Abstract
Nine modified urethane acrylate oligomers including polyether-modified polyurethane acrylate, epoxy-modified polyurethane acrylate and hyperbranched polyurethane acrylate have successfully been synthesized and characterized by Fourier transform infrared spectra. The properties of UV-cured films formed by these oligomers were also investigated. The effects of the molecular structure of the oligomers on the properties of cured film, including tack-free time, pencil hardness, impact resistance, adhesion and boiling water resistance, have been discussed. The results indicate that the UV-cured film with good performance can be designed by the modification of the molecular structure of the oligomer. Among the oligomers prepared in this paper, the cured film formed using HBPUA-50%OA as oligomer exhibits a relatively short tack-free time, high hardness, good impact resistance, great adhesion and boiling water resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
UV-curable coatings and their process have received great attention on account of environmental-friendly characteristics. They can cure very fast, reduce volatile solvents and decrease the energy required to cure coatings compared with conventional thermal curing [1–4]. The general chemical formulation of the UV-curable coatings contains a photoinitiator, reactive monomers and functionalized oligomers whose structure mainly determine the properties of the cured film [5].
The major types of oligomers used in free radical UV-curable coatings include epoxy acrylate, acrylated polyester and urethane acrylate. Among them, urethane acrylate has been widely used as oligomers for UV-curable coatings, as a consequence of excellent mechanical properties and chemical resistance [6–10]. However, a drawback of the urethane acrylate is its strong odor, skin and eye irritancy. Due to its low molecular weight, there could be high amount of extractables and considerable shrinkage which cause poor adhesion to substrate [11, 12]. Therefore, great efforts have been paid to modify the urethane acrylate [13–31]. The modified materials mainly included silicon-based, fluorine-based, epoxy-based, hyperbranch-based and biobased ones. The relationship between the structure of the modified molecule and the properties of the prepared urethane acrylate oligomers have been discussed by some researchers. For example, Han et al. synthesized a series of UV-curable hyperbranched polyurethane acrylate oligomers and characterized their UV-curing properties. The effects of different functional groups on the properties of the film were discussed [15–18]. In another case, an aqueous dispersions of castor oil-based polyurethane/aromatic polyamide sulfone block copolymers were successfully synthesized by Mohamed et al. [22]. The effects of castor oil-based polyurethane block on the characters of the copolymers were discussed in detail. In their very recent work, castor oil-based polyol was used to prepare polyurethane/polyamide copolymer dispersions [23]. Recently, to improve both hardness and flexibility of coating films, Eissa et al. prepared amino-terminated hyperbranched polymers which showed satisfactory results [32, 33]. These efforts will help researchers to develop coatings that can meet the needs of practical applications.
Coatings used for tinplate has extensive application [34–36]. Unfortunately, due to the performance gap between UV-curable coatings and conventional thermal curable coatings on tinplate, the application of the UV-curable coatings on tinplate is very limited. To meet the requirements for coating application on tinplates, it is very necessary to understand the relationship between the modified molecular structure of the oligomer and the properties of the UV-curable polyurethane acrylate coatings. For this goal, nine modified urethane acrylate oligomers including polyether-modified polyurethane acrylate, epoxy-modified polyurethane acrylate, and hyperbranched polyurethane acrylate were synthesized in this paper according to the commonly used modified molecular structure of polyurethane acrylate coatings in the literature. The physical properties of the coatings made by these modified oligomers were also investigated. In addition, the relationship between the modified molecular structure and properties of the coating films is discussed in this paper.
