Abstract
In every age group, urinary tract infection (UTI) is found as a major recurrence infectious disorder. Bio-films produced by bacteria perform a vital role in causing infection in the tract of the urinary system, leading to recurrences and relapses. The purpose of this review is to present the role and mechanism of bio-film producing MDR Gram-negative bacteria causing UTI, their significance, additionally the challenges for remedy and prevention of catheter-associated UTI. This work appreciates a new understanding of bio-film producers which are having multi-drug resistance capability and focuses on the effect and control of bio-film producing uropathogenic bacteria related to catheterization. We have tried to analyze approaches to target bio-film and reported phytochemicals with anti-bio-film activity also updated on anti-bio-film therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
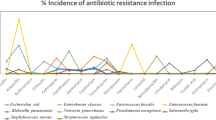
Urinary tract infections (UTIs) are common in both community and healthcare settings which leads to associated comorbidities along with high hospitalization costs [1]. Generally, UTI affects women more than men and creates complications like cystitis, pyelonephritis, and urethritis leading to even blood stream infection (BSI) and hematuria. Pathogens that cause UTIs include Gram-negative and Gram-positive bacteria, as well as fungi [2]. There are two types of UTIs: complicated and uncomplicated. Even though healthy people have no neurological urinary tract abnormalities, they are still impacted by uncomplicated UTIs [2,3,4]. Uncomplicated UTIs are most likely to infect women, children, and healthy elderly persons. Complicated UTIs are frequently caused by the combination of indwelling catheters, anomalies in the urinary system, and prolonged antibiotic exposures (or immunosuppression) [2]. The most prevalent causative agent is uropathogenic Escherichia coli (UPEC), which is responsible for both uncomplicated and complicated UTIs. Klebsiella pneumoniae, Staphylococcus saprophyticus, Enterococcus faecalis, group B Streptococcus (GBS), Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida spp. are the other causative agents for uncomplicated UTIs, according to the prevalence order. Enterococcus spp., K. pneumoniae, Candida spp., S. aureus, P. mirabilis, P. aeruginosa, and GBS are the other causative agents for complicated UTIs in order of prevalence [2].
According to previous reports, it has been estimated that 36% of UTIs occur due to nosocomial origin among which 80% were associated with indwelling urinary catheterization [5, 6]. The risk factors for UTI enhance with diabetes, kidney failure, corticosteroid use, chronic illness, organ transplant recipients, cancer, and immunocompromised diseases. Moreover, the other elements responsible for the obstruction of the urinary tract are prostate augmentation, rehashed pregnancies, tumors, renal calculi, and bladder catheterization. Microorganisms are ubiquitous in nature and growing or colonized on the superficial part of indwelling medical apparatus, and living tissues such as the urinary tract, the middle part of the ear, valves of the heart and outer membrane of lungs [7]. Urinary catheterization increases the chance of the occurrence of UTI. Moreover, worldwide approximately 150 million people get affected by infection in the urinary tract and catheter-associated urinary tract infection (CAUTI) is the most common hospital acquired infection affecting around 15–20% of hospitalized patients with prolonged catheterization. Among 40% of nosocomial UTI, 80% causes due to CAUTI [8]. Around 75% of hospital-acquired UTIs are caused by indwelling urinary catheters. Like many infections, UTI has encountered the second most common global health problem found in medical practice which is microbial infection. The infection might be caused by genitourinary tract microbe invasion and may extend from the kidney to the urethra. During the twenty-first century, several researches have been conducted on this infection, and it has been proved that one of the most common reasons for the infection in the urinary tract is the use of indwelling catheters during staying in the hospital. According to a recent survey, most of the UTIs are nosocomial or hospital-acquired CAUTI [9].
Role of Catheter and Catheter Infection Sources
Catheters are thin tubes used to drain urine for patients when naturally they are unable to do urinary retention due to some obstruction in the urinary tract, such as renal calculi, swollen prostate, any renal surgery, or medication. It is generally used to remove the urine artificially from the bladder. The catheter may become contaminated upon insertion when bacteria easily get entered into the urinary tract through catheterization. The long-term storage of urine in the catheter bag leads to the contamination of microorganisms, and the infection may easily retrograde into the bladder and leads to a complicated infection [5].
Indwelling urinary catheters are the major clinical concern for causing CAUTI [10]. The vast majority of UTI can be caused by Enterococcus species, Acinetobacter species, Pseudomonas species, etc. Most of them can form a protecting layer, though antibiotics cannot easily penetrate the cell wall and that has become a multi-drug resistant organism. Besides bio-film producers, some of the uropathogenic strains are not capable of forming the bio-film layer. Between these two classes, microbes without any protective layer can easily be cured by some of the renowned antibiotics, such as amikacin, imipenem, cotrimoxazole, and ciprofloxacin [11].
On the other hand, microbes with protective layers are difficult to treat with antibiotics. The protective layer is commonly known as bio-film. The microbes forming the bio-films are a major concern for catheterization, and there is less work on this problem available. Thus, by targeting this complicated infection from the aspect of the multi-drug resistance bio-film producers, this work appreciates a new understanding of bio-film producers which are having multi-drug resistance capability and focuses on the effect and control of bio-film producing uropathogenic bacteria related to catheterization [6, 12].
Bio-film
Microbes developed resistance over antimicrobial drugs by forming thin robust capsules as a protective layer adhering to mucilage considered as bio-film. Bio-film formation in microbes has been considered as the vital cause of so many epidemic episodes. Moreover, UTI is one of the major clinical concerns for developing MDR-bio-film–producing strains through recurrence episodes. However, the exact defense mechanism of this mucilage adhering to a thin robust layer against antimicrobial is not yet clearly proven [13]. Mostly, CAUTI is found in hospital-admitted patients because of the poor handling and unhygienic condition of medical indwelling devices [14, 15]. The main reason behind the formation of bio-film by colonizing infectious pathogenic organisms are usually found in catheter-associated infections since many communicate an arrangement of adhesion and exopolysaccharides promoting attachment leading to the formation of bio-film on the superficial part of the catheter. Microbes’ growth within this thick coherent protective layer confers the failure of antibiotic therapy [13].
Intense UTI brought about by microorganisms can prompt intermittent septicemia, which is characterized as a “reinfection” once it includes a strain aside from that inflicting the primary infection, or it is characterized as a “relapse” when it is brought about by a similar strain as that associated with the first UTI. Intermittent UTIs are basic among youthful, healthy women, despite their urinary tracts, by and large, being physiologically and anatomically ordinary. Around twenty-fifth of women with a history of intense cystitis can create intermittent UTI, which speaks to a significant weight to the health care system. As a result, the quantity of studies to clarify the elements inclining intermittent UTI to create compelling strategies for counteraction and treatment is urged to be expanded [16]. It is a significant general medical issue and the common form of the microbial irresistible disease in the network with a high pace of dreariness. UTI can be grouped into simple and complicated based on their decision of treatment [17]. UTI is mainly found in the case of the female uterine line than male due to the short distance of the urethra and genital tract and feminine unhealthy habits and using contraception. The likelihood is that the monthly cycle and its tidiness of the heads make curiously moist conditions in the urogenital that may progress microbial attack. The other rule factors make females more [17] susceptible to urinary tract infections. Explicitly dynamic young ladies are at more genuine peril of presenting UTIs (particularly, simple cystitis) on account of their life structures (short urethra) and certain social variables. Backslides are requested as tangled UTIs and require longer courses of anti-infection agents. Backslides in ladies have been identified with the limit of the microbes to shape bio-film [18].
