Abstract
Polymer electrolyte (PE) composed of poly(vinylidene fluoride-co-hexafluoropropylene) P(VdF-co-HFP) and triethylsulfonium bis(trifluoromethylsulfonyl)imide (SEt3TFSI) ionic liquid (IL) had been evaluated in lithium ion battery for the first time in order to improve its performance and cycle life. X-ray diffraction analysis (XRD) reveals that incorporation of the IL (20 and 25 wt%) into the polymer matrix results in the change of state of the material from semi-crystalline to amorphous nature. Thermo-gravimetric and differential thermal analysis (TG/DTA) of the PE sample with 25 wt% of the IL shows high thermal stability. The nature of functional groups present in the PE was investigated by Raman spectrum. Surface morphological characteristics indicate that increase in the loading of the IL into the polymer matrix leads to maximum number of pores with good interconnected network. Polymer/IL electrolyte (wt. ratio of 75:25) having a maximum ionic conductivity of 6.93 × 10−5 S/cm at 303 K with an activation energy of 0.23 eV shows excellent electrochemical potential stability of 4.4 V vs Li, as revealed by cyclic voltammetry (CV). Charge–discharge characteristics of the coin cell containing the above optimized ratio of PE with LiFePO4 cathode and Li anode shows a discharge capacity of 133 mAh/g, which is stable up to ten cycles.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Employment of polymer electrolytes in lithium ion battery has been widely investigated as an alternative to liquid combustible systems for their particular properties such as high mechanical stability, non-inflammability and non-leakage [1–5] and their usage avoids the evolution of gaseous materials during solvent decomposition. Further, Li ion battery can be made very compact, lightweight and highly reliable with the incorporation of thin film electrolytes. It is important to note that lithium ion polymer batteries had been proposed since early 1980s as being a potential solution to the safety issues. On the other hand, these polymer electrolyte materials have very poor ionic conductivities at room temperature (i.e. <10−5 S/cm), resulting in the limitations on their viability by significantly lowering the amount of current that can be drawn from a battery. In order to improve their room temperature ionic conductivity, alternative approaches have been developed by the incorporation of liquid plasticizers or low molecular weight polymers [6, 7], black copolymers [8], high conductivity inorganic nanofillers [9] and room temperature ionic liquids (RTILs) [10–12]. Among these, RTILs offer a promising approach for overcoming these drawbacks, which can act both as solvent and electrolyte, into the polymer electrolytes.
The RTILs have recently been considered as alternative electrolytes to carbonate based systems because they possess high oxidation potential, non-flammability, a low vapor pressure, good thermal stability, low toxicity and affordable boiling points [13–15]. Additionally, ionic liquids play dual character as electrolyte salts and organic liquids and are eco friendly in nature [16]. The absence of volatility is one of the most important benefits of ionic liquids, offering less toxicity, when compared to low boiling solvents. Hence, these salts are indeed extraordinary safety assets and the replacement of the conventional, flammable and volatile organic solvents by ionic liquid based electrolytes may greatly reduce the risk of thermal runaways. Imidazolium [17–20], piperidinium [21–24] and pyrrolidinium cation [25–28] based ILs are highly conducting and used as electrolytes for Li ion battery. However, problems associated with these ILs are their instability at low voltages and intercalation into the graphite anode resulting in exfoliation as well as rapid capacity fade. Moreover, pyrrolidinium cation based ILs are poor solvents for lithium and show limited lithium conductivity values. Hence, a different strategy has been adopted by the incorporation of IL into polymer matrix, thereby enhancing their ionic conductivity and electrochemical stability and this can meet performance standards of a battery electrolyte with good capacity and cycle life. Among the different polymers employed so far, fluorinated polymer namely PVDF-co-HFP [poly(vinylidene fluoride-co-hexafluoropropylene)] has been widely employed as an excellent polymer matrix, on account of their good thermal and oxidative stability. Moreover, it has good mechanical stability and film formation ability and concomitant low surface energy, due to presence of fluorine network, justifying it is choice for IL-based polymer electrolyte (PE). Recently, Hwang et al. have reviewed the employment of PVDF-co-HFP PE matrices incorporated with different imidazolium cations based ILs on their synthetic pathways, ion sources, IL contents and proton conductivities [29]. The mechanical and dimensionally stable solid electrolyte containing ionic liquid has comparable conductivity and electrochemical stability with that of liquid electrolytes [30–32]. Literature studies clearly reveal that most of the research work involving ILs incorporated into PVDF–HFP matrix are restricted to nitrogen based structures and recent investigations demonstrate that sulfur and phosphorous based systems have showed superior electrochemical stability and conductivity values, when compared to latter [33, 34]. In particular, sulfonium based ionic liquids received great attention owing to their low viscosity, high conductivity and electrochemical stability [35, 36]. Zhang et al. [31] reported that the ethyl based sulfonium ionic liquid possessed higher conductivity, when compared to methyl and butyl alkyl groups. Further, the nature of the anion influences the viscosity of the ionic liquid. For example, TFSI anion exhibits high charge delocalization within the S–N–S backbone, resulting in low viscosity [37, 38]. Moreover, ionic liquid containing TFSI anion is highly hydrophobic in nature and has low moisture sensitive and exhibits high thermal stability, molar conductivity and electrochemical stability with low viscosity, and much safer than the conventional organic electrolytes [39, 40].
