Abstract
Antibiotic resistance of bacteria is causing clinical and public health concerns that are challenging to treat. Infections are becoming more common in the present era, and patients admitted to hospitals often have drug-resistant bacteria that can spread nosocomial infections. Urinary tract infections (UTIs) are among the most common infectious diseases affecting all age groups. There has been an increase in the proportion of bacteria that are resistant to multiple drugs. Herein is a comprehensive update on UTI-associated diseases: cystitis, urethritis, acute urethral syndrome, pyelonephritis, and recurrent UTIs. Further emphasis on the global statistical incidence and recent advancement of the role of natural products in treating notorious infections are described. This updated compendium will inspire the development of novel phycocompounds as the prospective antibacterial candidate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections from multidrug-resistant (MDR) bacteria, mycobacteria, and fungi are wearisome health problems, causing several comorbidities, even physical disabilities from Hansen’s disease and ultimate mortalities [1]. Urinary tract infections (UTIs) are among the most commonplace infectious diseases [2], affecting all age groups, and even children were seen infected in hospitals and communities [3]. It would not be out of place to state that every female gets a UTI at least once in her lifetime. It is often infected in adult females with a challenged immune system. The short physical distance between vaginal and rectal openings is the contrivance to super-infections from rectal microbiota [2]. About 150 million people are diagnosed with UTIs yearly [4].
The occurrence of any infection depends on several factors, such as improper functioning of the immune system and the bacterial/fungal virulent status, i.e., MDR strains of infectious bacteria that survive in the body. The most common causative agents of UTI-MDR bacteria are Escherichia coli, Staphylococcus aureus, and Acinetobacter baumannii [5]. Thus, it became resistant to the most common antibacterial, which no longer remains in mainstream medicine today; therefore, chemical modifications were done [1, 6].
Moreover, the bloodstream infection leads to the spread of the infecting bacteria to all innards through blood, wherein those colonize; sometimes, the bacterial growth leads to toxic shock syndrome (TSS) and bacteremia (Fig. 1) [7, 8]. As it is known, both TSS and bacteremia lead to fatality without a timely use of some suitable antibacterial(s), assessed from the MDR nature of bacterial strains [9]. Staphylococcus sp., such as S. aureus or S. saprophyticus, is the second most common genus of the causative bacteria of acute UTI, after E. coli, and it accounts for 5–15% of the reported UTIs and usually affects younger women [10]. Indeed, Staphylococcus sp. is mostly a cutaneous infection that can spread to the urethra through sexual intercourse. Moreover, the complicated UTIs were attributed to Klebsiella sp.; pregnant women are more prone to this infection because the enlarged uterus blocks the required free urinary passage to wash out the infecting bacterial microbiota [11] (Fig. 2).
Recent trends in the study of plant products and the development of new drugs have established natural products as a significant source of druggable compounds against multiple diseases [12]. It is a common belief that natural medicines are usually potentially less toxic than their synthetic-prepared drugs [1]. A broad range of naturally occurring antimicrobial chemicals has been found in marine environments. These have shown remarkable increases in bioactive compounds, and concomitant algal-based products are significant therapeutic activities. However, conventional antimicrobials prefer those derived from natural sources because these are more effective, safer for the environment, and have fewer adverse effects [13].
Modern medicine’s most challenging issue is preventing the spread of more dangerous MDR bacterial pathogens. MDR bacterial infections account for over 700,000 annual fatalities worldwide, but estimates suggest that number might increase to 10 million by 2050 [9]. An extensive search for novel antimicrobial compounds that could reduce the existing dependence of modern medicine on standard antibiotics is essential to halt the trend of adding newer antibiotic classes that are gradually resistant to the newly generated bacterial strains [10].
This review considers a comprehensive update on UTI-associated diseases, namely cystitis, urethritis, acute urethral syndrome, pyelonephritis, and uncomplicated, complicated, and recurrent UTIs. Further emphasis on the global statistical incidence and recent advancement of the role of natural products in treating notorious infections are described. This updated compendium would inspire the development of novel phycocompounds as the prospective antibacterial candidate(s).
Urinary Tract Infection: A Global Threat
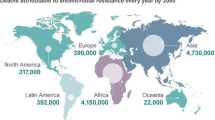
Post-COVID infection cases have increased, and patients admitted to hospitals carry drug-resistant bacteria that spread nosocomial. The antibiotic resistance of MDR bacteria produces clinical and public health concerns that are difficult to treat. This is because of the rise in the prevalence of multidrug-resistant bacteria (Fig. 3) [5]. There have been several reports of the emergence of MDR bacterial strains in various geographical places; this phenomenon is related to the local use of antibiotics. MDR microorganisms travel across regions, leading to global infection scenarios of individual harmful bacteria. These strains are more dangerous than drug-sensitive variants, and MDR bacteria are more antibiotic-resistant [8]. The administration of antibiotics for the treatment of a broad range of infections leads to the gradual development of antibiotic resistance in bacterial strains (Fig. 4). Even among bacteria that are not linked to one another phylogenetically, the bacterial consortia facilitate the interchange of genetic characteristics, which is necessary for the establishment of MDR bacterial microbiota [12]. Recently, the resistance mechanisms have resulted in the concurrent development of resistance to several antibiotic classes, resulting in hazardous MDR bacterial strains, some of which are also known as superbugs, the MDR-MRSA (multidrug-resistant-methicillin-resistant S. aureus) (Fig. 2). Because of the recent emergence of newer initially harmless microbes, the emergence of multidrug resistance in pathogenic strains occurred; eventually, the potential use of MDR bacilli became a possibility [7].
The clinical manifestations of UTI include harmless conditions as well as those that are potentially fatal. Any component of the urinary tract, including the urethra (which can develop urethritis), the bladder (which can develop cystitis), the ureters, and the kidneys, is susceptible to infection (pyelonephritis). In certain patients with lower UTIs, the disease could even ascend or cause pyelonephritis as well as male genital infections, including such prostatitis and epididymis-orchitis, or it can progress to severe and life-threatening urosepsis if it is not treated or if the case cannot be resolved with antibiotics (Fig. 1). This can happen in the absence of treatment or in issues where the infection cannot be resolved with antibiotics [11].