Bio-films and Infectious Diseases
Infections in Animals and Humans
Bio-films are liable for many non-curable recurrent infections.
Infections in animals
Additionally, bio-film is related to most diseases in animals. So, the study of the role of bio-film needs to be carefully studied within the field of animal sciences. Significant topics such as sanitation, welfare and well-being of animals, and animal disease control are snared into the capacity to manage bacterial majority detecting known as quorum sensing and bio-film.
Infections in humans
Researchers perceive that a person with a diabetic condition, ulcer, recurring infections, and wound breakdown has minimum chances to get cured by antibiotics. At that time, researchers have found that bio-film-related infection has maximum chances or might be the real problem of that situation. Research upon microbiology has been going on for a long duration and found that many microbes are having resistant infection–occurring capability with the help of bio-film formation. Bio-films are easily accessible and can cause many infections in humans. Many infections can occur in human beings due to the bio-film formation capability of bacteria. Those are urinary tract infection, cystic fibrosis, circulation system infection, hospital-acquired infections, septicemia, gum infection, etc. [19].
Bio-film Producing MDR Gram-Negative Bacteria Causing UTI
The recent studies and researches on bio-film producers and their causes discovered harmful pathogens on Gram-negative bacteria, and the most significant bacteria are bio-film producers and multidrug-resistant that causes recurring infections in human beings. Bacteria in the majority cannot be evaded with drugs, and cannot even match the capability of bio-film [19, 20]. The risk factors increase for health care facilities, the elderly, diabetes patients, and immunocompromised people. These bio-films can directly come in contact with wounds that cannot be healed. Bio-film can cause recurring infection due to its multi-drug resistant capability and has already been found in GNB. Penicillin is the most renowned drug because of its target potency against the peptidoglycan layer and is considered more effective against Gram-positive bacteria. The murein coating on the cell wall of Gram-positive bacteria makes it more effective than Gram-negative bacteria with a thin layer of peptidoglycan. Most bacteria that are present in bio-film are resistant to several antibiotics due to mutation. Multi-drug opposition Gram-negative bacteria caused an ascendant UTI, being the microorganisms, most habitually included are Escherichia coli, Klebsiella, P. aeruginosa, and Acinetobacter spp. [21]. In hospitals, some Gram-negative uropathogens were found to be highly resistant and discovered to be allied to harmful pathogenic strains [21].
E. coli
It is an aerobic and facultative anaerobic bacillus. These bacteria can grow at an optimum temperature of 37°C. Some of the strain shows beta hemolytic on blood agar medium and on MacConkey medium; it shows pink color due to lactose fermentation. It is part of normal intestinal flora. These bacteria can cause mainly four major infections in human beings. Those are diarrhea, urinary tract infection, pyogenic infection, and septicemia. It is a common uropathogenic organism that can cause infection in the urinary tract. It has transformed itself as the most successful commensal microorganism owing to its higher adaptability rate and versatility behavior that colonizes multiple body parts [22]. UTI often emanates inside the intestine of a patient. It infects patients with UTI through either hematogenous route or ascending route through fecal flora growth in the stool. Women are mainly at high risk of getting affected by UTI due to the short gap of the urethral site and spreading to the perineum, and then they easily access the bladder. E. coli is also responsible for recurrent and hospital-acquired infection and is also associated with indwelling medical catheter–related infections and can cause catheter-associated UTIs. In that case, there are many phytochemicals from effective plant extracts that have been found sensitive against bacterial bio-film [23].
P. aeruginosa
There are many different types of Pseudomonas species found in the environment as an encapsulated, Gram-negative, oxidase positive, non-lactose fermenting rod–shaped opportunistic pathogenic species can cause mild to moderate infections which are motile by polar flagella. Among 200 species of Pseudomonas, one of the most causative pathogenic strains which is responsible for causing infection in humans is P. aeruginosa. These bacteria can cause infection in various parts of the human body, i.e., the lungs, urinary tract, blood, ear, eye, and skin [24]. These bacteria survive in the environment and can spread through different sources in day-to-day life. Mainly, the resistance capability of the bacteria can increase in the hospital environment due to the formation of protective layers or the host’s immune defense called bio-film. This type of bio-film-producing bacteria can cause non-treatable recurring infections in patients who are admitted in the hospital for a long period for treatment or people who are having less immunity. Bio-film can easily grow on both living and non-living surfaces. It mainly grows on contaminated hand gloves, equipment, catheters, etc. [25, 26].
A. baumannii
It is a typically non-lactose fermenting rod–shaped coccobacilli, Gram-negative opportunistic bacteria found in people who are having less immunity. This is nowadays found as an MDR condition due to the ability to produce bio-film. It can also cause non-curable recurrence infections in human beings [27].
Bio-film Producing Microbes for CAUTI
Various infections are associated with bacterial colonization inside the urinary catheter surface, which forms bio-films. The community of microbes that are physically connected with the superficial layer refers to bio-film. The major characteristics of bio-film are the aggregation of bio-film with cells, exchange of resistant genes with the plasmids, endotoxin production is resistant over host defense mechanism, as well as antimicrobial more resistant to the planktonic bacteria which can survive in diverse environmental conditions. Bio-film is composed of multi-species like E. coli, P. aeruginosa, E. faecalis, and Klebsiella [28, 29]. The risk of developing symptomless bacteriuria with symptomatic UTI is usually increased by urinary catheterization through typical uropathogens, for example, Enterococcus spp., Pseudomonas spp., and Staphylococcus spp. [29].
The Centers for Disease Control and Prevention and the Department of Health and Human Resources revealed that UTI is the most common infection associated with healthcare as reported to the National Healthcare Safety Network (NSHN). Seventy-five percent of UTIs in Clinical admitted patients are related to urinary catheters. Fifteen to twenty-five percent of hospitalized patients get urinary catheters in a hospital stay. It is assessed that clinics spend more than $1 billion in overseeing catheter-related urinary tract infections or CAUTI [30]. Hospital-acquired catheter-associated urinary tract infections represent more than 1 million cases every year. As per the Centers for Disease Control NHSN, infections inferable from inhabiting urinary catheters represent 75% of UTIs acquired in hospitals and medical clinics.
Bio-film-Associated UTIs
Ongoing Bacterial Prostatitis
The bacteria have a protective climate by the prostatic ducts and acini (a small sac-like cavity) to facilitate the reaction. If the bacteria are not annihilated (destroyed) by any invulnerable (impossible to harm or damage) response, it tends to the multiplication/formation of bacterial colonies.
UTI Through the Use of Catheter
CAUTI can provoke complexities, for instance, renal inflammation by microbial infection, inflammatory condition of the urinary bladder, many septicemias, joint agony (extreme physical suffering), endophthalmitis, inflammation of endocardium, and meningitis. Moreover, CAUTIs also achieve delayed hospital stay, expanded expenses, and mortality [31, 32].