In the present work, PE containing PVdF-co-HFP incorporated with triethylsulfonium bis(trifluoromethylsulfonyl) imide (SEt3TFSI) ionic liquid, which has not been reported previously for Li battery application, has been tried as the electrolyte for improving its performance. The physico-chemical properties and electrochemical characteristics of the thin film are presented. The optimal ratio of PVDF-co-HFP and ILs are carefully investigated which facilitates the improvement of the interphase stability between the electrolyte and anode material.
Experimental
Preparation of IL incorporated polymer electrolyte (PE)
All the electrolytes have been prepared using the solution casting technique. PVdF-co-HFP and ionic liquid SEt3TFSI were purchased from Aldrich USA. Tetrahydrofuran (THF) and N-methyl 2 pyrrolidone (NMP) was purchased from SRL India and used without further purification. Required quantity of polymer was dried under vacuum (1 × 10−3 Torr) at 100 °C for 10 h in order to remove the moisture. It was completely dissolved in THF and calculated amount of ionic liquid was mixed with the polymer solution, which was stirred continuously in order to obtain homogeneity. The homogeneous solution was casted onto flat bottom petri plates. Then the solution was dried to form film at 30 °C in the vacuum oven for overnight. Further, the films were dried at 60 °C under vacuum for 5 h in order to get the flexible freestanding film. Table 1 shows the different wt% of IL incorporated PE.
Characterizations of thin film PE
The film was characterized for its crystal structure by X-ray diffraction analysis using the PANalytical X’Pert PRO powder X-ray Diffractometer using Cu-Kα radiation as source and operated at 40 kV. Laser Raman spectra were done with STR 500 Laser Raman spectrometer, SEKI, Japan. Thermo-gravimetric analysis of the gel electrolyte was performed using STA 409 PL Luxx at a heat rate of 10 K/min within the temperature range from room temperature to 900 °C under nitrogen atmosphere. The surface morphology of the electrolytes was characterized by SEM Model JEOL-JSM-6500F scanning electron microscope at an accelerating voltage of 5 and 15 kV after sputtering platinum over the samples and AFM with Agilent Technology Inc., (A100SGS).
Electrochemical studies
Two stainless electrodes (2 cm × 2 cm) were used for conductivity measurement, where the electrolyte was sandwiched between the parallel blocking electrodes. The impedance spectra were measured in the frequency and the temperature ranges from 1 Hz to 500 kHz and 303 to 353 K, respectively, using a computer-controlled micro Autolab III Potentiostat/Galvanostat. The evaluated ohmic resistance value was converted into conductivity. The electrochemical studies were carried out using Autolab electrochemical workstation (GPES, PGSTAT 302 N) and the charge/discharge life cycle was carried out using WonAtech (WBCS3000S) automatic charge/discharge testing system. For linear voltammetric studies, a CR-2032 coin cell was fabricated with Li metal as counter as well as reference electrodes and stainless steel as working with the IL-incorporated PE. The coin cell for the electrochemical characterization had been assembled with the electrode composed of 80:10:10(LiFePO4: PVdF: Super p carbon). The slurry was made using NMP and was coated on alumina foil. After coating, the electrodes were dried at 80 °C for about 6 h. The mass of the active substance was nearly 1 mg.