UTIs can be divided into three categories: lower, which affects only the bladder; upper, which affects the pyelonephritis; and either uncomplicated as well as complicated. An uncomplicated urinary tract infection (UTI) develops in a usual host that is not pregnant, does not have any structural or physiological abnormalities, and has not been outfitted (for example, with a catheter) [12, 13]. The urethra serves as the exit point for urine and allows the entry of microbiota into the urinary tract. However, these microbes are regularly flushed out through frequent urination in both men and women (Fig. 1). Urine acts as a portal for releasing microbes from the urethra into the urinary tract. In addition, the opening of the urethra in women is close to vaginal cavities and even the rectum, contributing to the development of UTIs. Although E. coli is responsible for eighty percent of all cases of infection, in addition to the illness caused by E. coli, gram-positive cocci and other organisms can also cause urinary tract infections (UTIs) (Fig. 2) [4, 11]. When a bladder infection takes place, it is frequently followed by a condition of the kidneys. This, in turn, leads to a blood-borne disease, which, in extreme cases, can result in fatal consequences, including death. Because of this, severe cases of urinary tract infection (UTI) have the potential to take lives, but with the proper treatment, patients can make a speedy recovery and avoid spreading the infection. Cystitis is an infection of the lower urinary system caused by bacteria usually found in the bladder. This illness is often followed by pyelonephritis, which is an infection of the upper urinary tract. This may result from BSI, and the antibiotics are resistant to UTIs (Fig. 4) [13].
Associated Diseases
Cystitis
Inflammation of the urinary bladder causes pain and discomfort from the infection by bacteria found in the fecal microbiota. These bacteria colonize the vaginal and periurethral openings and then enter the bladder. Urine is slightly acidic, with urea and uric acid contributing to the demise of infectious bacteria and fungi. Infection from E. coli can be confirmed by the complex with a sugar called d-mannose, which is a kind of monosaccharide that is found in the bladder. Furthermore, enterococci and non-UTIs are caused by infection from 5 to 10% coliform aerobic Gram-negative rods. Moreover, the clinical manifestations are distinguished by symptoms such as dysuria, urine frequency, and urgency and may also be accompanied by discomfort in the suprapubic region. These are the signs of a lower UTI, which has still not moved beyond the bladder; infection is the most prevalent cause of inflammation [7].
Urethritis
Infections with microorganisms are typically the root cause of urethral annoyance. The related symptoms may be comparable to cystitis. Infectious causes, such as the fungus Chlamydia trachomatis or the bacterium Neisseria gonorrhoeae, are typically acquired via sexual intercourse and do not climb to the bladder. The urethra is the principal entry point for organisms into the bladder and BSI; the particular infections of the urethra are the typical course of UTI [8, 13].
Acute Urethral Syndrome
A 'urethral syndrome’ is diagnosed when the clinical signs and symptoms of a UTI are present. Still, the urine culture does not show any positive results for the infection from any bacterium. Patients visit the hospital complaining of a bacterial infection in their lower urinary tract, which may be caused by microorganisms or chronic diseases like diabetes. Patients may also be admitted to the facility complaining of pain in their lower urinary tract with fungi [5].
Pyelonephritis
Pyelonephritis is the infection of the kidneys from the beginning of the UTI; clinical manifestations of pyelonephritis are often more complex than those of lower urinary tract infections. Pyelonephritis occurs when bacteria from the infected bladder migrate to the kidneys. Symptoms of a lower urinary tract infection often precede an upper urinary tract infection [5, 11]; by empiric therapy or administration of some antibiotic of the higher generation or the ongoing drug of the regions, the lower UTIs are usually controlled being prompted by the unbearable pain in the lower abdomen.
Recurrent Urinary Tract Infection
A recurrence of the symptoms of a UTI after the infection may occur from the continued presence of MDR bacterial strains for the recurrence of symptoms. In adults, the recurrent UTI could be the occurrence of three or even more UTI episodes in one year [4, 6].
Statistics of Urinary Tract Infections
There were 274 million new UTIs worldwide in 2017 in people of all ages and sexes. Every year, symptomatic UTIs affect one or more episodes for about 10% of women in the USA. Women between the ages of 18 and 24 years who are sexually active have the highest prevalence of the infection. The symptoms disappear in approximately one-quarter of these women, and an equal ratio becomes infected with the causative microorganisms. The incidence of UTIs in males is lower than in women, particularly in men with urologic structural abnormalities [7, 11].
Biomass Production
Algal biomass production involves numerous phases, including culture, harvesting, and dehydration of the biomass. The open pond and closed photobioreactor (PBR) technologies are the two methods for generating or cultivating algal biomass. Then, two systems—natural waterways (ponds, lakes, and lagoons) and artificial ponds (circular and raceway)—are used to categorize the production of open ponds. The open pond is a less expensive way to produce vast amounts of algal biomass than the PBR. However, the PBR offers a tremendous and well-managed closed culture environment for growing, avoiding risk or contamination from molds, bacteria, protozoa, and competition by other algae. It is typically positioned outside to use the accessible energy sources from sunshine. PBR is divided into three categories: flat plate, vertical column, and tubular. However, the PBR offers a tremendous and well-managed closed culture environment for growing, avoiding risk or contamination from molds, bacteria, protozoa, and competition by other algae. To make use of the accessible energy sources from sunshine, it is typically positioned outside.
The algal biomass can be separated from the culture medium or harvested by four processes: biomass aggregation (flocculation and ultrasound), flotation, centrifugation, and filtration. Combining two or more procedures is sometimes done to boost efficiency. The selection of the harvesting technique is influenced by several algae-related factors, including the density, size, and intended end products. In the biomass dehydration process, as soon as algal biomass is removed from the growing medium, it is processed to the next stage to avoid rotting or to increase its shelf life. The three most common drying or dehydrating methods are sun drying, spray drying, and freeze-drying. The desired outcomes are determined by the technique picked [14, 15].
Extraction of Phycochemicals
Algae have minerals, lipids, proteins, and carbohydrates as components. The algal biomass is pre-treated to release the stored bioactive component in the cells to use the compounds for various applications, such as biofuels/energy and agricultural use. A variety of techniques, including physical, mechanical (bead milling, homogenization, microwave, ultrasonic, and pulsed electric field), chemical (solvent, acid, and alkali), and biological (enzymes) procedures, can be used to lyse the cell walls to extract all the necessary components. A pre-treatment process method is selected based on the required end products [14].