Experimental
Materials
Isophorone diisocyanate (IPDI), trimethylolpropane (TMP) and dimethylolpropionic acid (DMPA) were purchased from Ketian Electronic Materials Co., Ltd. Polyethylene glycol (PEG200, PEG400, PEG600), maleic anhydride (MA) and oleic oil (OA) were supplied by Lingfeng Chemical Reagent. Dibutyltin dilaurate (DBTDL), p-hydroxyanisole (HQMME) and p-methyl benzenesulfonic acid (p-TSA) were obtained from Sinopharm Chemical Reagent Co., Ltd. The diglycidyl ether of bisphenol A, epoxy resin (E51, E78 and E132 with an epoxide value of 0.51, 0.78 and 1.32, respectively) were obtained from Jiangsu Tetra New Material Technology Co., Ltd. Isobornyl acrylate (IBOA) used as reactive diluents was obtained from Shanghai Hechuang Chemical Co., Ltd. 1-Hydroxycyclohexyl phenyl ketone (Irgacure 184) used as photoinitiator was supplied by Nanjing Wali Chemical Technology Co., Ltd. 2-Hydroxypropyl methacrylate (HPA) was purchased from Jinsheng Auxiliaries Factory. All other common chemical reagents were of analytical grade and used as received.
Preparation of NCO-bearing adduct
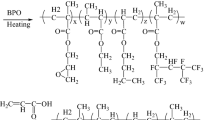
Isocyanate (NCO)- bearing adduct was obtained by the reaction between the cycloaliphatic NCO group of IPDI and the OH groups of HPA with DBTDL as catalyst and HQMME as inhibitor. The synthesis process has been described in Scheme 1. Briefly, IPDI (22.229 g, 0.1 mol), HQMME (0.053 g) and DBTDL (0.106 g) were mixed in a four-necked flask equipped with a stirrer, nitrogen inlet, a thermometer and a dropping funnel. Then HPA (13.014 g, 0.1 mol) was slowly dropped into the mixture by the dropping funnel and stirred at 30 °C. The isocyanate content of the system was determined by titration at various intervals. The reaction was stopped when the content of isocyanate reached half of its initial value. At that time, the value of monoisocyanate was half of its initial value in the mixture [16, 17, 37]. The conversion of the reaction was 99.0%.
Preparation of polyether-modified polyurethane acrylate
PEG (0.05 mol) with different molecular weight was slowly dropped into the resultant NCO-bearing adduct and the mixture was stirred at 50 °C. The reaction continued until the content of isocyanate was less than 0.1 wt%. The added PEG was 10.000 g (PEG200), 20.000 g (PEG400), 30.000 g (PEG600) to obtain the polyether-modified polyurethane acrylate of PUA (200) (conversion: 97.0%), PUA (400) (conversion: 96.6%), PUA (600) (conversion: 95.6%), respectively. The synthesis process is described in Scheme 2.
Preparation of epoxy-modified polyurethane acrylate
The epoxy-modified polyurethane acrylate (E-PUA) was synthesized via a three-step procedure. First, the NCO-bearing adduct was synthesized using the process described in Scheme 1. Second, the modified epoxy resin (MP–OH) was obtained. Briefly, MA (15.850 g, 0.165 mol), PEG200 (33.000 g, 0.165 mol) and HQMME (0.073 g) were mixed in a four-necked flask equipped with a stirrer, nitrogen inlet, a thermometer and a dropping funnel. The mixture was stirred at 75 °C until the content of acid value of the system was reduced to half of the initial one. The conversion of the reaction was 98.5%. Then, after a little triethylamine was added as the catalyst, epoxy resin was slowly dropped into the mixture and stirred at 100 °C until the content of acid value was less than 5 mg KOH/g by titration. Three kinds of epoxy resin (E51, E78 and E132) were used in this reaction. The added epoxy functional group was 0.66 mol. It meant that the addition content of E51, E78 and E132 was 129.413 84.612 and 50.002 g, respectively. The conversion of the reaction of E51, E78 and E132 was 95.5, 95.6 and 95.8%, respectively. Third, the epoxy-modified polyurethane acrylate (E-PUA) was prepared by the reaction between the NCO-bearing adduct obtained from the first step and the modified epoxy resin (MP–OH) prepared from the second step in the presence of DBTDL as catalyst and HQMME as polymerization inhibitor. The reaction was carried out at 50 °C under nitrogen atmosphere until the content of isocyanate was less than 0.1 wt%. The conversion of the reaction for E51-PUA, E78-PUA and E132-PUA was 96.7, 97.0 and 97.5%, respectively. The synthesis process is described in Scheme 3.