Devices Used in Medical Division–Related Bio-films
Both Gram-positive and Gram-negative microbes are always responsible for the formation of bio-films on medical devices, for example E. coli, P. aeruginosa, and A. baumannii. These bacteria are present on the skin of healthy patients and inside the water passage ports where they contaminate the medical equipment and apparatus. Indwelling apparatus are regularly colonized by multi-species bio-films. At first, bio-films are made from one animal category, later on, proceeded with additional openings cause multi-species bio-films. Various factors boost the speed and formation of bio-films. Firstly, the bacteria join the outside of the gadget sufficiently at the last. This underlying pace of connection relies upon the sum and kind of bacterial cells inside the liquid during the presence of the gadget, the course period through the device, and accordingly, the physicochemical attributes of the uncovered surface [33, 34]. However, several researches have been contributed in the relevant field and few of the important key outcomes are mentioned in Table 1.
Recurrent Cystitis
Infections are led by the bladder and kidney well-being deficiency. For example, stones in the kidney, abnormal urine drainage, or less drainage. An appointment with a doctor is to be taken for a laboratory check-up if such problems are suspected. While having sex, there is a chance of cystitis in females. Utilization of diaphragms and spermicide might be some reason for cystitis [33, 34]
Hormones
Vagina, bladder, and urethra are the organs that respond to the biochemical hormone called estrogens. The levels of estrogen in the body are reduced after menopause. The thickness of tissues of the organs gets reduced; it gets weaker and becomes dry. The increase in the risk of recurrent cystitis is seen after these changes. The changes in the urinary tract lead to cystitis, which is most common during pregnancy.
Pyelonephritis
On reaching the kidney, the bacteria cling to the uroepithelium and the structure slightly bio-films before attacking the urinary system. Moreover, the catheter stream gets blocked because of the development of translucent bio-films about bladder distension, urine spillage, and renal inflammation.
Renal Calculus and Its Infection
Simply, if there should be an occurrence of urease-positive bacteria, bio-film development is joined by the statement of calcium and magnesium gems. This crystallized state happens exclusively after the bio-film is made since the bio-film fills in as a nucleation site [46].
Prevalence of MDR Gram-Negative Bio-film Producers in CAUTI
CAUTI is the most common hospital-acquired infection affecting over 1 million hospitalized patients. Mostly, 75% of UTIs are acquired in the hospital by indwelling urinary catheters. This kind of infection is associated with the colonization of endogenic bacteria on the urinary catheter surface and forms bio-film inside the patients. Bio-film refers to a community of microbes physically connected with the superficial layer, where it plays a vital role in continuing bacterial infections. Bacteria residing within the bio-film are more resistant to the planktonic bacteria and can survive in diverse environmental conditions. The major properties of bio-film are an assemblage of bio-film with cells, exchange of resistant genes within the plasmids, endotoxin production is resistance over host defense mechanism as well as antimicrobial. At present, some of the well-known hospital pathogens are attributed to “ESKAPE” group of organisms (K. pneumoniae, E. faecium, A. baumannii, S. aureus, Enterobacter spp., and P. aeruginosa) [47, 48]. Bio-films of a urinary catheter is mainly composed of multi-species of bacteria like E. coli, P. aeruginosa, E. faecalis, and Klebsiella [27]. Hospital-admitted patients with any kind of minor or chronic disease were very much sensitive to these urinary tract infections, and detection of bio-film producers is slightly difficult because re-insertion of indwelling catheters was found most noticeable infection-causing agent [28]. The risk of developing symptomless bacteriuria with symptomatic UTI is usually increased by urinary catheterization through typical uropathogens, for example, Enterococcus spp., Pseudomonas spp., and Staphylococcus spp. [31]. Here, we have discussed and lightened up on some of the clinical investigations of UTI caused by MDR GNB. Table 2 presents the prevalence study of catheter-associated infection-causing pathogens.
In the year 2020, Almalki et al. [49] demonstrated that MDR E. coli are the increasing concern causing this CAUTI. However, the pathogens associated with CAUTI are mostly from the Enterobacteriaceae group. A study from Saudi Arabia reported that from 350 clinical samples were collected from catheterized UTI cases; 36% of the isolates were found to form bio-film. The significant bio-film producers were 20% E. coli, 19% of Klebsiella, 8% of Proteus mirabillis, 17% of P. aeruginosa, and 9% of Citrobacter sp. The most significant findings of this study were PAN drug-resistant E. coli, Klebsiella, and Pseudomonas were found with XDR activity [49].
In the year 2018–2019, Khalek et al. [13] researched catheterized patients. Total numbers of 350 urine samples were collected from UTI-affected catheterized patients admitted in the urology department and intensive care units of Tanta University Hospital. P. aeruginosa was isolated from 14.6% of CAUTI. All of them have highly resistant against cephoperazone, 82.5% of Pseudomonas isolates were resistant against chloramphenicol, 19.6% were meropenem resistant, 35% to ceftazidime, 25% were resistant to amikacin, 11% to ciprofloxacin, 8% to norfloxacin, 33% to ceftazidime, 35% to aztreonam, and 28% to cefepime [13, 58].
In Nepal during 2018, Maharjan et al. [50] executed work at Tribhuvan University Teaching Hospital. During that work period, 105 urine specimens were collected from catheter-associated infections in urinary tract affected patients. Among total collected samples, 62% pathogenic growth was significantly found with UTI confirmation in which female was the most predominantly affected category. Fifty-seven percent E. coli followed by 15% of K. pneumoniae, 12% P. aeruginosa, 8% S. aureus, 3% Enterobacter spp., and least numbers of P. mirabilis, Acinetobacter spp., and E. faecalis were found. Overall, 46% of strains were found as positive bio-film producers, and 54% were negative bio-film producers. Here, more than 90% bio-film-producing Gram-negative strains were resistant to ampicillin and amoxicillin–clavulanate, and from this study, we considered that bio-film producers were found highly resistant than non-bio-film producers [50].
In Indonesia in 2020, Gunardi et al. [54] performed one original research on bio-film producers. This work has been conducted out of collected 109 catheterized patients’ samples. Seventy-eight percent of catheters were found culture positive, which was greater than collected urine samples (38%). Moreover, 18% of Candida spp., 28% of E. coli, 16% of K. pneumoniae and 13% of E. faecalis were found from catheter culture. Analysis showed that mainly, catheterization was associated with developing risk factors for bio-film formation [59]. Moreover, this study concluded that 68% of patients had been resistant to mostly wide-ranging antibiograms, which means that there was no special effect of antibiotics found against bio-film producers [54].
In 2017, there was a study conducted by Biswas et al. [60] on bio-film producers at M. S. Ramaiah Medical College and Hospital, Bengaluru. In this study, there were 37 urinary specimens collected from catheterized indoor patients, which all acquired with E. coli infection. Around 25% of hospitalized patients were having infections with indwelling Foley catheters. Here, 50 numbers of samples out of 55 cases were identified as bio-film producers [60].
A research was conducted in 2019, at Koirala institute of Health Sciences, Nepal, by Bahadur et al. [32] for 6 months. There was a total number of 10,423 urine samples, and 1360 samples were obtained from indwelling catheter-associated patients and concluded that comparatively, CAUTI is highly infectious than common UTI. Among total 11,783 collected urine specimens, overall, 2216 positive cultured urine specimen isolates were found and from them; 1745 were GNB, and 471 were GPC. This study attributed to the fact that most tertiary care hospitals admitted catheterized patients were highly affected with this nosocomial infection, and many other researchers partially proved that indwelling catheters might have increased risk factors of UTI [32].