Synergistic Effect of Phycochemicals for UTI Prevention
There has been a concerning rise in MDR bacterial strains in recent years; it has become increasingly important to locate alternative sources of antibacterial for timely control, lest increasingly higher generations of antibiotics be used. Unfortunately, the use of any newer antibiotics would induce the emergence of deadlier MDR bacterial strains, as the phenomenon of the emergence of drug-resistance bacteria is an inducible event in nature, wherein the Darwinian principle of the ‘struggle for existence and survival of the fittest’ is operative in microbes was nor proved by Darwin or his followers. Massively exchanging genetic characters in bacterial consortia in the body or the environment is a natural process [12]. Furthermore, research in biomedical and natural products and the utilization of bioactive chemicals derived from medicinal plants as therapeutic agents has been a significant area of research. Likewise, algae also create an extensive range of primary and secondary metabolites with a wide range of biological activities, with inherent amino acid residues, terpenoids, phlorotannin, steroids, phenols, ketones, alkenes cyclic polysulfides, and a few more (Fig. 5) [8]. Natural compounds with pharmacological effects can be sourced from marine algal species due to their unique structural composition in blue-green algae. Incidentally, seaweed or macroalgae are highly valued because of their medicinal substances of curiosity [6].
Algae have emerged as a rich source of novel therapies since the 1940s. The three significant algae families, namely, Rhodophyceae, Phaeophyceae, and Chlorophyceae, account for most of the approximately 2400 natural products identified. Indeed, antibacterial activity was thought of as a way to identify algae as the notable medicinal potential for the production of pharmacologically active algal compounds (Table 1) [59] because of their naturally inherent secondary metabolites that have antibacterial activity against infectious bacteria invitro often envisaged. Apart from the antibacterial activity, anticoagulant and antifouling activity are identified with algal chemicals. Antimicrobial substances such as chlorellin derivatives, acrylic acid, halogenated aliphatic compounds, phenol inhibitors, and the more recently known guanine sesquiterpenes and labdane diterpenoids were also documented in macroalgae (Table 2) [60, 61]. These antimicrobial agents were found to inhibit the growth of microorganisms in vitro (Fig. 2).
Blithely, it was found that marine algae produce a wide variety of bioactive secondary metabolites that act as antimicrobial, antifeedant, antihelmintic, and cytotoxic agents. The bioactive substances produced by marine algae are alkaloids, polyketides, cyclic peptides, polysaccharides, phlorotannins, diterpenoids, sterols, diterpenoids, quinones, lipids, and glycerols [13]. Because of the prokaryotic photosynthesizing metabolic activity, these organisms have biotechnological advantages over other organisms like plants and fungi. These advantages include the production of several compounds of curiosity that have potential applications in food, bioenergy production, and health biomaterials [13, 60].
Pigments, carotenoids, C-phycobilin, peptides, fatty acids, and polysaccharides are biologically active compounds formed by cyanobacteria. These biologically active molecules present considerable promise for pharmaceutical and cosmeceutical applications due to their antioxidant, anticancer, antimicrobial activity, antiviral, skin-regenerative, immunomodulatory, and immune-stimulatory activities. Cyanobacterial pigments have inhibited various cell line activities (Fig. 1) [13, 61]. In addition, the utilization of polysaccharides derived from microalgae in the field of biomedicine has been investigated for the fact that these polysaccharides are biocompatible, biodegradable, and nontoxic, in addition to exhibiting specific therapeutic capabilities [62, 63]. Moreover, cyanobacteria are photoautotrophic and prokaryotic bacteria that are known to grow in several habitats. Those can remove nitrogen from the atmosphere, make phosphate more soluble, and are an excellent source of high-quality chemicals, renewable biofuels, and bioactive substances [13, 64]. To avoid the need for synthetic antimicrobial substances or sub-therapeutic doses of conventional antibiotics, microalgae biomass cell-free extracts have been evaluated as additions to food and feed composition (Table 1) [59]. Excessive use of antibiotics, as well as chemotherapeutics to treat infectious diseases, causes MDR variations. Microalgae are a safe, cost-effective bacterial infection treatment [13]. It has been reported that some green algae, Microcystis sp., Chlorella sp., Chroococcus sp., Anabaena sp., Oscillatoria sp., and Spirulina sp. have antibacterial properties [64].
Antibiotic-like compounds may be derived from seaweeds; the indicators of antimicrobial active chemicals in seaweeds include the synthesis/conglomeration of several metabolites. Indeed, antioxidants, antivirals, anti-inflammatories, and anticoagulants are just a few of the biologically active chemicals found in abundance in seaweeds [11]. As it is, seaweeds contain chemicals with comparatively high anti-proliferating and antioxidant action; those are low in fat but include a variety of vitamins and bioactive components, such as sulphated polysaccharides and terpenoids. Sulphated polysaccharides are a promising natural antioxidant without any cholesterol that is scarce in land plants [13]. These activities previously include anticoagulant, anti-hyperlipidemic, antiviral, antitumor, and antioxidant activity [65]. Because the brown alga Sargassum swartzii produces a natural product free of harmful side effects and displays antioxidant and antibacterial activity, this natural substance should be explored for synthesizing some newer medicines to control pathogenic microbes [13]. The bioactive compounds can be virulent factors or beneficial medications and chemicals that assist in creating industrial commodities. These metabolites play a role in determining how humans associate and value particular substances. Terpenoids comprise a significant portion of this category of natural products. They include thousands of structures involved in various biological process abnormalities [11].
Terpenes
Algae are the most common source of terpenes, a type of isoprenoid. These chemicals are responsible for controlling the aromas, flavours, and pigments that they produce. Terpenes have several different beneficial interactions within the body. These might be effective in warding off infections, predators, etc. Antibacterial terpenes are produced by cyanobacteria in low quantities [96]. Microcoleus lacustri has norbietane; Nostoc commune has noscomin and comnostin (A, B, C, D, E) had shown antibacterial activity against E. coli, S. aureus, B. cereus, and S. epidermidis (Fig. 5) [66, 67, 77, 89, 91]. Moreover, Udotea flabellum, U. conglutinata, Halimeda sp., and Laurencia sp. possess terpenoids (udoteafuran, flexilin, halimedatrial), and acetylmajapolene A, B, which were effective against S. aureus, P. mirabilis and P. vulgaris [75, 97].
Aromatic Compounds
Antibacterial activity was proven against E. coli, S. aureus, and Micrococcus luteus by aromatic bioactive compounds isolated from Nostoc sp., Fischerella sp., and Eucapsis sp., respectively. These compounds include carbamidocyclophanes and ambigol (A, B, C) [66, 67].
