel aromatic polyimides having 4,5-di(1,3-phenylene)imidazole structure. Polymer 39:6093–6098
Ghaemy M, Alizadeh R (2009) Synthesis of soluble and thermally stable polyimides from unsymmetrical diamine containing 2,4,5-triaryl imidazole pendent group. Eur Polym J 45:1681–1688
Rafiee Z, Rasekh M (2016) Preparation and characterization of polyimides containing triaryl imidazole side groups. Polym Adv Technol 27:533–540
Zolfigol MA, Baghery S, Moosavi-Zare AR, Vahdat SM (2015) Synthesis of 1,2,4,5-tetrasubstituted imidazoles using 2,6-dimethylpyridinium trinitromethanide [2,6-DMPyH]C(NO2)3 as a novel nanostructured molten salt and green catalyst. RSC Adv 5:32933–32940
Das PJ, Das J, Ghosh M, Sultana S (2013) Solvent free one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles catalyzed by secondary amine based ionic liquid and defective Keggin Heteropoly acid. Green Sustain Chem 3:6–13
Kumar D, Thomas KRJ (2011) Optical properties of pyrene and anthracene containing imidazoles: experimental and theoretical investigations. J Photochem Photobiol A Chem 218:162–173
Kulhanek J, Bures F (2012) Imidazole as a parent π-conjugated backbone in charge-transfer chromophores. Beilstein J Org Chem 8:25–49
Jaberi ZK, Barekat M (2010) One-pot synthesis of tri- and tetra-substituted imidazoles using sodium dihydrogen phosphate under solvent-free conditions. Chin Chem Lett 21:1183–1186
Boopathy M, Subramanian K (2016) Studies on photocrosslinking and flame-retardant properties of chalcone-based polyacrylamides. Polym Adv Technol 27:466–476
Iqbal A, Lee SH, Park OO, Siddiqi HM, Akhter T (2016) Synthesis and characterization of blue light emitting redox-active polyimides bearing noncoplanar fused carbazole-triphenylamine. New J Chem 40:5285–5293
Damaceanu MD, Rusu RD, Bruma M, Jarzabek B (2010) Blue fluorescent polyamides containing naphthalene and oxadiazole rings. J Polym 42:663–669
Do JY, Jang B (2013) The efficient synthesis of N-fused coronene analogs and a related polyimide with near-infrared absorption. Polym J 45:1177–1182
Lindner JP (2016) Imidazolium-based polymers via the poly-Radziszewski reaction. Macromolecules 49:2046–2053
Faghihi K, Ashouri M, Feyzi A (2013) Synthesis and characterization of new polyimide/organoclay nanocomposites containing benzophenone moieties in the main chain. J Mex Chem Soc 57:133–136
Revathi R, Prabunathan P, Devaraju S, Alagar M (2015) Synthesis of soluble polyimides based on ether-linked cyclohexyldiamine and their ultraviolet shielding behavior. High Perform Polym 27:247–253
Kato SI, Yamada Y, Hiyoshi H, Umezu K, Nakamura Y (2015) Series of Carbazole–pyrimidine conjugates: syntheses and electronic photophysical, and electrochemical properties. J Org Chem 80:9076–9090
Sharma BK, Shaikh AM, Agarwal N, Kamble RM (2016) Synthesis, photophysical and electrochemical studies of acridone-amine based donor–acceptors for hole transport materials. RSC Adv 6:17129–17137
Truong MA, Nakano K (2016) Syntheses of dibenzo[d,d’]benzo[2,1-b:3,4-b’]difuran derivatives and their application to organic field-effect transistors. Beilstein J Org Chem 12:805–812
Sharma BK, Shaikh AM, Kamble RM (2015) Synthesis, photophysical, electrochemical and thermal investigation of triarylamines based on 9H-Xanthen-9-one: yellow–green fluorescent materials. J Chem Sci 27:2063–2071
Brouwer AM (2011) Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl Chem 83:2213–2228
Wurth C, Gonzalez MG, Niessner R, Panne U, Haisch C, Gengera UR, Resch GU (2012) Determination of the absolute fluorescence quantum yield of rhodamine 6G with optical and photoacoustic methods—providing the basis for fluorescence quantum yield standards. Talanta 90:30–37
Chen CJ, Hung Ju, Yen HJ, Hu YC, Liou GS (2013) Novel programmable functional polyimides: preparation, mechanism of CT induced memory, and ambipolar electrochromic behavior. J Mater Chem C 1:7623–7634
Hu YC, Chen CJ, Liou GS (2012) Novel triphenylamine-containing ambipolar polyimides with pendant anthraquinone moiety for polymeric memory device, electrochromic and gas separation applications. J Mater Chem 22:20394–20402
Acknowledgements
The authors acknowledge the financial support of the University Grants Commission, New Delhi, India through grant UGC F. No: 39-784/2010. The author A. Hariharan acknowledges the BSR fellowship from the University Grants Commission, New Delhi, India through grant UGC-BSR doctoral fellowship.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hariharan, A., Kumar, S., Alagar, M. et al. Synthesis, photophysical and electrochemical properties of polyimides of tetraaryl imidazole. Polym. Bull. 75, 93–107 (2018). https://doi.org/10.1007/s00289-017-2015-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2015-1