Preparation of hyperbranched polyurethane acrylate
The hyperbranched polyurethane acrylate (HBPUA-OA) was synthesized via a three-step procedure. First, the NCO-bearing adduct was synthesized using the process described in Scheme 1. Second, the oleic acid-modified hyperbranched polyester (HBP-OA) was obtained. Briefly, TMP (13.417 g, 0.1 mol) and p-TSA (0.536 g) were added in a four-necked flask equipped with a stirrer, nitrogen inlet, a thermometer and a dropping funnel. After the mixture was melted at a temperature of 140 °C, DMPA (120.717 g, 0.9 mol) was added in batches to the melted mixture. The reaction was continued at 140 °C until the content of acid value was less than 10 mg KOH/g by titration. The conversion of the reaction was 97.2%. Then the temperature of the system was raised to 170 °C and the oleic acid was slowly dropped into the mixture. The reaction was continued until the content of acid value was less than 10 mg KOH/g by titration. The added oleic acid was 84.741 g (0.3 mol), 169.482 g (0.6 mol) and 254.223 g (0.9 mol) to obtain the different proportions of oleic acid-modified hyperbranched polyesters as HBP-25%OA (conversion: 95.6%), HBP-50%OA (conversion: 96.6%) and HBP-75%OA (conversion: 97.0%), respectively. Third, the hyperbranched polyurethane acrylate (HBPUA-OA) was prepared by the reaction between the NCO-bearing adduct obtained from the first step (70.486 g, 0.2 mol) and the oleic acid-modified hyperbranched polyester obtained from the second step with DBTDL as catalyst and HQMME as polymerization inhibitor. The reaction was carried out at 50 °C under nitrogen atmosphere until the content of isocyanate was less than 0.1 wt%. The conversion of the reaction for HBPUA-25%OA, HBPUA-50%OA and HBPUA-75%OA was 97.1, 96.1 and 95.2%, respectively. The synthesis process is described in Scheme 4.
Film preparation and UV curing
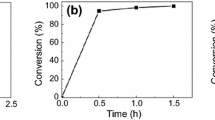
The curing experiments were performed in air. The prepared oligomer (50 wt%) with 45 wt% IBOA was mixed with 5 wt% Irgacure 184. The UV source used for irradiation was a UV lamp (INTELLI-RAY 400) made by Uvitron International Inc., USA. The light intensity was 100 mW cm−2. The time of exposure to UV irradiation of the different UV-curable coatings was just their tack-free time as in Fig. 4.
Characterization
Chemical analysis
The isocyanate content (NCO%) was determined according to the di-n-butylamine acetone method [38]. The content of acid value, epoxy value and hydroxyl value was determined according to the Chinese National Standards which was GB6743-86, GB/T4612-84 and GB/T12008.3-2009, respectively. The determination procedure can be found in the literature [39].
Fourier transform infrared spectra
Fourier transform infrared (FTIR) spectra were obtained on a Nicolet-750 FTIR spectrometer. The samples were pressed into potassium bromide pellets. The scanning range was from 4000 to 400 cm−1.
Properties of the cured films
Considering that the purpose of the modification is to meet the practical application in the tinplate, the properties of the coating should achieve the standard of the practical product. Therefore, the properties include pencil hardness, impact resistance, adhesion and boiling water resistance, measured according to the Chinese National Standards (GB) by some researchers [39–41]. Specifically, the thickness of the cured films was measured with a thickness gauge (Qua Nix 4500). The pencil hardness was tested according to GB/T 6739-1996 using a Pencil Hardness Tester (BGD 506/3). The impact resistance of the cured films (GB/T1732-1993) was measured with a DuPont Impact Tester (BGD 304). The adhesion of the cured films (GB1720-79) was tested with an automatic circle adhesion tester (BGD 501/2). All of the mentioned equipment was supplied from Biuged Laboratory Instruments (Guangzhou) Co., Ltd. In addition, the boiling water resistance of the film was tested in accordance with GB/T1877-2007.