Alkaloid
Most of the antibacterial alkaloids that have been identified from cyanobacteria are compounds that contain indole. The antibacterial activity of alkaloids (ambiguine, hapalindole, laminarin, nostocarboline, and tjipanazole D) isolated from cyanobacteria Fischerella sp., Hapalosiphon fontinalis, Nostoc sp., and Westiellopsis sp., as well as brown algae, Ascophyllum nodosum, and Laminaria hyperborea were effective against pathogenic bacteria revealed the presence of brominated indoles and norharman. These two types of indole alkaloids were effective against harmful microorganisms (Fig. 5) [59]. Many compounds in the hapalindole class exhibit potent antibacterial action against Gram-positive and negative bacteria. It was revealed that the antibacterial activity of 12-epi-hapalindole E isonitrile was due to the ability of the compound to block the RNA polymerase of bacteria. It was found that the cyanobacterial alkaloid calothrixin A, which was isolated from the cyanobacterium Calothrix sp., blocked bacterial RNA polymerase [13].
Lipids
It was found that certain fatty acids derived from marine algae possessed antimicrobial properties. The lipids had antibacterial properties, and those also acted as QS inhibitors. The fatty acids coriolic acid, -linolenic acid, and -dimorphecolic acid isolated from Fischerella sp. and Oscillatoria sp. could inhibit the growth of the pathogenic bacteria M. flavus and S. aureu`s [93].
Proteins
Some methods are mainly extracting proteins like conventional protein extraction methods, current protein extraction methods, and enrichment methods—membrane filtration, previously reported [99]. Moreover, marine algae are an excellent source of proteins, mainly green and blue-green alga, which have 40–60 percent proteins and offer various health benefits to men and animals. The proteins C-phycocyanin and phycobiliprotein derived from Anabaena sp., Spirulina sp., Westiellopsis sp., Synechocystis sp., and Streptomyces sp. were active against E. coli, K. pneumoniae, and Pseudomonas sp. [63].
Peptides
Peptides inhibit the growth of bacteria and are an essential functioning component of the immune system. Cyclic peptides are either ribosomally synthetically produced and post-translationally altered (RiPPs) or non-ribosomal peptides resembling a significant class of bioactive cyanobacterial bioactive molecules. RiPPs are made by ribosomes, and RiPPs are modified post-translationally. The cyanobactins constitute the most prominent family of RiPPs, and most of its members have undergone post-translational alterations, such as the formation of a macrocyclic core structure [89]. Kawaguchipeptins A and B, discovered from Microcystis aeruginosa are two particularly antimicrobial examples. Antibacterial properties were demonstrated by bioactive peptides such as tiahuramide (A, B, and C) and borophycin, which were derived from Lyngbya sp. and Nostoc sp. Respectively (Fig. 5). These peptides had promising activity on M. luteus and E. coli. Antibacterial activity was demonstrated by muscoride A, a linear peptide, as well as by schizotrin A and scytonemin A, lipopeptides derived from Nostoc sp., Schizothrix sp., and Scytonema sp. Respectively (Table 2). In addition, lyngbyazothrin, pahayokolide, scyptolin, and tenuecyclamide can potentially prevent bacterial infection growth in vitro [17, 80].
Polyphenols
Algae are responsible for the production of polyhalogenated compounds (PHCs), such as the chlorinated ambigols A, B, and C, which have been isolated from Fischerella ambigua and had potent activity against Gram-positive bacteria and mammalian cancer cell lines (Table 2). The cyanobacterium Leptolyngbya crossbyana was used to isolate polybrominated crossbyanols. Crossbyanol B, which has two sulfo substituents, was the only chemical that demonstrated substantial effectiveness in the brine shrimp lethality test and against MRSA [86].
Polyketides
The three different kinds of polyketide synthases (PKSs) contribute to the production of polyketides, leading to natural products with a wide range of chemical possibilities. Moreover, PKSs gene clusters responsible for producing these chemicals in cyanobacteria have been discovered. In a recent study, cyanobacteria were designed to create polyketides. The biosynthesis of alkylresorcinols is a chemical family that has received the most attention. Nostocyclyne A, 1,8-dihydroxy-4-methyl anthraquinone, carbamidocyclophanes, and cylindrocyclophanes are examples of other members of this chemical family with antibiotic activity (Table 2) [61]. The above compound could prevent the growth of several Gram-positive human pathogenic strains, such as Streptococcus pyogenes, Staphylococcus aureus, and Bacillus subtilis; it also had antiplasmodial activity. Malyngolide was the first polyketide antibiotic isolated from cyanobacteria in 1981 from Lyngbya majuscule [95].
The less recent research was focused on isolating alkyne-containing polyketides, synthesizing them, and determining whether these are effective against MRSA. These instances illustrate the possibility that cyanobacterial polyketides could be used to treat infections caused by resistant strains [97].
Other Classes
In addition to chlorellin from Chlorella sp., endophytic actinomycetes from Caulerpa sp., Turbinaria sp., and Sargassum sp., and halogenated compounds from Laurencia sp. demonstrated antibacterial action against E. coli, K. pneumoniae, and Pseudomonas sp. in vitro [11, 59].
Challenges for Phycocompounds
Microalgae are a rich source of valuable active bio-compounds such as carotenoids, C-phycocyanin, phenolics, amino acids, polyunsaturated fatty acids, sulphated polysaccharides, pigments, lipids, phlorotannins, polysaccharides, peptides, terpenes, polyacetylenes, sterols, indole alkaloids, these chemicals, which were associated with various pharmacological actions, such as the control of bacteria, viruses, tumors, inflammation, and allergies (Table 2) [30]. The fact that these compounds are only found in trace amounts in the extracts is one of the limitations. It is essential to study the many approaches that can be taken to boost the synthesis of these compounds [13].
Since algal slow-growth, periodicity, and low extraction yields are additional fundamental limitations, one of the significant issues in this area is the sustainable synthesis of these chemicals to supply enough for preclinical, clinical, and future commercialized drugs. By such a need, the whole chemical synthesis can ensure the long-term and steady production of the bioactive molecules, given the expansion of the scope of their uses [87].
Future Prospective
New methods in molecular biology, such as CRISP/ Cas9 and genomic research, could make it possible to obtain larger quantities of compounds without the need for many reagents or a significant amount of time, which would result in a reduction in the cost related to the mass production of these molecules [7, 59].
To design novel extraction processes, explore untouched marine sources with hidden medicinal values, and investigate structural interactions between active constituents from the same and multi-source. The elucidation of intermediate interactions of bioactive cofactors and their potential applications for marine-derived bioactive compounds shall be considered in the future [13]. As the source of several therapeutically beneficial chemicals, cyanobacteria have significant therapeutic potential. Numerous studies have been conducted on the pharmacological activities of cyanobacteria, including their bioactive components [62]. However, more in vivo and in vitro investigations employing various animal models and clinical studies will be required to bring cyanobacteria with their bioactive constituents into the translational mode for society.