In 2019, Aygun et al. [51] revealed that infections, especially carbapenem-resistant bacterial infections, were associated with poor results among serious children in pediatric intensive care units. Among all infections, carbapenem resistance was found in approximately one-third of GNB culture outcomes, and total parental nutrition was a risk factor for developing carbapenem-resistant infections. Here, it is concluded that carbapenem-resistant infections are a serious issue among pediatrics and help to reduce the inappropriate utilization of antibiogram, especially carbapenem, which will be important in preventing MDR bacterial infection [51].
In 2020, Wabe et al.’s [61] research commenced on UTI at Saint Paul’s Hospital Millennium College with asymptomatic infection of 290 pregnant women. The predominant bacteria were E. coli, and most of them were resistant to amoxicillin (87%) and cotrimoxazole (78%). Fifty-seven percent of isolates were asymptomatic MDR, and most of them have previously been infected with catheter-associated UTI [61].
From 2014 to 2018, in Beijing Tsinghua Changgung Hospital, research conducted by Wang et al. [35] upon MDR bacteria can cause UTI which were having urinary stones. In this 4-year study, 1655 samples were collected from urinary stones having patients among which 367 patients had positive microbial growth [35, 62]. E. coli was found as the most common isolate with a prevalence of 29%, followed by 12% E. faecalis, 10.5% P. mirabilis, and 6.8% Klebsiella pneumoniae 44.4% isolates were identified as significantly associated with MDR formation [63]. The three most common GNB were E. coli, P. mirabilis, and Klebsiella pneumonia, with a MDR rate of 84%, 63%, and 48% respectively. Drug resistance rates varied between MDR and non-MDR for ampicillin, cefazolin, ceftriaxone, cefepime, gentamicin, amikacin, and levofloxacin [63].
At Chennai, in a multidisciplinary hospital, the total number of admissions during 2012, 2013, and 2014 were 221, 236, and 116 respectively [56]. Nagvekar et al. [56] found during his research the elevated level of positive cases, and 26 to 34% antimicrobial resistant bio-film–producing strains among these notified positive samples were identified. Positive cases are mostly found in the hospitalized patients during staying in hospitals. Acinetobacter and Klebsiella were found as the most prevalent microbes than E. coli among those positive antimicrobial-resistant cultures [56].
The study upon bio-film-producing K. pneumoniae published in Saudi Journal of Biological Sciences, by Rajivgandhi et al. [64] concludes that the K. pneumoniae strain was carbapenem-resistant, which was based on the method of phenotypic disc diffusion in the year 2021. The level of resistivity is confirmed by the MIC method [37]. Additionally, using the multiplex PCR method, the presence of the carbapenemase genes IMP and MRP in the bacterial sample was confirmed. The selected Klebsiella strain was carbapenemase and bio-film producer, which was further confirmed by the resistivity characteristics features of carbapenem-resistant K. pneumoniae [64].
In between 2018 and 2019, Umema et al. [52] collected 80 urine samples from 80 pregnant women, and the research took place at Taibah University. Out of 80, there was 65 numbers of women who had UTI, which reflect an aggregate of 80% prevalence of UTI in pregnant women [65]. After the investigation, the results reflect that 67% of uropathogenic bacteria strains belonged to 31% numbers of E. coli, 23% were of Klebsiella, 16% of Pseudomonas, Streptococcus was 4%, 4% of Enterococcus, and 3% Proteus genera, which were identified using the characterization, biochemically. Against amoxicillin, pipemidic acid, and ampicillin, E. coli has the overall highest resistivity; against pipemidic, ampicillin, and ciprofloxacin and cefotaxime, the Klebsiella bacteria have resistivity next to E. coli; for Pseudomonas species, the resistivity was seen against ciprofloxacin and cefotaxime. The highest MDR were identified using 16S rRNA as P. aeruginosa strain UA17, E. coli strain UA32, and K. pneumoniae strain UA 47 in the three strains [52].
The association between the antimicrobial resistance and bio-film and hemagglutinin, produced by uropathogenic E. coli, was assessed in research in the year 2019 by Hagos et al. [53]. About 27.3% among the study participants was found as UTI; the major etiologic agent was found as uropathogenic E. coli, followed by coagulase-negative Staphylococcus. In the catheterization history, UTI along with the risk factors were found significantly. It was associated with MDR, bio-film formation and recurrent UTI. Nitrofurantoin was listed as the most effective drug for uropathogenic E. coli, after the detection of antibiotic susceptibility of drugs. The resistivity towards norfloxacin, cotrimoxazole, and gentamicin was found in approximately 100% of producers of bio-film [53].
In 2020, Anusha et al. [66] conducted research on ESBL and those species of uropathogenic pathogens which produce bio-film. The Beta-lactamase and the ability to produce bio-film were the major virulence determinant of uropathogens, proven by many studies and researches. The recurrence and the level of chronicity in infections is a serious concern among uropathogens in the emergence of antibiotic resistance and bio-film producers. Extended spectrum beta-lactamase production and antibiotic susceptibility pattern among uropathogenic E. coli isolate and the determination of association in bio-film formation were the main objective in the overall studies and research. A total of 11 antibiotic-resistant groups were detected among the 127 E. coli isolates; six isolates possess ESBL ability among the 11 isolates. Except for E1 and E32, all the other strains possessed the ability of bio-film production; urinary pathogens have the most virulence factors in the formation of bio-film. So, there is a necessity in the detection of bacteria that produce bio-film to conduct the surveillance of the antibiotic susceptibility, and the device is effective for the measures in the control of infection [66].
In 2019, Muhammad et al. [5] conducted a study with the urine sample and that resulted in 43 numbers positives samples, and their isolates were found 100% bio-film producers. A total of 43 E. coli isolations were undergoing the process of the formation of bio-film. Thirteen (30.23%) out of 43 have shown a strong bio-film formation whereas the rest 25 (58.13%) and 5 (11.62%) were moderate and weak bio-film producer isolates respectively. The predominance of the infection was 61.85% as observed in this study. Females were suffering more predominately 62.61%, despite lacking statistical significance. These results for the prevalence of CAUTI were 61.9% and 66.2% for females. There was evidence in a review of (Nicolle, 2014), which recognized the similar incident in most developing nations. There could be attributes to the anatomical differences of urogenital position, anal proximity, and shorter urethra because females are suffering more predominantly [5].
Mechanisms
Mechanism of Pathogenesis of Urinary Tract Infections
Colonization After Adherence
The pathogenesis of a urinary tract infection begins with the adhesion of the pathogenic bacteria. The infection of the urinary tract begins with periurethral contamination caused by gut swelling, followed by colonization of the uterine wall and subsequent migration of the microbe (pathogen) to the bladder (Fig. 1) [2, 59, 67]. The success of uropathogen colonization inside the bladder is usually determined by the host pathogens and the outcome of their intricate interplay. The pathogenic organisms can travel towards the kidneys by aggregating and overcoming host immune surveillance, reattaching by the adhesion mechanism of pilli, colonizing the renal epithelium, and ultimately causing tissue damage through the release of toxins. Many known uropathogenic bacteria, such as K. pneumoniae and S. saprophyticus, cause uncomplicated UTIs, and UPEC can attach to the bladder’s epithelial cell. The principal protein components of superficial facet cells, such as K. pneumoniae and UPEC, attach to uroplakins, which create a crystalline array to protect mammalian bladder tissue from harmful substances [68, 69]. In addition to uroplakins, the other receptors for UPEC are α3β1 integrins, which are found on the surface of urepithelial cells. By distinction, as the bacteria travel through the urinary tract, it becomes attached to the bladder stone or renal stone, or the associated catheter by any physical obstruction, resulting in the onset of complicated UTIs [70]. Others, however, such as P. mirabilis, P. aeruginosa, and Enterococcus spp., are more likely to cause complicated UTIs (Fig. 1). Following that, these uropathogenic bacteria frequently form a protective layer, i.e., bio-film, which is responsible for the colonization and growth of resistant infections [65, 71].