Results and discussion
X-ray diffraction (XRD)
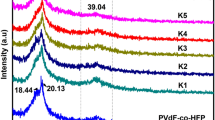
The crystalline peak properties of IL incorporated PVDF-co-HFP were characterized by XRD. Figure 1 shows the XRD diffraction pattern of the prepared polymer electrolyte (PE) samples containing different wt% of IL such as 5, 10, 15, 20, 25, denoted as PE-IL1, PE-IL2, PE-IL3, PE-IL4 and PE-IL5. The pure PVdF-co-HFP is a semi-crystalline polymer and there are two broad peaks at 20.4° and 39.4° corresponding to its crystalline planes [41]. With the incorporation of IL into the polymer matrix, the peak intensity of both the peaks decreases (sample IL1). Further addition of IL makes the peak weak and wide (PE-IL2 and PE-IL3). For both PE-IL4 and PE-IL5 samples, the high-intensity region peak disappears and the peak noted at a 2θ value of 20.4 becomes low, which reveals that the high amount of ionic liquid plasticizers the electrolyte and produces more amorphous phase in the polymer network [42, 43].
Raman spectroscopy
Raman spectroscopy is particularly appropriate to characterize PE and the bond present in the spectrum corresponds to vibrational frequency of the molecules. Figure 2 represents such spectra for different PE samples. Pure polymer PVdF-co-HFP shows the major Raman peak at 795 cm−1 assigned to CH2 ν rocking and the rocking mode of CF2 and CH2 at 413 cm−1 disappears in all the electrolyte samples, as a result of the effect of addition of the SEt3TFSI [44]. Another major peak of 877 cm−1 corresponds to the combination of symmetric C–C and CCCδ skeletal bending of C(F)–C(H)–C(F) of the host polymer PVdF-co-HFP is shifted to high frequency value of 886 cm−1 reflecting the major change in the bare polymer. A peak at 600 cm−1 indicates the CF scissoring CCCδ skeletal bending of C(H)–C(H)–C(F) [40]. The CH2 ν rocking (832 cm−1), ν s symmetric stretching (1424, 2976 cm−1) and ν s anti-symmetric stretching (3011) are also noted in the Raman spectrum. The most intense band of the anion appearing at 741 cm−1 was attributed to the CF3 bending of the SCF3 group of TFSI [45]. The SO2 band of NSO2CF3 is out of phase anti-symmetric stretching, located at 1335 cm−1. Bands specifically associated with TFSI− are still observed at 1125 cm−1 with ν s symmetric stretching. The peak at 1046 cm−1 has been noted as ν a SNS anti-symmetric stretching vibration of triflate anion [46].
Raman spectra of pure PVdF-co-HFP, the PEs namely PE-IL1, PE-IL2, PE-IL3, PE-IL4 and PE-IL5. The PE compositions are the same as in Fig. 1
Surface morphologic studies using scanning electron microscope (SEM) and atomic force microscope (AFM)
Figure 3 shows the SEM images obtained for different PE samples containing various IL contents. The micropores noted in the SEM images are directly related to its conductivity values. The scanning images of the PE samples differ from their number of pores, pore size and uniform distribution of micropores. The appearance of pores corresponds to the process associated with the evaporation of the THF solvent during vacuum oven drying. The increased number of pores enhances the absorption of non-volatile liquid electrolyte, leading to high conductivity of PE [12, 47]. The sample PE-IL1 [PVDF-co-HFP:SEt3TFSI (95:5 wt%)] shows granular shape indicating the semiconducting nature of the polymer. Further incorporation of the IL content into the polymer results in the decrease of the pore size as well increase of number of pores with interconnected network and increase of amorphous nature of the PE [48]. Highly amorphous nature leads to high conductivity and large amount of absorbed IL. From the SEM pictures, it is observed that the PE-IL5 (PVDF-co-HFP:SEt3TFSI (75:25 wt%) provides a well closed interconnected network with maximum number of pores.