Results and discussion
FT-IR analysis
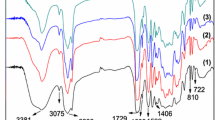
Figure 1 shows the FT-IR spectra of PUA (200), PUA (400) and PUA (600). Obviously, the peak for –NCO at 2275 cm−1 disappears, while the peak for N–H stretching vibration at 3337 cm−1 and the N–H bending vibration at 1535 cm−1 can be found, indicating that NCO-bearing adduct is reacted completely. The peaks at 1721 and 1112 cm−1 are assigned to C=O and –C–O–C–, respectively. Three absorption peaks are observed at around 1637, 1450 and 810 cm−1, indicating the existence of double bonds of the acrylate groups. The peaks at 2956 and 2871 cm−1 can be assigned to the stretching vibration for –CH2 and –CH3. These FT-IR assignments demonstrate that the polyether-modified polyurethane acrylate oligomers are successfully prepared.
Figure 2 shows the FT-IR spectra of E51-PUA, E78-PUA and E132-PUA. Some peaks in Fig. 2 are similar to Fig. 1. For example, the peak disappeared at 2275 cm−1 for –NCO indicating that the NCO-bearing adduct reacted completely, the peaks at 1620, 1450 and 810 cm−1 prove the existence of the double bonds of the acryl groups, and the peaks at 2956 and 2863 cm−1 belong to the stretching vibration for –CH2 and –CH3. Compared with Fig. 1, the much stronger absorption peak around 3387 cm−1 indicates that there is a certain amount of hydroxyl groups residual in the molecule. These FT-IR assignments demonstrate that the epoxy-modified polyurethane acrylate oligomers are successfully prepared.
Figure 3 shows the FT-IR spectra of HBPUA-25%OA, HBPUA-50%OA and HBPUA-75%OA. Similar absorption peaks as in Fig. 2 appeared indicating the presence of C=C, –OH, C=O, –C–O–C– and N–H groups. This in turn proves that the hyperbranched polyurethane acrylate oligomers are successfully prepared.
Properties of cured film
Tack-free time
Figure 4 shows the tack-free time of the UV-curable coating systems. The results indicate that the tack-free time depends on the content of the double bond structure of the systems. Benefit for the highest content of the double bond structure, the coating system consisting of epoxy-modified polyurethane acrylate has the minimal tack-free time. The same phenomenon could be also observed during the curing process of the same oligomer type with different degrees of modification. For example, the tack-free time of coating films cured with hyperbranched polyurethane acrylate system decreases in the order of HBPUA-25%OA, HBPUA-50%OA and HBPUA-75%OA, which just followed the content of the active double bond structure in the oligomer.
It should be noted that the double bond structure of maleic anhydride and oleic acid segments in the oligomer molecular have little effect on the tack-free time of the coating system. The main reason is that the activity of the double bond of maleic anhydride and oleic acid segments is much lower than that of the acrylate segment. In short, the increased content of the active double bond in the oligomer decides its tack-free time.
Pencil hardness and impact resistance
The pencil hardness and the impact resistance of coatings mainly depend on the chain flexibility of the molecule and cross-linking density of the coating [42]. Specifically, the improved flexible structure of the oligomer enhances the impact resistance of the film, but leads to reduction of hardness of the film. However, the improved cross-linking density of the coating plays the opposite effect. In our knowledge, it is very hard to improve the hardness and impact resistance at the same time by the modification of the oligomer. In general, the curing film with good flexibility and low cross-linking density represents the good impact resistance but poor pencil hardness, while the properties of good hardness and poor impact resistance would be obtained with poor flexibility and high cross-linking density. In our coating systems, the content of the double bond of the system determines the cross-linking density of the film. And the polyethylene glycol segment, maleic anhydride polyethylene glycol monoester segment and oleic acid segment present the flexible structure of polyether-modified polyurethane acrylate, epoxy-modified polyurethane acrylate and hyperbranched polyurethane acrylate, respectively.
Figure 5 shows the pencil hardness and impact resistance of coating films cured with different oligomers. The results demonstrate the previous discussion about the effect of the chain flexibility and cross-linking density on the property of pencil hardness and impact resistance. Figure 5 also indicates that the hardness and flexibility of the coating films can be controlled by adjusting the content of the double bond and the flexible structure of the coating systems.