Mechanism of Virulence Factors of Uropathogenic Bacteria
Elements that are produced by microbes and evoke diseases are called the virulence factor. Virulence is the ability to spread the infection to the host which can cause diseases. These factors are either secretory, membrane-associated, or cytosolic [72].
There are many virulence factors of bacteria that can cause diseases:
-
i)
Adherence and colonization: Adherence is an essential step in bacterial pathogenesis, required for colonizing a new host. Fimbriae or pili attached to the living surface or other cells are required for the virulence mechanism of some bacterial pathogens.
-
ii)
Invasion factors: Pathogenic organisms invade the host cell
-
iii)
Capsules and other surface components: This factor act like a protective cover that may prevent them from phagocytosis.
-
iv)
Endotoxins: The endotoxins on Gram-negative bacteria can cause fever, inflammation, and many other virulent incidents.
-
v)
Exotoxins: These are including several types of toxin proteins and enzymes released from pathogenic microbes.
-
vi)
Siderophores: These are iron-binding elements that permit some microbes to compete with the host for iron that is bound with hemoglobin, lactoferrin, transferrin, etc.
UPEC and K. pneumoniae are the most common uncomplicated UTI pathogens. Distinct from other uropathogens, UPEC and K. pneumoniae have dedicated mechanisms for:
-
(a)
Recognition, adherence, and invasion of urothelial cells
-
(b)
Replication inside the cell to form intracellular bacterial communities
-
(c)
Bacterial dispersion and host cell re-invasion [73].
The adhesive properties of UPEC have been found the most important factor of pathogenicity. Adhesins of UPEC can contribute to virulence mechanisms in different ways: (a) activate straight away to the signaling pathways of bacterial and host cells; (b) other bacterial products delivered to the host tissues; and (c) promote invasion of bacteria [74].
In 2012, Bein et al. state that the type 1 fimbriae or pili shown virulence activity in animal, but in human, still those functions remain not clarified [74]. In 2015, Flores-Mireles et al. revealed type 1 pili of UPEC is essential for colonization, persistence, and invasion.
Bacteria generally use various types of virulent elements to evade phagocytosis by cells of the immune system. Uropathogenic bacteria initially use type 1 pili to form bio-film and colony on the surface of the bladder. Most commonly, we found K. pneumoniae, UPEC, P. mirabilis, and P. aeruginosa. The FimH is the mannose-binding adhesin property of bacterial pilus, which is highly similar but having difficult binding specificities. FimH-mediated bio-film formation is inhibited by helpful mannose, as appeared to the methyl mannose–mediated inhibition of UPEC FimH. Overall, K. pneumoniae FimH has weaker adherence capability to the bladder than UPEC FimH.
Stages of mechanism of virulence factors (Fig. 2) in bacterial pathogenesis [75] are given below:
-
1.
Entrance of uropathogens through the urinary tract
-
2.
A group of microbes denied the immune system cells of the host with the use of phage-encoded modification proteins such as LPS altering proteins, outer membrane proteins, effector proteins, coat protein, and effector proteins.
-
3.
Reaching the active site of the pathogen, it can either remain extracellular and complicated toxins which may be taken up by targeted host cell or, in the case of intracellular microbes, are internalized within the target eukaryotic cell. A range of virulence factors that are phage-encoded is essential for entrance and survival within the host cell such as enterotoxic, cytotoxic, cardiotoxic, and neurotoxic.
-
4.
Phage-encoded microbial toxins and protein effectors can cause a range of effects.
Mechanisms of Pathogenesis During Catheter-Associated Urinary Tract Infections
Microbes may move towards the urinary bladder through the contaminated catheter tip during catheterization with urethral distal flora or from microbes ascending the outside or the inside of the catheter. Urine remains in the urinary bladder of catheterized patients [76].
Bio-film layer is so preventive for microorganisms on urinary catheters due to it provides a protective survival shell-like atmosphere to the microbes, so the bio-film-associated urinary catheter is too difficult to exterminate with the use of different drugs. Microbes function as a community inside the bio-film layer and communicate closely with one another.
Flores-Mireles et al. explained the mechanism of pathogenesis during catheter-associated urinary tract infection. In this study, research on CAUTIs mediated by P. mirabilis depends on mannose-resistant proteus-like pili for initial attachment and for formation of bio-film on the catheter and inside the wall of bladder [2].
Bacteria trigger an inflammatory reaction on the inner surface of the bladder, which results in the influx of neutrophils and sloughing of epithelial cells with bound bacteria. In contrast, surface of the catheter has an absence of inherent defense mechanisms.
The initiation of bio-film formation on the surface of the catheter is the deposition of a conditioning film of host urinary components, including electrolytes, proteins, and many other organic molecules.
This conditioning film can change the surface of the urinary catheter and counteract any anti-adhesive properties. Some free-swimming bacteria stick to the surface with the hydrophobic and electrostatic interactions and through the utilization of pili or flagella. Attachment or adhesion is followed by cell division, recruitment of additional planktonic bacteria, and secretion of extracellular matrix.
Not only does the urinary catheter invite bio-film formation, but the insertion of the catheter itself impairs many of the normal defense mechanisms of the bladder. The urinary catheter provides adhesion space to microbes, and the catheter gives rise to microbes for entering with the external and internal surface to the urinary system initiates from urinary tract opening. Urine often flows in the bladder and catheter itself, and urinary stasis helps to the multiplication of bacterial cells [77]. Though triggering an inflammatory response by mechanical erosion, the catheter also damages the mucosa of the bladder [78, 79].
Patients with long-term indwelling catheterization culture their urine and catheter monthly, which show that the bacterial flora is constantly shifting and changing, regardless of antibiotic use [80].
The process of hydrolysis of urea in urine produces a subsequent amount of urease, and the formation of calcium crystals and magnesium ammonium phosphates gets induced by the production of urease, with increasing pH level. Bacteria produce extracellular polymeric substances which adhere to the tips of the urinary catheter (Fig. 3) for trapping crystals and permit the formation of crystalline bio-film. These bio-films are the protective layer of microbes that protects the community of microbes from the immune system of the host cell and multiple antibiograms. In addition, these systems prevent urine drainage properly and resulting in reflux and encouraging to occur different abnormal conditions like pyelonephritis, septicemia, and shock. Finally, the bacterial toxins hemolysin (HpmA) and toxic agglutinin are responsible for the destruction of tissue and dissemination of microbes to the renal system. HpmA inserts itself into the cell membrane and encourages it to form the pore. These HpmAs make the host cell unstable, causing exfoliation and releasing nutrients as well as damaging the tissue. Toxic agglutinin is the most responsible element for the destruction of the cell wall and reduces the immunity of host cells, causing crack of cytoplasm and gives rise to the osmotic stress and causing depolymerization of filaments of actin, thus compromising the structural integrity of the cells. Through these toxins, all nutrients are released and, using siderophores, allow the microbes to scavenge iron [2, 78, 79].