Further research is required to assess the compounds in more intricate biological systems, such as in vivo models. The potential for 3D chemical structure modeling approaches to be used in the search for new biological targets for algae-derived chemicals has yet to be fully realized [11]. Furthermore, the potential of medicinal co-adjuvants generated from algae should be studied. Some algae-derived substances have limits that could be mitigated, and their efficacy could be enhanced by applying cutting-edge techniques like nanotechnology to create novel nano-formulations. However, more advanced technologies are required to verify whether their antimicrobial efficacy could be improved in nano-formulations [98].
Despite the literature addressing female UTIs and their risk factors, much remains to be learned. Methods to reduce the danger of UTIs are under scrutiny. Strategies for managing several essential issues, including pregnancy, diabetes, anaemia, etc., are still being researched and developed. Despite growing interest from public health groups, research into public education about UTIs and their causes has been sparse. There should be more nontoxic antibacterial through which MDR microorganisms can be controlled [6].
Conclusion
The gargantuan types of secondary metabolites produced by algae have the potential to serve as a rich source of new entities that can lead to the formulation of new medications. Those metabolites can also have peptides, alkaloids, indole alkaloids, polyketides, and terpene chemical structures, and many of these molecules have a wide range of pharmacological properties. The enormous chemical diversity and biological activities of algal products have prepared those sources an appealing candidate for developing novel drugs for application in various therapeutic domains; nonetheless, the historical underappreciation of algae compared to other microbial sources of natural products. Thus, the pharmacological potential of algae advantages increased attention from the scientific community and research that draws from various fields. In addition, the diverse algal strains originating from environments that still need to be well investigated can be promising candidates for use in the pharmaceutical industry.
The oxygenic photosynthetic blue-green algae are found in various biological niches worldwide. Over the past two decades, researchers have recognized algae as a promising resource for developing novel therapeutic lead compounds due to the wide range of actions displayed by the bioactive molecules isolated from them. Because it can be grown with relatively inexpensive inorganics, cyanobacteria offer another benefit as the microbiological source for drug development. As a result, the blue-green algae may treasure broader application in drug research.
Data and Material Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Catalano A, Iacopetta D, Ceramella J et al (2022) Multidrug resistance (MDR): a widespread phenomenon in pharmacological therapies. Molecules 27:616. https://doi.org/10.3390/molecules27030616
El-Shouny WA, Gaafar RM, Ismail GA et al (2017) Antibacterial activity of some seaweed extracts against multidrug resistant urinary tract bacteria and analysis of their virulence genes. Int J Curr Microbiol Appl Sci 6:2569–2586. https://doi.org/10.20546/ijcmas.2017.611.302
Colborn KL, Bronsert M, Hammermeister K et al (2019) Identification of urinary tract infections using electronic health record data. Am J Infect Control 47:371–375. https://doi.org/10.1016/j.ajic.2018.10.009
Timsit JF, Ruppé E, Barbier F et al (2020) Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med 46:266–284. https://doi.org/10.1007/s00134-020-05950-6
Walker AC, Bhargava R, Vaziriyan-Sani AS et al (2021) Colonization of the Caenorhabditis elegans gut with human enteric bacterial pathogens leads to proteostasis disruption that is rescued by butyrate. PloS Pathog 17:e1009510. https://doi.org/10.1371/journal.ppat.1009510
Nguyen SN, Le Thi HT, Tran TD et al (2022) Clinical epidemiology characteristics and antibiotic resistance associated with urinary tract infections caused by E. coli. Int J Nephrol 2022:1–5. https://doi.org/10.1155/2022/2552990
Najmi A, Javed SA, Al Bratty M et al (2022) Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules 27:349. https://doi.org/10.3390/molecules27020349
Church NA, McKillip JL (2021) Antibiotic resistance crisis: challenges and imperatives. Biologia 76:1535–1550. https://doi.org/10.1007/s11756-021-00697-x
Wagenlehner FM, Bjerklund Johansen TE, Cai T et al (2020) Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol 17:586–600. https://doi.org/10.1038/s41585-020-0362-4
Chakrabarty S, Mishra MP, Bhattacharyay D (2022) Targeting microbial bio-film: an update on MDR gram-negative bio-film producers causing catheter-associated urinary tract infections. Appl Biochem Biotechnol 5:1–35. https://doi.org/10.1007/s12010-021-03711-9
Klein RD, Hultgren SJ (2020) Urinary tract infections: microbial pathogenesis, host–pathogen interactions and new treatment strategies. Nat Rev Microbiol 18:211–226. https://doi.org/10.1038/s41579-020-0324-0
Vanneste BG, Van Limbergen EJ, Marcelissen TA et al (2022) Development of a management algorithm for acute and chronic radiation urethritis and cystitis. Urol Int 106:63–74. https://doi.org/10.1159/000515716
Bishoyi AK, Sahoo CR, Padhy RN (2022) Recent progression of cyanobacteria and their pharmaceutical utility: an update. J Biomol Struct Dyn 6:1–34. https://doi.org/10.1080/07391102.2022.2062051
Balasubramaniam V, Gunasegavan RD, Mustar S, Lee JC, Mohd Noh MF (2021) Isolation of industrial important bioactive compounds from microalgae. Molecules 26:943. https://doi.org/10.3390/molecules26040943
Karki S, Shrestha K, Gautam R, Narayan R (2020) Phytochemical screening, FT-IR and GC-MS analysis of Euphorbia hirta. J Pharmacogn Phytochem 9:1883–1889