SEM images of the PEs namely PE-IL1, PE-IL2, PE-IL3, PE-IL4 and PE-IL5 with the magnification of 1 K. The PE compositions are the same as in Fig. 1
Based on the SEM analysis, surface topography of the sample PE-IL5 was characterized by AFM and is shown in Fig. 4. From the figure, it appears that homogeneous nature of the PE had been found to be enhanced with the addition of IL electrolyte [49], where this can increase the contact between the electrolyte and electrode. The topographical image shows large number of pores, which is responsible for the ion migration.
Conductivity studies
Impedance measurement was performed for finding out the ionic conductivity of the polymer electrolytes starting from PE-IL1 to PE-IL5 films at different temperatures starting from 303 to 353 K with an increment of 10 K and the spectra are shown in Fig. 5. For both PE-IL1 and PE-IL2, the impedance spectra shows a semicircle, which is due to the effect of bulk resistance [50]. It is noted that for the PE with low ionic liquid content (5 and 10 wt%) follows a solid polymer electrolyte (SPE) behavior. The increase of the ionic liquid (15 and 20 wt%) results in a depressed semicircle with a spike. This is due to the effect of electrolyte/blocking electrode interface. Further increase of the ionic liquid content (25 wt%) results in a complex impedance plot with a spike. Low-frequency straight line towards the real axis is caused by the effect of the capacitive electrode behavior. The disappearance of semicircle in high content of ionic liquid is due to the decrease of ionic resistance of the electrolyte. This causes high degree of disorder in the polymer electrolyte, favoring high ionic transport. The semicircle in the high-frequency range is related to the conduction process in the bulk of the complex and the linearity in the low-frequency region is due to the effect of blocking electrode.
Room temperature complex impedance plot of PEs namely PE-IL1, PE-IL2, PE-IL3, PE-IL4 and PE-IL5. The PE compositions are the same as in Fig. 1
Conductivity values of the different contents IL incorporated polymer had been measured from the following equation:
where σ is the ionic conductivity, l is the thickness of the electrolyte sample, A is the area of the prepared electrolyte, R b is the bulk resistance. The conductivity values for the samples from PE-IL1 to PE-IL5 at different temperatures are shown in Table 1. From the table, it is noted that with the increase of ionic liquid content, the conductivity of the PE increases linearly and the polymer electrolyte with 25 wt% ionic liquid (PE-IL5) has the maximum ionic conductivity of 6.93 × 10−5 S/cm. Further the conductivity also increases with the increase of temperature from 303 to 353 K, as revealed from the plot of log conductivity versus inverse temperature (1000/T) for different contents of IL (Fig. 6) and this may be correlated with the influence of internal activation of molecules [48]. The activation energy (E a) values were calculated from the slop of the straight line. A low activation energy (E a = 0.23 eV) had been obtained for the sample having maximum ionic conductivity. Decrease in the E a value suggests more amorphous nature of the polymer electrolyte, as shown in Table 1. A similar trend in the increase of conductivity value with temperature is also noted for the IL incorporated polymers [29]. Typically, the addition of polymers to liquid electrolytes containing lithium salts to form gels results in a significant drop in the ionic conductivity [51, 52]. It is noted that the increase in the electrolyte conductivity is due to the addition of a liquid salt with ions possessing plasticizing nature that have only a weak interaction with the polymer, leading to easy migration of ions. Further, the enhanced conductivity may also be associated with large number of charge carriers for the ionic transport, where the large size of imide anion discourages the ion pair formation [53]. Ionic transport is caused by the diffusion of carrier ions through the free volume of polymer matrix so that the large size of the imide disperses the carrier ions in the polymer domain at the molecular level, inducing high conductivity.