Mechanism of Antibiotic Resistance
Antibiotics work to destroy bacterial cells, and this can be done by either destroying genomic content or by destroying bacterial cell’s organelles [81, 82]. Antibiotics are having a specific mode of action for destroying the cell wall and interrupting the cell growth to spread infection. Overall, the process of specific shifting inside the cells of microbes is done in different ways, which are impairment synthesis of cell wall, impairment of protein biosynthesis, impairment of DNA replication, alteration of cell membrane, and anti-metabolite activity [81, 83].
Antimicrobial resistance has been defined as the adopted ability of microorganisms to develop resistance against the specific antimicrobial agents to which they were once susceptible [84, 85]. The disappointment or confined infiltration of antimicrobial specialists into bio-films, the section in moderate developing or starvation expresses, the assurance of persisters, or other pressure lenient aggregates are known to be anti-infection resilience instruments of bio-films that achieve infections that are difficult to treat and require complex multi-drug treatment methodology, especially when bio-films are poly-microbial [58]. Mechanism of antibiotic resistance described herewith figure (Fig. 4) [86]. Bacterial anti-toxin obstruction is likewise one of the results of the bacterial bio-film networks which add to the ongoing infections. These bio-film networks have barely any extra obstruction systems when contrasted with planktonic ones which hamper the therapies choice and prompt rise similarly as spreading of the constant awful bugs [6]. Clinical observations and test studies showed plainly that anti-toxin treatment alone is much of the time deficient to annihilate bio-film infections. Consequently, to successfully treat bio-film infections with presently accessible anti-toxins and assess the results become significant and pressing for clinicians [87].
Bacterial bio-films are portrayed as exceptionally impervious to anti-toxin treatment and insusceptible responses. Although, notably, anti-infection treatment is at present generally a significant and viable measure for the control of microbial infections, notwithstanding, anti-infection medicines are practically difficult to destroy bio-film infections. In vitro and in vivo analyses exhibited that the minimum inhibitory concentration (MIC) and the base bactericidal concentration (MBC) for bio-film bacterial cells were normally a lot higher (roughly 10–1000 occasions) than the planktonic bacterial cells [87]. The powerful anti-microbial MBC in vivo for bio-film annihilation is in this manner, which is difficult to reach by conventional anti-microbial administrations because of the toxicities and the results of anti-toxins and the limit of renal and hepatic functions. Treatment of bio-film infections turns out to be subsequently tested and attracts fundamentally logical consideration. Various clinical investigations have been performed, which would benefit the control of bio-film [87, 88].
Mechanism of Bio-film Formation
Many unicellular cells prefer to form surface attachments, or they form a surface-attached multicellular environment called bio-film, and the formation of this thin protective layer requires close contact with the surface. For example, in the rainy season, we can see a mat-like growth on a substrate that is bio-film. Depending upon the type of microorganism involved, it may be heterogeneous or homogeneous. Bio-film formation is very important for microorganisms to survive in a natural environment. There are many steps and factors involved in this process like quorum sensing is a responsible factor for bio-film formation. It is very useful for spreading infections and clinically very important to study bio-film.
Bio-film is communities of bacteria that combine making themselves resistant against broad-spectrum antibiotics, by organizing local area of bio-film typified inside a self-created polymeric grid and disciple to an inactive and living surface. Bio-film is mainly formed on surfaces submerged in or in contact with water. Microbial aggregates and floccules as well as adherent populations within the pore spaces of porous media are also considered as bio-film. The development of molding film is promoted by the testimony of urinary segments of biomaterial in the arrangement of a bio-film. The ions, polysaccharides, and different parts generally diffuse to embed before the appearance of the principal life forms or first organisms. A considerable amount of particles are proteinaceous, which gives a destination to the receptor of which encourages. Along these lines, the making of a molding film changes the surface qualities of inserts. It is therefore that inserts with modified surface attributes can be ineffectual as systems of forestalling microbial connection. The part of the molding film is indispensable as numerous pathogens do not have systems to follow straightforwardly onto exposed embedded surfaces.
The subsequent stage is the methodology and connection of pathogenic microbes. With the end for bacteria to respond to a surface, they should have the option to “sense” their vicinity to these surfaces. A bio-film forms slime and proteins adhering to a surface. A bio-film forms when bacterial cells adhere to a surface and produce a matrix of extracellular polymeric substances (EPS) which is a sticky type of glue-like material. The bacteria remain embedded within EPS which protects them [89]. A developed bio-film is developed of three layers (Fig. 5) [90]:
-
1.
Microbes attached to the convenient surface.
-
2.
Formation of layer and start producing bio-film.
-
3.
Maturation of bio-film layer.
-
4.
Dispersion of bio-film and detachments and reversion to planktonic growth, starting a new cycle.
In Table 3, there are all the proposed mechanisms, and their key features are mentioned shortly.
Strategies Against UTI and Antimicrobial Resistance
Co-existence of Multi-drug Resistance with Bio-film
Antibiotics are used in the field of medical microbiology for the prevention of infection against pathogenic bacteria, for more than a few decades. Antibiotic resistance is a complex, multifaceted, and multi-sectoral ecosystem problem that threatens the “one health” framework. So, in this context, understanding of drivers of antibiotic resistance holds the key towards addressing the global menace in lower- and middle-income group countries [91]. There are certain bacteria that capsule themselves with a protective layer of bio-film that prevents itself from antibiotics. So, it plays a key role as a multidrug-resistant strain.
Approaches to Target Bio-film
Bacterial colonize and receive nutrients inside the light organs a structure located in the squid’s mantle cavity. The microbes glow just under specific conditions the genes are expected to make light are off when the densities of the bacterial cell are low, such as in sea water, but they glow up even more under denser conditions such as in the light organ of the squid when enough cells are together to have an enhanced visualization how do cells know they have accomplished a sufficient number of individuals that is quorum before turning on the luminescence genes. Incidentally, this phenomenon is termed quorum sensing (QS). It analyzes the production of auto-inducer phenomenon by which bacterial cells being able to sense their surrounding population [92]. Its flagging is answerable to the gene expression which coordinates at bacterial local area, incorporating to control the outflow of destructiveness factors, just as impacting bio-film formation. Numerous common products go about as quorum sensing inhibitors, and in this way, have gainful impacts towards lessening the formation of bio-film [93].The procedures to grow new medicines are focused in this survey, which targets the methodologies on the development of bio-film that is utilized to treat the infections related to bio-film.
One of the ways to deal with restraining the bio-film formation is to target the quorum sensing (QS) regulatory system that eventually causes the hindrance of bio-film [94]. Plants are the rich asset of a number of mixes that can meddle with the QS framework, where the receptors got inactivated in the pathway. Those mixes with their optional metabolites have antibacterial just as anti-bio-film potential. Flavonoids and terpenoids just as the crude extract got from Senegalia nigrescens have shown against quorum sensing action [26]. These methodologies mean to lessen or restrain bio-film formation, or to increment bio-film scattering.
Another methodology utilized by scientists for distinguishing molecules active in forestalling bio-film formation is the screening of enormous chemical libraries. One of the soonest HTS utilized a luminescence-based way to deal with quantities P. aeruginosa. Bio-film biomass framed on 384-well configuration pin devices, rather than conventional crystal violet (CV) bio-film staining [95]. After screening 66,095 mixes, 30 molecules were recognized that hindered bio-film connection by more noteworthy than half when utilized at concentrations under 20 μM [95].