Pradhan J, Das S, Das BK (2014) Antibacterial activity of freshwater microalgae: a review. Afr J Pharmacy Pharmacol 8:809–818. https://doi.org/10.5897/AJPP2013.0002
Rojas V, Rivas L, Cárdenas C et al (2020) Cyanobacteria and eukaryotic microalgae as emerging sources of antibacterial peptides. Molecules 25:5804. https://doi.org/10.3390/molecules25245804
Demiriz T, Cokmus C, Pabuccu K (2011) Antimicrobial activity of some algal species belonging to cyanobacteria and chlorophyta. Asian J Chem 23:1384–1386
Singh U, Singh P, Singh AK et al (2021) Identification of antifungal and antibacterial biomolecules from a cyanobacterium. Arthrospira platensis Algal Res 54:102215. https://doi.org/10.1016/j.algal.2021.102215
Chowdhury MM, Kubra K, Hossain MB et al (2015) Screening of antibacterial and antifungal activity of freshwater and marine algae as a prominent natural antibiotic available in Bangladesh. Int J Pharmacol 11:828–833. https://doi.org/10.3923/ijp.2015.828.833
Azeez R (2014) Growth and biochemical parameters of selective cultured cyanobacteria and exploiting antibacterial potency against human bacterial pathogens. Appl Bot 72:25537–25543
Rao D (2015) Antibacterial activity of fresh water Cyanobacteria. J Algal Biomass Util 6:60–64
Nainangu P, Antonyraj AP, Subramanian K et al (2020) In vitro screening of antimicrobial, antioxidant, cytotoxic activities, and characterization of bioactive substances from freshwater cyanobacteria Oscillatoria sp. SSCM01 and Phormidium sp. SSCM02. Biocatal Agric Biotechnol 29:101772. https://doi.org/10.1016/j.bcab.2020.101772
Prakash JW, Marimuthu J, Jeeva S (2011) Antimicrobial activity of certain fresh water microalgae from Thamirabarani River, Tamil Nadu, South India. Asian Pac J Trop Biomed 1:S170-173. https://doi.org/10.1016/S2221-1691(11)60149-4
Hamdy AD (2018) Determination of the effect of some biological products of Synechocystis pevalekii on some pathogenic bacteria isolated from wounds and urinary tract. Tikrit J Pure Sci 23:9–17. https://doi.org/10.25130/tjps.23.2018.022
Skočibušić M, Lacić S, Rašić Z (2019) Evaluation of antimicrobial potential of the marine Cyanobacterium, Rivularia mesenterica. J Adv Microbiol 16:1–1. https://doi.org/10.9734/JAMB/2019/v16i430128
Tyagi R, Kaushik BD, Kumar J (2014) Antimicrobial activity of some cyanobacteria. In Microbial diversity and biotechnology in food security. 463–470. https://doi.org/10.1007/978-81-322-1801-2_41
Yi Z, Yin-Shan C, Hai-Sheng LU (2001) Screening for antibacterial and antifungal activities in some marine algae from the Fujian coast of China with three different solvents. Chin J Oceanol Limnol 19:327–331. https://doi.org/10.1007/BF02850736
Thamilvanan D, Karthikeyan D, Muthukumaran M et al (2016) Antibacterial activity of selected microalgal members of Chlorophyceae. World J Pharm Pharm Sci 5:718–729
Shaima AF, Yasin NH, Ibrahim N et al (2022) Unveiling antimicrobial activity of microalgae Chlorella sorokiniana (UKM2), Chlorella sp. (UKM8) and Scenedesmus sp. (UKM9). Saudi J Biol Sci 29:1043–1052. https://doi.org/10.1016/j.sjbs.2021.09.069
Ghalem BR, Zouaoui B (2018) Antibacterial activity of diethyl ether and chloroform extracts of seaweeds against Escherichia coli and Staphylococcus aureus. Int. J Avian Wildl 3:310–313. https://doi.org/10.15406/ijawb.2018.03.00111
El Zawawy N, El Shafay S, Abomohra AE (2020) Macroalgal activity against fungal urinary tract infections: in vitro screening and evaluation study. Rend Lincei Sci Fis Nat 31:165–175. https://doi.org/10.1007/s12210-019-00856-y
Christabell J, Lipton AP, Aishwarya MS et al (2011) Antibacterial activity of aqueous extract from selected macroalgae of southwest coast of India. Sea Res Util 33:67–75
Manikandan S, Ganesapandian S, Singh M et al (2011) Antimicrobial activity of seaweeds against multi drug resistant strains. Int J Pharm 7:522–526. https://doi.org/10.3923/ijp.2011.522.526
Moubayed NM, Al Houri HJ, Al Khulaifi MM et al (2017) Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf). Saudi J Biol Sci 24:162–169. https://doi.org/10.1016/j.sjbs.2016.05.018
Selvaraj P, Neethu E, Rathika P et al (2020) Antibacterial potentials of methanolic extract and silver nanoparticles from marine algae. Biocatal Agric Biotechnol 28:101719. https://doi.org/10.1016/j.bcab.2020.101719
Rajivgandhi G, Ramachandran G, Maruthupandy M et al (2018) Antibacterial effect of endophytic actinomycetes from marine algae against multi drug resistant gram-negative bacteria. Exam Mar Biol Oceanogr 1:1–8. https://doi.org/10.31031/EIMBO.2018.01.000522
El-deen N (2011) Screening for antibacterial activities in some marine algae from the red sea (Hurghada, Egypt). Afr J Microbiol Res 5:2160–2167. https://doi.org/10.5897/AJMR11.390
Al-Judaibi A (2014) Antibacterial effects of extracts of two types of Red Sea Algae. J Biosci Med 2:74. https://doi.org/10.4236/jbm.2014.22012
Navarro F, Forján E, Vázquez M et al (2017) Antimicrobial activity of the acidophilic eukaryotic microalga Coccomyxa onubensis. Phycol Res 65:38–43. https://doi.org/10.1111/pre.12158
Challouf R, Dhieb RB, Omrane H et al (2012) Antibacterial, antioxidant and cytotoxic activities of extracts from the thermophilic green alga, Cosmarium sp. Afr J Biotechnol 11:14844–14849. https://doi.org/10.5897/AJB12.1118
Kilic NK, Erdem K, Donmez G (2019) Bioactive compounds produced by Dunaliella species, antimicrobial effects and optimization of the efficiency. Turkish J Fish Aquat Sci 19:923–933. https://doi.org/10.4194/1303-2712-v19_11_04
Kavita K, Singh VK, Jha B (2014) 24-Branched Δ5 sterols from Laurencia papillosa red seaweed with antibacterial activity against human pathogenic bacteria. Microbiol Res 169:301–306. https://doi.org/10.1016/j.micres.2013.07.002
Vairappan CS (2003) Potent antibacterial activity of halogenated metabolites from Malaysian red algae, Laurencia majuscula (Rhodomelaceae, Ceramiales). Biomol Eng 20:255–259. https://doi.org/10.1016/S1389-0344(03)00067-4