Thermal analysis
Thermo-gravimetric and differential thermal analysis (TG/DTA) of the samples of pure PVdF-co-HFP, Pure IL, PE-IL4 and PE-IL5, having higher conductivity then the others, are shown in Fig. 7. Thermal analysis shows three types of response in the temperature range between room temperature and 900 °C. At first stage, a small weight loss around 70 °C is responsible for the evaporation of the moisture at the time of loading of the sample. It is well known from the literature that the IL is stable up to 240 °C. The TG graph of the polymer electrolyte confirms its stability up to 440 °C. Significant decomposition at 240 °C represents the thermal stability of IL with the mass change of 19 wt%. After 500 °C, a gradual decrease of weight is observed and at 900 °C, a residual mass of 19 wt% remains due to the presence of carbon in the polymer electrolyte. When compared to PE-IL4 electrolyte, PE-IL5 however, shows better thermal stability. The results noted in the DTA results are in good agreement with the TG [54]. In the temperature regions of 250, 450 and 600 °C, the exothermic curve shows the decomposition of the PE. The TGA and DTA results infer that PE containing PVdF-co-HFP:SEt3TFSI (75:25, PE-IL5) possess high thermal stability [55] and this composition had been investigated for further characterization related to voltammetry and charge–discharge studies.
TG/DTA plot for the PEs namely PE-IL4 and PE-IL5. The PE compositions are the same as in Fig. 1
Electrochemical characterization
Linear sweep voltammetry
The electrochemical stability window of the PE (PE-IL5) was studied using linear sweep voltammetry (LSV) and the voltammogram recorded at a scan rate of 5 mV/s is shown in Fig. 8. From the figure, it can be seen that until 4.5 V vs Li, the current flow is found to be negligible and after that it increases sharply with applied voltage, indicating the decomposition of the PE. [56]. This shows that the PE is stable up to the potential region of 4.4 V vs Li.
Cyclic voltammetry (CV)
The electrochemical characteristics of the coin cell containing LiFePO4 and the PE (PE-IL5) were studied using CV. Figure 9 shows the first, second and third cycle of the CV for the polymer electrolyte at a scan rate of 5 m/Vs within a voltage range of 2.4–4.4 V vs Li. An anodic and a cathodic peak appear at a potential of 3.55 and 3.3 V vs Li [57], respectively, indicating the strong reversible behavior of the electrochemical system. The insert shows the magnified view of anodic peak. Multiple scan for the three cycles shows the overlapping of the curves, which may be associated with the reversibility [58]. Beyond 4.5 V, the anodic current rises due to the decompositions of the polymer electrolyte and further decrease in the current for the second and third cycle indicates the formation of solid electrolyte interface (SEI) on the electrode surface [59]. The SEI formation prevents further reaction of SEt3TFSI with the lithium electrode.
Charge–discharge profile
The charge–discharge characteristics of the coin cell containing LiFePO4 cathode and the PE (PE-IL5) were investigated and capacity vs voltage curves recorded for ten cycles for such cell is shown in Fig. 10. The cell gives excellent initial discharge capacity of 133 mAh/g with the working voltage of 3.40 V. The charge–discharge profile shows very flat voltage range of 3.39 V in the first cycle and a similar pattern is followed for other cycles, reflecting good electrochemical properties. The discharge capacity in the subsequent cycles slowly decreases with the increase of cycle numbers. The capacity decrease is mainly attributed to the formation of passive layer on the cathode material. The cell delivers good reversibility with discharge capacity of 122 mAh/g at 10th cycle.
Conclusions
The PVdF-co-HFP containing different weight percentages of IL, SEt3TFSI had been prepared and characterized. The XRD results confirm the appearance of amorphous nature of the substance with the addition of IL (20 and 25 wt %) to the polymer matrix and the PE is stable up to 450 °C, as revealed by TG/DTA analysis. The nature of different functional groups present PE had been identified using Raman spectroscopy. The maximum number of pores with good interconnected network leads to high ionic conductivity, as evidenced by SEM and AFM images. Polymer/IL electrolyte (wt. ratio of 75:25, PE-IL5) with a maximum ionic conductivity of 6.93 × 10−5 S/cm and activation energy of 0.23 eV shows an excellent electrochemical potential stability of 4.4 V vs Li, as revealed from LSV and CV. A discharge capacity of 133 mAh/g was obtained for the coin cell containing PE-IL5 electrolyte and LiFePO4 as the cathode, which is stable up to ten cycles.