Another conceivable methodology for treating bio-films associated infection is the utilization of mixes that scattering from enzymes that corrupt the polymers of the ECM and accordingly disintegrate bio-films. One of the most punctual dispersal specialists was the disclosure of cis-2-decenoic acid (C2DA), an unsaturated fat delivered by a few sorts of bacteria [96].
Through bacteriophages, it might be possible to treat infection-related to bio-film. Bacteriophage treatment has really been utilized for more than 50 years, yet the rise of multi-drug resistant bacteria and the proceeded advancement of opposition in numerous bacteria have incited more studies into the utilization of bacteriophage as a method for treating infections [97]. The benefit of utilizing bacteriophages is that they can contaminate and slaughter both pathogenic and nonpathogenic strainsa [87].
Formation of bio-film, an assortment of pathogenic microbes, can represent a genuine well-being risk that is very difficult to combat. Nano-technique, notwithstanding, addresses another promising way to deal with act against and destroying bio-film-framing pathogenic microbes due to the low cytotoxicity and novel mechanism of action. In the current study, a reasonable blend and portrayal of biological compatible nanoparticles made up of silver nitrate and medicinal leaf extraction was investigated. The blended AgNPs were screened for antibacterial and hostile to bio-film action against pathogenic infection getting from different gram-negative bacteria. The utilization of nanoparticles has emerged as a promising approach and being a preventive measure against bio-film formation by destroying the exopolysaccharides (EPS) of the bio-film matrix and killing the bacteria [92].Consequences of the investigation exhibit the capability of phyto-integrated silver nano-particle to go about as against bio-film specialists and for other biomedical applications [98].
Therapeutic Approaches of Phytochemicals Against Bio-film Producers
Phytochemicals are the compounds that are produced by plants through plant metabolism and found in leaves, fruits, vegetables, etc. Medicinal plants are very renowned to treat different kinds of infection in Ayurveda. According to a survey report of the World Health Organization (WHO), 80% of the third world populations depend on traditional herbal therapies [99]. Based on the current study, there are some pathogenic microbes found which can survive on the live surface and caused many recurrent infections over there. The reason behind microbial recurrent infection is the multi-drug resistance capability due to the formation of a peptidoglycan layer called bio-film. Multi-drugs resistance means many of the high spectrum antibiotics do not work to kill the bio-film-coated bacteria due to the presence of that protective layer. Researchers found that many of the phytochemicals and antioxidant compounds which are present in different parts of the body are very much helpful to cure severe infections [100]. A study in 2016 about antimicrobial and anti-bio-film activities of 8 methanolic plant extracts was thought about in contrast to clinically isolated microbes. The antimicrobial movement of Prosopis laevigata, Opuntia ficus-indica, and Gutierrezia microcephala was demonstrated by the primer screening dispersion. From 0.7 to >15 mg/ml, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were resolved. The expansion of plant extracts (MBC × 0.75) was accessed to the specific bio-film formation file (SBF). In a partial subordinate way, there is a significant decrease in SBF caused by Opuntia ficus-indica. By the utilization of saltwater shrimp (Artemia salina L.) lethality test, the cytotoxin action of plant extract was resolved [101].
Antimicrobial resistance (AMR) has a very significant role in the health issue globally; however, there is a gradual expansion of role among the AMR and advancement of new antimicrobial. From the plant extracts, there might be great development per may be wellsprings of compelling antimicrobial mixes which can act against planktonic and additionally bio-films of pathogens [102]. For the treatment of various bacterial and parasitic infections, and pharmacological improvement, bio-film hindrance or inhibition is considered a significant medication that is presently broadly contemplated [89]. In addition, 80% of the total populace and over 30% of pharmaceutical formulations are reliant upon therapeutic plants, as detailed by WHO [103]. There are many phytochemicals’ analyses already done against infections and bio-film; those are tannins, saponins, flavonoids, quinines, glycosides, cardiac glycosides, terpenoids, terpenes, beta-sitosterol, phenol, coumarins, steroids, alkaloids, anthocyanin, betacyanin, ellagic acid, phenolic acid, and gallic acid. In 2020, Das et al. [104] explained that these phytochemicals are found in different medicinal plants and using different basic methods like agar well diffusion, disk diffusion, or minimum inhibitory concentration with crude plant extract. Vaccinium macrocarpon Aiton (cranberry) has been found best remedy against UTI. Some evidences suggested that proanthocyanins present in cranberry, which prevent bacteria from adhering to the walls of the tract of urinary system. Probiotics such as Lactobacillus and Bifidobacterium are beneficial microorganisms that may act by the competitive exclusion principle to defend against infections in urinary tracts [104]. In 2019, Lagha et al. [105] found that in T. zygis, an essential oil is found that has high antimicrobial activity against E. coli followed by Origanum majorana and Rosmarinus officinalis. R. officinalis oil had the highest anti-bio-film activity followed by T. zygis and O. majorana. Hence here, author concluded the above-tested oils showed highly effective antibacterial and anti-bio-film characteristics against UTI caused by E. coli. These oils could be considered as effective alternative treatment or substitution of antibiotics [105].
Recent Update on Anti-bio-film Therapy upon Catheterized UTI
Findings for Control CAUTI
There are numerous compelling strategies that have been adopted to treat catheter-associated UTIs, and those treatments and prevention are not yet proven 100% effective. The anti-uropathogenic, antibacterial, and anti-bio-film activities of many plants’ extracts have been reported by many researchers, which includes many preliminary antibacterial studies using different basic techniques with crude plant extracts [104].Combination therapies have also been started against this infectious disease, and further, more studies are required to know which antibiotic combination efficacy works better together. As adherence has an importance in every step of causing UTI, so one interesting strategy for the development of anti-virulence therapies, including vaccines has been developed to target CUP pili as well as bacterial toxins, siderophores, and proteases [2]. Nowadays, the utilization of reminder facilities and usage of infection control projects can successfully diminish catheter-associated UTIs, although their presence can be challenging. There is still no proof to help the routine utilization of antimicrobial-impregnated catheters; however, the utilization of hydrophilic-covered catheters for clean irregular catheterization can adequately decrease infections. Fundamental outcomes with chlorhexidine-covered catheters are promising. In instances of genuine catheter-associated UTI in patients with a background marked by past anti-microbial treatment or medical care–associated bacteremia, exact anti-infection therapy ought to be started with the movement against multi-resistant uropathogens. Suprapubic catheterization is not better than urethral catheters regarding reducing the pace of catheter-related bacteriuria [106].
Prevention of CAUTI
Great efforts have been contributed and a wide range of approaches have been researched over the most recent couple of a very long time to prevent. Although an ideal solution has not yet been distinguished, numerous significant issues with respect to catheter care and catheter-related infections have been explained. Table 4 summarizes the strategies for prevention and treatment.
The accompanying general recommendations are usually utilized:
-
a)
A closed catheter system ought to be utilized.
-
b)
The length of catheterization ought to be insignificant.
-
c)
In antiseptic conditions, catheters are to be present. The chances of bacteriuria are high if there is the utilization of disinfectant gel.
-
d)
The drainage pouch ought to be kept beneath the bladder position and the attached tube.
-
e)
An indwelling catheter ought to consistently be presented via prepared staff or training personnel.
-
f)
Urethral injury ought to be limited through the utilization of appropriate enough emollient and the littlest conceivable indwelling medical devices.