Amorim RD, Rodrigues JA, Holanda ML et al (2012) Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed Gracilaria ornata. Braz Arch Biol Technol 55:171–181. https://doi.org/10.1590/S1516-89132012000200001
Sasidharan S, Darah I, Noordin MK (2010) In vitro antimicrobial activity against Pseudomonas aeruginosa and acute oral toxicity of marine algae Gracilaria changii. New Biotechnol 27:390–396. https://doi.org/10.1016/j.nbt.2010.02.002
Dayuti S (2018) Antibacterial activity of red algae (Gracilaria verrucosa) extract against Escherichia coli and Salmonella typhimurium. IOP Conf Ser: Earth Environ Sci 137:012074. https://doi.org/10.1088/1755-1315/137/1/012074
Baliano AP, Pimentel EF, Buzin AR et al (2016) Brown seaweed Padina gymnospora is a prominent natural wound-care product. Rev Bras Farmacogn 26:714–719. https://doi.org/10.1016/j.bjp.2016.07.003
Nagayama K, Iwamura Y, Shibata T et al (2002) Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother 50:889–893. https://doi.org/10.1093/jac/dkf222
Choi JG, Kang OH, Brice OO et al (2010) Antibacterial activity of Ecklonia cava against methicillin-resistant Staphylococcus aureus and Salmonella spp. Foodborne Pathog Dis 7:435–441. https://doi.org/10.1089/fpd.2009.0434
Lee DS, Kang MS, Hwang HJ et al (2008) Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol Bioprocess Eng 13:758–764. https://doi.org/10.1007/s12257-008-0162-9
Vijayabaskar P, Vaseela N, Thirumaran G (2012) Potential antibacterial and antioxidant properties of a sulfated polysaccharide from the brown marine algae Sargassum swartzii. Chin J Nat Med 10:421–428. https://doi.org/10.1016/S1875-5364(12)60082-X
Poongothai E (2018) Antimicrobial activity of aqueous and cow-urine extracts of Sargassum wightii (sea weed) on multiple drug resistant pathogens. Int J Rec Adv Pharm Res 2:4–87
Sangeetha J, Gayathri S, Rajeshkumar S (2017) Antimicrobial assessment of marine brown algae Sargassum whitti against UTI pathogens and its phytochemical analysis. Res J Pharm Technol 10:1905–1910. https://doi.org/10.5958/0974-360X.2017.00334.1
Setyati WA, Pramesti R, Susanto AB et al (2020) In vitro antibacterial study and spectral analysis of brown seaweed Sargassum crassifolium extract from Karimunjawa Islands, Jepara. IOP Conf Ser: Earth Environ Sci 530:012028. https://doi.org/10.1088/1755-1315/530/1/012028
Tajbakhsh S, Pouyan M, Zandi K et al (2011) In vitro study of antibacterial activity of the alga Sargassum oligocystum from the Persian Gulf. Eur Rev Med Pharmacol Sci 15:293–298
Akremi N, Cappoen D, Anthonissen R et al (2017) Phytochemical and in vitro antimicrobial and genotoxic activity in the brown algae Dictyopteris membranacea. S Afr J Bot 108:308–314. https://doi.org/10.1016/j.sajb.2016.08.009
Corona E, Fernandez-Acero J, Bartual A (2017) Screening study for antibacterial activity from marine and freshwater microalgae. Int J Pharma Bio Sci 8:189–194. https://doi.org/10.22376/ijpbs.2017.8.1p189-194
Kini S, Divyashree M, Mani MK et al (2020) Algae and cyanobacteria as a source of novel bioactive compounds for biomedical applications. Adv Cyanobacterial Biol. https://doi.org/10.1016/B978-0-12-819311-2.00012-7
Bishoyi AK, Sahoo CR, Sahoo AP et al (2021) Bio-synthesis of silver nanoparticles with the brackish water blue-green alga Oscillatoria princeps and antibacterial assessment. Appl Nanosci 11:389–398. https://doi.org/10.1007/s13204-020-01593-7
Sahoo CR, Maharana S, Mandhata CP et al (2020) Biogenic silver nanoparticle synthesis with cyanobacterium Chroococcus minutus isolated from Baliharachandi sea-mouth, Odisha, and in vitro antibacterial activity. Saudi J Biol Sci 27:1580–1586. https://doi.org/10.1016/j.sjbs.2020.03.020
Singh RK, Tiwari SP, Rai AK et al (2011) Cyanobacteria: an emerging source for drug discovery. J Antibiot Res 64:401–412. https://doi.org/10.1038/ja.2011.21
Carpine R, Sieber S (2021) Antibacterial and antiviral metabolites from cyanobacteria: their application and their impact on human health. Curr Res Biotechnol 3:65–81. https://doi.org/10.1016/j.crbiot.2021.03.001
Molina-Grima E, García-Camacho F, Acién-Fernández FG et al (2021) Pathogens and predators impacting commercial production of microalgae and cyanobacteria. Biotechnol Adv 8:107884. https://doi.org/10.1016/j.biotechadv.2021.107884
Osman A, Salama A, Ghany AA et al (2015) Antibacterial activity and mechanism of action of phycocyanin extracted from an egyptian strain of Anabaena oryzae SOS13. Zagazig J Agric Res 42:309–321
Wright AD, Papendorf O, König GM (2005) Ambigol C and 2, 4-dichlorobenzoic acid, natural products produced by the terrestrial cyanobacterium Fischerella ambigua. J Nat Prod 68:459–461. https://doi.org/10.1021/np049640w
Kadam SU, O’Donnell CP, Rai DK et al (2015) Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: ultrasound assisted extraction, characterization and bioactivity. Mar Drugs 13:4270–4280. https://doi.org/10.3390/md13074270
Kubota T, Iwai T, Sakai K et al (2014) Amphidinins C-F, amphidinolide Q analogues from marine dinoflagellate Amphidinium sp. Org Lett 16:5624–5627. https://doi.org/10.1021/ol502685z
Najdenski HM, Gigova LG, Iliev II et al (2013) Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int J Food Sci 48:1533–1540. https://doi.org/10.1111/ijfs.12122
Sarada DV, Sreenath Kumar C, Rengasamy R (2011) Purified C-phycocyanin from Spirulina platensis (Nordstedt) Geitler: a novel and potent agent against drug resistant bacteria. World J Microbiol Biotechnol 27:779–783. https://doi.org/10.1007/s11274-010-0516-2
Vairappan CS, Suzuki M, Ishii T et al (2008) Antibacterial activity of halogenated sesquiterpenes from Malaysian Laurencia spp. Phytochemistry 69:2490–2494. https://doi.org/10.1016/j.phytochem.2008.06.015
Swamy MA (2011) Marine algal sources for treating bacterial diseases. Adv Food Nutr Res 64:71–84. https://doi.org/10.1016/B978-0-12-387669-0.00006-5