References
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Yuan F, Chen HZ, Yang HY, Li HY, Wang M (2005) PAN–PEO solid polymer electrolytes with high ionic conductivity. Mater Chem Phys 89:390
An YX, Zuo PJ, Cheng XQ, Liao LX, Yin GP (2011) The effects of LiBOB additive for stable SEI formation of PP13TFSI-organic mixed electrolyte in lithium ion batteries. Electrochim Acta 56:4841–4848
Brissot C, Rosso M, Chazalviel J-N, Lascaud S (1999) Dendritic growth mechanisms in lithium/polymer cells. J Power Sources 81–82:925–929
Farrington MD (2001) Safety of lithium batteries in transportation. J Power Sources 96:260–265
Chintapalli S, Frech R (1996) Effect of plasticizers on ionic association and conductivity in the (PEO) 9LiCF3SO3 system. Macromolecules 29:3499
Saito Y, Stephan AM, Kataoka H (2003) Ionic conduction mechanisms of lithium gel polymer electrolytes investigated by the conductivity and diffusion coefficient. Solid State Ionics 160:149
Ghosh A, Kofinas P (2008) Nanostructured block copolymer dry electrolyte. J Electrochem Soc 155:A428–A431
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456
Shin JH, Henderson WA, Passerini S (2005) PEO-based polymer electrolytes with ionic liquids and their use in lithium metal-polymer electrolyte batteries. J Electrochem Soc 152:A978–A983
Shin JH, Henderson WA, Passerini S (2005) An elegant fix for polymer electrolytes. Electrochem Solid State 8:A125–A127
Shin JH, Henderson WA, Passerini S (2003) Ionic liquids to the rescue? Overcoming the ionic conductivity limitations of polymer electrolytes. Electrochem Commun 5:1016–1020
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Fisher AS, Khalid MB, Widstrom M, Kofinas P (2012) Anion effects on solid polymer electrolytes containing sulfur based ionic liquid for lithium batteries. J Electrochem Soc 159(5):A592–A597
Xiong S, Xie K, Blomberg E, Jacobsson P, Matic A (2014) Analysis of the solid electrolyte interphase formed with an ionic liquid electrolyte for lithium–sulfur batteries. J Power Sources 252:150–155
Deraman K, Mohamed NS, Subban RHY (2013) Conductivity and electrochemical studies on polymer electrolytes based on poly vinyl (chloride)–ammonium triflate–ionic liquid for proton battery. Int J Electrochem Sci 8:1459–1468
Yang P, Cui W, Li L, Liu L, An M (2012) Characterization and properties of ternary P (VdF–HFP)–LiTFSI–EMITFSI ionic liquid polymer electrolytes. Solid State Sci 14:598–606
Sekhon SS, Lalia BS, Park J-S, Kim CS, Yamada K (2006) Physicochemical properties of proton conducting membranes based on ionic liquid impregnated polymer for fuel cells. J Mater Chem 16:2256
Fernicola A, Panero S, Scrosati B, Tamada M, Ohno H (2007) New types of Brönsted acid-base ionic liquids-based membranes for applications in PEMFCs. Chem Phys Chem 8:1103
Sutto TE (2007) Hydrophobic and hydrophilic interactions of ionic liquids and polymers in solid polymer gel electrolytes. J Electrochem Soc 154:P101–P107
Kim K, Cho Y-H, Shin H-C (2013) 1-Ethyl-1-methyl piperidinium bis(trifluoromethanesulfonyl)imide as a co-solvent in Li-ion batteries. J Power Sources 225:113–118
Baranchugov V, Markevich E, Pollak E, Salitra G, Aurbach D (2007) Amorphous silicon thin films as a high capacity anodes for Li-ion batteries in ionic liquid electrolytes. Electrochem Commun 9:796–800
Markevich E, Baranchugov V, Salitra G, Aurbach D, Schmidt MA (2008) Behavior of graphite electrodes in solutions based on ionic liquids in in situ Raman studies. J Electrochem Soc 155(2):A132–A137