Adhesion
The adhesion of the cured films is presented in Fig. 6. It indicates that the adhesion of the films formed by the epoxy-modified polyurethane acrylate and hyperbranched polyurethane acrylate are better than those formed by the polyether-modified polyurethane acrylate. The reason might due to the plenty of hydroxyl groups in the epoxy-modified polyurethane acrylate molecule and the hyperbranched polyurethane acrylate molecule. These hydroxyl groups can form the hydrogen bonding between the film and the tinplate, resulting in improving the adhesion of the film. At the same time, the increased content of the flexible chain segment in the oligomer molecule can reduce the shrinkage force during the curing process. It can also improve the adhesion of the cured film. Oleic acid acts as the flexible segment for the hyperbranched polyurethane acrylate. Therefore, the enhanced content of oleic acid in the hyperbranched polyurethane acrylate molecule can improve the adhesion of the film; however, it will reduce the hydroxyl group content in the coating at the same time. For these reasons, optimized adhesion could be preferred by adding the appropriate amount of oleic acid for the hyperbranched polyurethane acrylate.
Boiling water resistance
The boiling water resistance of the cured films is shown in the right side column of Table 1. It is observed that the cured films formed by the hyperbranched polyurethane acrylate system show the best boiling water resistance among the tested films. In our knowledge, the boiling water resistance is mainly dependent on the adhesion and the cross-linking density of the film. Good boiling water resistance can be obtained by films with good adhesion and high cross-linking density which can be supplied by the rich content of hydroxyl groups and double bond structure in the oligomer molecule of our UV-curable coating system. Therefore, due to their highest content of hydroxyl group and double bond structure among the tested oligomers, the films cured by hyperbranched polyurethane acrylate are found to have the best boiling water resistance.
Summary of the coating film properties
Table 1 summarizes the determined properties of the coating films formed by the oligomers prepared in this manuscript. The effects of the oligomer structure on the properties of the coating films have been discussed in the previous paragraph. It can be concluded that (a) the active double bond determines the tack-free time; (b) the chain flexibility and double bond content decide the hardness and impact resistance; (c) the hydroxyl group and the flexible chain segment in the oligomer molecule can improve the adhesion; and (d) the adhesion and cross-linking density affect the boiling water resistance. The film with good performance can be predicted and designed by modification of the oligomers obeying the above conclusions. Due to its appropriate molecular structure, the curing film formed by the HBPUA-50% OA as oligomer is observed to have the best comprehensive performance among all the films prepared in this research.
Conclusion
To reveal the relationship between the modified molecular structure and properties of the UV-curable polyurethane acrylate films used for inplate coating, nine modified urethane acrylate oligomers including polyether-modified polyurethane acrylate, epoxy-modified polyurethane acrylate and hyperbranched polyurethane acrylate were successfully synthesized in this study. The properties of the UV-curable films formed by these oligomers were determined. The effects of the molecular structure (e.g., active double bond, flexible chain segment, hydroxyl group) of the oligomers on the properties of cured film, which are tack-free time, pencil hardness, impact resistance, adhesion and boiling water resistance, are discussed. The results indicate that the film with good performance can be predicted and obtained by modifying the molecular structure of the oligomer. Among the oligomers synthesized in this paper, the coating film formed by HBPUA-50%OA as oligomer is observed to have the best comprehensive performance and exhibits a relatively short tack-free time, high hardness, good impact resistance great adhesion and boiling water resistance.