Thajuddin N, Subramanian G (2005) Cyanobacterial biodiversity and potential applications in biotechnology. Curr Sci 89:47–57
Falch BS, König GM, Wright AD et al (1995) Biological activities of cyanobacteria: evaluation of extracts and pure compounds. Planta Med 61:321–328. https://doi.org/10.1055/s-2006-958092
Mo S, Krunic A, Chlipala G et al (2009) Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J Nat Prod 72:894–899. https://doi.org/10.1021/np800751j
Banker R, Carmeli S (1998) Tenuecyclamides A− D, Cyclic Hexapeptides from the cyanobacterium Nostoc spongiaeforme var. tenue. J Nat Prod 61:1248–1251. https://doi.org/10.1021/np980138j
Thanh Doan N, Rickards RW, Rothschild JM et al (2000) Allelopathic actions of the alkaloid 12-epi-hapalindole E isonitrile and calothrixin A from cyanobacteria of the genera Fischerella and Calothrix. J Appl Phycol 12:409–416. https://doi.org/10.1023/A:1008170007044
Bui HT, Jansen R, Pham HT et al (2007) Carbamidocyclophanes A− E, chlorinated paracyclophanes with cytotoxic and antibiotic activity from the vietnamese cyanobacterium Nostoc sp. J Nat Prod 70:499–503. https://doi.org/10.1021/np060324m
Mundt S, Kreitlow S, Jansen R (2003) Fatty acids with antibacterial activity from the cyanobacterium Oscillatoria redekei HUB 051. J Appl Phycol 15:263–267. https://doi.org/10.1023/A:1023889813697
Choi H, Engene N, Smith JE et al (2010) Crossbyanols A− D, toxic brominated polyphenyl ethers from the Hawai’ian bloom-forming Cyanobacterium Leptolyngbya crossbyana. J Nat Prod 73:517–522. https://doi.org/10.1021/np900661g
Ishida K, Matsuda H, Murakami M et al (1997) Kawaguchipeptin B, an antibacterial cyclic undecapeptide from the cyanobacterium Microcystis aeruginosa. J Nat Prod 60:724–726. https://doi.org/10.1021/np970146k
Zainuddin EN, Jansen R, Nimtz M et al (2009) Lyngbyazothrins A− D, antimicrobial cyclic undecapeptides from the cultured cyanobacterium Lyngbya sp. J Nat Prod 72:1373–1378. https://doi.org/10.1021/np8007792
Swain SS, Paidesetty SK, Padhy RN (2017) Antibacterial, antifungal and antimycobacterial compounds from cyanobacteria. Biomed Pharmacother 90:760–776. https://doi.org/10.1016/j.biopha.2017.04.030
Nagatsu A, Kajitani H, Sakakibara J (1995) Muscoride A: A new oxazole peptide alkaloid from freshwater cyanobacterium Nostoc muscorum. Tetrahedron Lett 36:4097–4100. https://doi.org/10.1016/0040-4039(95)00724-Q
Pérez Gutiérrez RM, Martínez Flores A, Vargas Solís R et al (2008) Two new antibacterial norabietane diterpenoids from cyanobacteria, Microcoleous lacustris. J Nat Med 62:328–331. https://doi.org/10.1007/s11418-008-0238-z
Volk RB, Furkert FH (2006) Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol Res 161:180–186. https://doi.org/10.1016/j.micres.2005.08.005
Jaki B, Orjala J, Heilmann J et al (2000) Novel extracellular diterpenoids with biological activity from the cyanobacterium Nostoc commune. J Nat Prod 63:339–343. https://doi.org/10.1021/np9903090
Becher PG, Keller S, Jung G et al (2007) Insecticidal activity of 12-epi-hapalindole J isonitrile. Phytochemistry 68:2493–2497. https://doi.org/10.1016/j.phytochem.2007.06.024
Hirata K, Yoshitomi S, Dwi S et al (2003) Bioactivities of nostocine a produced by a freshwater cyanobacterium Nostoc spongiaeforme TISTR 8169. J Biosci Bioeng 95:512–517. https://doi.org/10.1016/S1389-1723(03)80053-1
Ploutno A, Carmeli S (2000) Nostocyclyne A, a novel antimicrobial cyclophane from the cyanobacterium Nostoc sp. J Nat Prod 63:1524–1526. https://doi.org/10.1021/np0002334
An T, Kumar TK, Wang M et al (2007) Structures of pahayokolides A and B, cyclic peptides from a Lyngbya sp. J Nat Prod 70:730–735. https://doi.org/10.1021/np060389p
Pergament I, Carmeli S (1994) Schizotrin A; a novel antimicrobial cyclic peptide from a cyanobacterium. Tetrahedron Lett 35:8473–8476. https://doi.org/10.1016/S0040-4039(00)74436-4
MacMillan JB, Molinski TF (2005) Majusculoic acid, a brominated cyclopropyl fatty acid from a marine cyanobacterial mat assemblage. J Nat Prod 68:604–606. https://doi.org/10.1021/np049596k
Matern U, Schleberger C, Jelakovic S et al (2003) Binding structure of elastase inhibitor scyptolin A. Chem Biol 10:997–1001. https://doi.org/10.1016/j.chembiol.2003.10.001
Helms GL, Moore RE, Niemczura WP et al (1988) Scytonemin A, a novel calcium antagonist from a blue-green alga. J Org Chem Res 53:1298–1307. https://doi.org/10.1021/jo00241a033
Ishibashi M, Moore RE, Patterson GM et al (1986) Scytophycins, cytotoxic and antimycotic agents from the cyanophyte Scytonema pseudohofmanni. J Org Chem Res 51:5300–5306. https://doi.org/10.1021/jo00376a047
Prinsep MR, Caplan FR, Moore RE et al (1992) Tolyporphin, a novel multidrug resistance reversing agent from the blue-green alga Tolypothrix nodosa. J Am Chem Soc 114:385–387. https://doi.org/10.1021/ja00027a072
Moore RE, Patterson GM, Mynderse JS et al (1986) Toxins from cyanophytes belonging to the Scytonemataceae. Pure Appl Chem 58:263–271. https://doi.org/10.1351/pac198658020263
Bleakley S, Hayes M (2017) Algal proteins: extraction, application, and challenges concerning production. Foods 6:33. https://doi.org/10.3390/foods6050033
Acknowledgements
The authors are grateful to the Dean, Dr. S. Mishra, Institute of Medical Sciences & Sum Hospital, Bhubaneswar, for the facilities.
Funding
This work was supported by the SOADU-PhD fellowship of AK Bishoyi (Regd. No.1981611008/2019), Siksha ‘O’ Anusandhan Deemed to be University, Bhubaneswar, Odisha, India.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Informed Consent
This article contains no studies with human participants or animals performed by authors.
Publication Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bishoyi, A.K., Lakra, A., Mandhata, C.P. et al. Prospective Phycocompounds for Developing Therapeutics for Urinary Tract Infection. Curr Microbiol 81, 35 (2024). https://doi.org/10.1007/s00284-023-03535-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03535-2