Gao K, Song X-H, Shi Y, Li S-D (2013) Electrochemical performances and interfacial properties of graphite electrodes with ionic liquid and alkyl-carbonate hybrid electrolytes. Electrochim Acta 114:736–744
Kaga Y, Katayama Y, Miura T, Komaba S (2010) Anode reactions of a tin thin film electrode modified with an ion-conductive polymer in a room-temperature ionic liquid electrolyte. ECS Trans 25(36):91–98
Hassoun J, Fernicola A, Navarra MA, Panero S, Scrosati B (2010) An advanced lithium-ion battery based on a nanostructured Sn–C anode and an electrochemically stable LiTFSi-Py 24 TFSI ionic liquid electrolyte. J Power Sources 195:574–579
Lux SF, Schmuck M, Jeong S, Passerini S, Winter M, Balducci A (2010) Li-ion anodes in air-stable and hydrophobic ionic liquid-based electrolyte for safer and greener batteries. Int J Energy Res 34:97–106
Lewandowski A, Swiderska-Mocek A (2009) Properties of the lithium and graphite–lithium anodes in N-methyl-N-propylpyrrolidinium bis (trifluoromethanesulfonyl) imide. J Power Sources 194:502–507
Ye Y-S, Rick J, Hwang B-J (2013) Ionic liquid polymer electrolytes. J Mater chem A 1:2719
Singh PK, Bhattacharya B, Mehra RM, Rhee HW (2011) Plasticizer doped ionic liquid incorporated solid polymer electrolytes for photovoltaic application. Curr Appl Phys 11:616–619
Zhang Q, Liu S, Li Z, Li J, Chen Z, Wang R, Lu L, Deng Y (2009) Novel cyclic sulfonium-based ionic liquids: synthesis, characterization, and physicochemical properties. Chem Eur J 15:765–778
Anuar NK, Subban RHY, Mohamed NS (2012) Properties of PEMA–NH4CF3SO3 added to BMATSFI ionic liquid. Materials 5:2609–2620
Matsumoto H, Matsuda T, Miyazaki Y (2000) Room temperature molten salts based on trialkylsulfonium cations and bis(trifluoromethylsulfonyl) imide. Chem Lett 29:1430–1431
Tsunashima K, Sugiya M (2007) Physical and electrochemical properties of low-viscosity phosphonium ionic liquids as potential electrolytes. Electrochem Commun 9:2353–2358
Zhao D, Fei Z, Ang WH, Dyson PJ (2007) Sulfonium-based ionic liquids incorporating the allyl functionality. Int J Mol Sci 8:304–315
Fisher AS, Khalid MB, Widstrom M, Kofinas P (2011) Solid polymer electrolytes with sulfur based ionic liquid for lithium batteries. J Power Sources 196:9767–9773
Hapiot P, Lagrost C (2008) Electrochemical reactivity in room-temperature ionic liquids. Chem Rev 108:2238–2264
Sirisopanaporn C, Fernicola A, Scrosati B (2009) New, ionic liquid-based membranes for lithium battery application. J Power Sources 186:490–495
Kim GT, Jeong SS, Xue MZ, Balducci A, Winter M, Passerini S, Alessandrini F, Appetecchi GB (2012) Development of ionic liquid-based lithium battery prototypes. J Power Sources 199:239–249
Liew CW, Ong YS, Lim JY, Lim CS, Teoh KH, Ramesh S (2013) Effect of ionic liquid on semi-crystalline poly(vinylidene fluoride-co-hexafluoropropylene) solid copolymer electrolytes. Int J Electrochem Sci 8:7779–7794
Wu F, Feng T, Bai Y, Wu C, Ye L, Feng Z (2009) Preparation and characterization of solid polymer electrolytes based on PHEMO and PVDF–HFP. Solid State Ionics 180:677–680
Yu B, Zhou F, Wang C, Liu W (2007) A novel gel polymer electrolyte based on poly ionic liquid 1-ethyl 3-(2-methacryloyloxy ethyl) imidazolium iodide. Eur Polym J 43:2699–2707
Singh PK, Sabin KC, Chen X (2016) Ionic liquid–solid polymer electrolyte blends for supercapacitor applications. Polym Bull 73:255–263
Aravindan V, Vickraman P, Krishnaraj K (2008) Lithium difluoro (oxalate) borate-based novel nanocomposite polymer electrolytes for lithium ion batteries. Polym Int 57:932–938