References
Seo J, Jang ES, Song JH, Choi S, Khan SB, Han H (2010) Preparation and properties of poly(urethane acrylate) films for ultraviolet-curable coatings. J Appl Polym Sci 118:2454–2460
Decker C (1998) The use of UV irradiation in polymerization. Polym Int 45:133–141
Jaworek T, Bankowsky HH, Koniger R, Reich W, Schrof W, Schwalm R (2000) Radiation curable materials—principles and new perspectives. Macromol Symp 159:197–204
Andrzejewska E (2001) Photopolymerization kinetics of multifunctional monomers. Prog Polym Sci 26:605–665
He JY, Zhou L, Soucek MD, Wollyung KM, Wesdekmiotis C (2007) UV-curable hybrid coatings based on vinylfunctionalized siloxane oligomer and acrylated polyester. J Appl Polym Sci 105:2376–2386
Khudyakov IV, Swiderski KW, Greer RW (2006) Structure-property relations in UV-curable urethane acrylate oligomers. J Appl Polym Sci 99:489–494
Patel HV, Raval JP, Patel PS (2009) Preparation and performance of UV curable polyurethane coating for metal surfaces. Arch Appl Sci Res 1:294–305
Lee BH, Kim HJ (2006) Influence of isocyanate type of acrylated urethane oligomer and of additives on weathering of UV-cured films. Polym Degrad Stabil 91:1025–1035
Inan TY, Ekinci E, Yildiz E, Kuyulu A, Gungor A (2001) Preparation and characterization of novel UV-curable urethane methacrylate difunctional monomers and their structure-property relationships, 1. Macromol Chem Phys 202: 532–540
Kwon JY, Yoo HJ, Kim HD (2001) Effect of chemical structure on the properties of UV-cured polyurethane acrylates films. Fibers and Polymers 2:141–147
Decker C, Moussa K (1993) Recent advances in UV-curing chemistry. J Coat Technol 65:49–57
Masson F, Decker C, Jaworek T, Schwalm R (2000) UV-radiation curing of waterbased urethane-acrylate coatings. Prog Org Coat 39:115–126
Milinaviciute A, Jankauskaite V, Narmontas P (2011) Properties of UV-curable hyperbranched urethane-acrylate modified acrylic monomer coatings. Mater Sci Medzg 17:378–383
Cheng XE, Liu SY, Shi WF (2009) Synthesis and properties of silsesquioxane-based hybrid urethane acrylate applied to UV-curable flame-retardant coatings. Prog Org Coat 65:1–9
Han WS, Lin BP, Yang H, Zhang XQ (2012) Synthesis and properties of UV-curable hyperbranched polyurethane acrylate oligomers containing carboxyl groups. Polym Bull 68:1009–1022
Han WS, Lin BP, Yang H, Zhang XQ (2013) Synthesis of UV-curable hyperbranched polyurethane (meth)acrylate oligomers via thiol-ene "click" chemistry. J Appl Polym Sci 128:4261–4270
Han WS, Lin BP, Zhou YD, Song JG (2013) Synthesis and properties of UV-curable hyperbranched polyurethane acrylate oligomers containing photoinitiator. Polym Bull 68:729–743
Han LJ, Dai JY, Zhang, LS, Ma SQ, Deng J, Zhang RY, Zhu J (2014) Diisocyanate free and melt polycondensation preparation of bio-based unsaturated poly(ester-urethane)s and their properties as UV curable coating materials. RSC Adv 4:49471–49477
Kim BS, Bae JE, Lee S, Kim DK, Rhee H (2012) Preparation and properties of photocurable, high refractive, 2-naphthol epoxy-modified urethane acrylate. Polym Bull 68:2097–2105
Kim D, Jang M, Seo J, Nam KH, Han H, Khan SB (2013) UV-cured poly(urethane acrylate) composite films containing surface-modified tetrapod ZnO whiskers. Compos Sci Technol 75:84–92
Sabani S, Onen AH, Gungor A (2012) Preparation of hyperbranched polyester polyol-based urethane acrylates and applications on UV-curable wood coatings. J Coat Technol Res 9:703–716
Mohamed HA, Badran BM, Rabie AM, Morsi SMM (2014) Synthesis and characterization of aqueous (polyurethane/aromatic polyamide sulfone) copolymer dispersions from castor oil. Prog Org Coat 77:965–974
Mohamed HA, Morsi SMM, Badran BM, Rabie AM (2016) Polyurethane/aromatic polyamide sulfone copolymer dispersions from transesterified castor oil. Polym Bull. doi:10.1007/s00289-016-1728-x
Grigale-Sorocina Z, Kalnins M, Simanovska J, Vindedze E, Birks I, Brazdauska E (2016) Effect of additives on UV-activated urethane acrylate polymerization composite coatings. Mater Sci Medzg 22: 54–59
Chun JH, Cheon JM, Jeong BY, Jo NJ (2016) The effect of 3-isocyanato-1-propene on adhesive properties of UV-curing urethane/siloxane acrylate resin. J Nanosci Nanotechnol 16:2687–2691
Tathe DS, Jagtap RN (2015) The effect of 3-isocyanato-1-propene on adhesive properties of UV-curing urethane/siloxane acrylate resin. J Coat Technol Res 12:187–196
Park JM, Jeon JH, Lee YH, Lee DJ, Park H, Chun HH, Do Kim H (2015) Synthesis and properties of UV-curable polyurethane acrylates containing fluorinated acrylic monomer/vinyltrimethoxysilane. Polym Bull 72:1921–1936
Mishra V, Mohanty I, Patel MR, Patel KI (2015) Development of green waterborne UV-curable castor oil-based urethane acrylate coatings: preparation and property analysis. Int J Polym Anal Charact 20:504–513
Grigale-Sorocina Z, Kalnins M, Simanovska J, Vindedze E, Birks I, Brazdauska E (2015) Additives in UV-activated urethane acrylate polymerization composite coatings. Proc Est Acad Sci 64:88–93
Park JM, Lee YH, Park H, Kim HD (2014) Preparation and properties of UV-curable fluorinated polyurethane acrylates. J Appl Polym Sci 131:40603
Najafi F, Bakhshandeh E, Hadavand BS, Saeb MR (2014) Toward UV-curable urethane acrylate/silica hybrid coatings: introducing urethane methacrylate trimethoxysilane (UAMS) as organic-inorganic coupling agent. Prog Org Coat 77:1957–1965
Eissa MM, Youssef MSA, Ramadan AM, Amin A (2013) Poly(ester-amine) hyperbranched polymer as toughening and co-curing agent for epoxy/clay nanocomposites. Polym Eng Sci 53:1011–1020
Eissa MM, Samy M, Ramadan AM, Amin A (2015) Amino-terminated hyperbranched polymer for toughness improvement of epoxy/clay nanocomposites. Polym Bull 72:3147–3168
Fang YQ, Wang D, Jing X, Xue BS (2015) Synthesis and characterization of fluorinated organic-inorganic hybrid coatings on tinplate. J Appl Polym Sci 132:42428
Liu JG, Xu L, Fang YQ (2014) Hybrid organic-inorganic sol-gel coatings with interpenetrating network for corrosion protection of tinplate. J Sol-Gel Sci Technol 71:246–253
Zumelzu E, Rull F (2003) Characterisation and performance of the protection of epoxyphenolic coatings on tinplates. Surf Coat Int Part B Coat Trans 86:203–207
Bao FF, Shi WF (2010) Synthesis and properties of hyperbranched polyurethane acrylate used for UV curing coatings. Prog Org Coat 68:334–339
Xiong J, Sun F, Du H (2007) Determination of isocyanate group in polyurethane by di-n-butylamine-acetone method. Chin J Anal Lab 26:73–76
Zhang LY, Jiao QZ, Zhao Y, Zhou MJ, Feng W, Ge YR (2011) Synthesis and application on UV-curable epoxy/dendritic maleate resin. Polym Bull 67:1583–1594
Lin JN, Zeng XR, Hou YJ, Shi HJ (2008) Synthesis and characterization of UV-curable hyperbranched urethane acrylate. Polym Plast Technol Eng 47:237–241
Gao Q.Z., Li HQ, Zeng XR (2011) Preparation and characterization of UV-curable hyperbranched polyurethane acrylate. J Coat Technol Res 8:61–66
Tasic S, Bozic B, Dunjic B (2004) Synthesis of new hyperbranched urethane-acrylates and their evaluation in UV-curable coatings. Prog Org Coat 51:321–328
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangsu Province (BK20130602), Collaborative Innovation Center of Suzhou Nano Science and Technology, the priority academic program development of Jiangsu higher education institutions and Jiangsu Himonia Technology Co., LTD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiao, Z., Wang, X., Yang, Q. et al. Modification and characterization of urethane acrylate oligomers used for UV-curable coatings. Polym. Bull. 74, 2497–2511 (2017). https://doi.org/10.1007/s00289-016-1847-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1847-4