Kim JK, Matic A, Ahn JH, Jacobsson P (2010) An imidazolium based ionic liquid electrolyte for lithium batteries. J Power Sources 195:7639–7643
Duluard S, Grondin J, Bruneel JL, Campet G, Delville M-H, Lassegues J-C (2008) Lithium solvation in a PMMA membrane plasticized by a lithium-conducting ionic liquid based on 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide. J Raman Spectroscopy 39:1189–1194
Han HS, Kang HR, Kim SW, Kim HT (2002) Phase-separated polymer electrolyte based on poly(vinyl chloride)/poly(ethyl methacrylate) blend. J Power Sources 112:461–468
Ramesh S, Liew C-W, Ramesh K (2011) Evaluation and investigation on the effect of ionic liquid onto PMMA–PVC gel polymer blend electrolytes. J Non Cryst Solids 357:2132–2138
Jung H-R, Ju D-H, Lee W-J, Zhang X, Kotek R (2009) Electrospun hydrophilic fumed silica/polyacrylonitrile nanofiber-based composite electrolyte membranes. Electrochem Acta 54:3630–3637
Saikia D, Chen-Yang YW, Chen YT, Li YK, Lin SI (2008) Investigation of ionic conductivity of composite gel polymer electrolyte membranes based on P (VDF–HFP), LiClO4 and silica aerogel for lithium ion battery. Desalination 234:24–32
Sekhon SS (2003) Conductivity behaviour of polymer gel electrolytes: role of polymer. Bull Mater Sci 26:321–328
Abraham KM, Alamgir M (1993) Ambient temperature rechargeable polymer-electrolyte batteries. J Power Sources 43:195–208
Stephan AM, Kumar SG, Renganathan NG, Kulandainathan MA (2005) Characterization of poly(vinylidene fluoride–hexafluoropropylene)(PVdF–HFP) electrolytes complexed with different lithium salts. Eur Polym J 41:15–21
Gerbaldi C, Nair JR, Ahmad S, Meligrana G, Bongiovanni R, Bodoardo S, Penazzi N (2010) UV-cured polymer electrolytes encompassing hydrophobic room temperature ionic liquid for lithium batteries. J Power Sources 195:1706–1713
Missan HPS, Lalia BS, Karan K, Maxwell A (2010) Polymer–ionic liquid nano-composites electrolytes: electrical, thermal and morphological properties. Mater Sci Eng B 175:143–149
Noor ISM, Majid SR, Arof AK, Djurado D, Neto SC, Pawlicka A (2012) Characteristics of gellan gum–LiCF3SO3 polymer electrolytes. Solid State Ionics 225:649–653
Ye H, Huang J, Xu JJ, Khalfan A, Greenbaum SG (2007) Li ion conducting polymer gel electrolytes based on ionic liquid/PVDF–HFP blends. J Electrochem Soc 154:A1048–A1057
Raghavan P, Zhao X, Manuel J, Chauhan GS, Ahn JH, Ryu H-S, Ahn H-J, Kim K-W, Nah C (2010) Electrochemical performance of electrospun poly(vinylidene fluoride-co-hexafluoropropylene)-based nanocomposite polymer electrolytes incorporating ceramic fillers and room temperature ionic liquid. Electrochim Acta 55:1347–1354
Nittani T, Shimada M, Kawamura K, Dokko K, Rho Y-H (2005) Synthesis of Li+ ion conductive PEO–PSt block copolymer electrolyte with microphase separation structure. Electrochem solid State Lett 8:A385–A388
Acknowledgments
The author M. Sivakumar gratefully acknowledges for the financial support to carry out this work by University Grants Commission (UGC), New Delhi, Govt. India, under major research project (F.No.41-839/2012(SR)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthupradeepa, R., Sivakumar, M., Subadevi, R. et al. Sulfonium cation based ionic liquid incorporated polymer electrolyte for lithium ion battery. Polym. Bull. 74, 1677–1691 (2017). https://doi.org/10.1007/s00289-016-1796-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1796-y