Conclusion
Bio-films comprise a significant commitment to the high rate, re-occurrence, and enlargement of UTIs, subsequently requiring proficient avoidance and prevention measures. Bio-film examination will provoke a prevalent understanding of the infection cycle and will therefore prompt the headway of new evasion and treatment choices. An ideal strategy against bio-film molecules, with hostile to pathogenesis, dynamic towards control, prevention of spreading and minimize resistant capability of external virulent microbes [6].
Various methodologies have been attempted to decrease the frequency of CAUTI, yet few have demonstrated efficacy. As of late, the part of bio-films was found in the pathogenesis of CAUTI, and the latest techniques including novel urinary catheters to upset the bio-film have been researched. Antibiotic-coated catheters could forestall or defer the beginning of CAUTI during transient catheterization and hold a guarantee for the chance of concealment of CAUTI [21]. Our present review article focused on an overview of different Gram-negative bacteria–causing CAUTI, which can become multi-drug-resistant with the co-existence of bio-film, their origin, and persistence. But if we fail to discover treatment against bio-film-forming MDR, then deadly recurrent UTI might be a possibility in near future.
Broadly perceived hospital-acquired infection on urinary tract commonly found due to reuse of unhygienic intravascular catheter. Catheterization represents a major source of growth of bacteria on its surface, and many resistance growths are achieved through bio-film formation. Notwithstanding, an extensive comprehension of bacterial bio-film arrangement, pathogenic state, and other key variables basic for the advancement of UTIs would help in the improvement of novel and successful infection regulate procedures [46].
Most bacteria in nature exist as bio-films. For the medical profession, bio-films represent a consideration profession, as not exclusively are they connected with most infections in people, yet they are likewise incredibly hard to treat because of their characteristic resilience to invulnerable reactions and antimicrobials. Notwithstanding, most drugs are created and tried against free-living microbes [22].
Bio-films establish a significant commitment to the high occurrence and non-curable UTIs, accordingly requiring proficient counteraction and regulate spreading. Along these lines, the current study is concerned about considering the occurrence of opposition, the discovery of most as often as possible uropathogenic bacteria and their profile film–creating capacities to a better comprehension of the bacterial infection consequently growing new system for counteraction and treatment. An ideal decision will incorporate a blend of anti-bio-film with viable antibacterial medications [19]. Bio-films establish a significant commitment tore-occurrence, and complicated infections in the urinary tract, along these lines which required productive counteraction and controlling measures. Bio-film exploration will incite a prevalent cognizance in disease occurring, and it will in this manner lead to the headway of latest avoidance and therapy possibility. A unique strategy will consolidate a mix or combination of anti-bio-film molecules, with an enemy of contagious impact, dynamic towards excitatory concentration to lessen the danger of creating resistance and with less toxicity for the external virulent factor [58].
Being utilized at low MIC levels in artificial culture in the laboratory, few phytochemicals have been found with anti-infection resistance–altering abilities along with the synergistic impact; these mixture with anti-toxins can give rise to the treatment against resistance bacteria effectively. Catheter-associated bio-film–forming infections’ wide range spreads in the overall world. Through this study, we enlightened the reason behind multi-drug resistance catheter-associated urinary tract infection and the mechanisms of the pathogenesis. Overall, from this review, we understood that the formation of bio-film is the important reason behind most of the untreatable MDR UTI and phytochemical-producing plants address a potential wellspring against these causative organisms [115]. Also, we mentioned some researches which have been conducted within 15 years on it. This review will be helpful to understand the novel cause, advancing the knowledge of prevention and treatment against this recurrent non-treatable condition.
References
Mishra, M. P., Rath, S., Rath, S., Swain, S. S., Ghosh, G., Das, D., & Padhy, R. N. (2017). In vitro antibacterial activity of crude extracts of 9 selected medicinal plants against UTI causing MDR bacteria. Journal of King Sound University, 29(1), 84–95. https://doi.org/10.1016/j.jksus.2015.05.007
Flores-Mireles, A., Walker, J., Caparon, M., & Hultgren, S. J. (2015). Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nature Reviews. Microbiology, 13, 269–284. https://doi.org/10.1038/nrmicro3432
Hooton, T. M. (2012). Uncomplicated urinary tract infection. New England Journal of Medicine, 366, 1028–1037. https://doi.org/10.1056/NEJMcp1104429
Nielubowicz, G. R., & Mobley, H. L. (2010). Host–pathogen interactions in urinary tract infection. Nature Reviews. Urology, 7, 430–441. https://doi.org/10.1038/nrurol.2010.101
Muhammad, I. A., & Ghareb, D. J. (2019). Bio-film forming capability, multidrug resistance and detection of associated genes in uropathogenicEscherichia coli isolated from catheterized patients. ZANCO Journal of Pure and Applied Sciences, 31(4), 9–22. https://doi.org/10.21271/zjpas.31.4.2
Sharma, D., Misba, L., & Khan, A. U. (2019). Antibiotics versus Bio-film: an emerging battleground in microbial communities. Antimicrobial Resistance and Infection Control, 8(76), 1–10. https://doi.org/10.1186/s13756-019-0533-3
Dash, D., Sarangi, G., Patro, P., & Chayani, N. (2018). Study of bio-film production in Escherichia coli causing urinary tract infection and its correlation with antimicrobial resistance. Journal of the Academy of Clinical Microbiologists, 20(2), 88–91. https://doi.org/10.4103/jacm.jacm_35_17
Majumder, M. M. I., Ahmed, T., Ahmed, S., & Khan, A. R. (2018). Microbiology of catheter associated urinary tract infection. Intech Open, 80080, 23–43. https://doi.org/10.5772/intechopen.80080
Menegueti, M. G., Ciol, M. A., Bellissimo-Rodrigues, F., Auxiliadora-Martins, M., Gaspar, G. G., Canini, S. R. M. D. S., Basile-Filho, A., & &Laus A. M. (2019). Long-term prevention of catheter-associated urinary tract infections among critically ill patients through the implementation of an educational program and a daily checklist for maintenance of indwelling urinary catheters: A quasi-experimental study. Medicine, 98(8), 1–5. https://doi.org/10.1097/MD.0000000000014417
Vargas-Cruz, N., Rosenblatt, J., Reitzel, R. A., Chaftari, A. M., Hachem, R., & Raad, I. (2019). Pilot ex vivo and in vitro evaluation of a novel foley catheter with antimicrobial periurethral irrigation for prevention of extraluminal bio-film colonization leading to catheter-associated urinary tract infections (CAUTIs). BioMed Research International, 20192869039, 1–10. https://doi.org/10.1155/2019/2869039
Chakrabarty, S., Choudhury, S., & Mishra, M. P. (2020). Prevalence of Gram-negative non-lactose fermenters causing urinary tract infections in a tertiary care hospital, Eastern India. Shodh Sarita, 28, 2348–2397.
Guggenbichler, P. J., Assadian, O., Boeswald, M., & Kramer, A. (2011). Incidence and clinical implication of nosocomial infections associated with implantable biomaterials - catheters, ventilator associated pneumonia, and urinary tract infections. GMS Krankenhaushygiene Interdisziplinä, 6(1), 1–19. https://doi.org/10.3205/dgkh000175
Khalek, S. A., Ramadan, M. O., & Radwan, M. H. (2020). Phenotypic and genotypic detection of efflux pump mediated meropenem resistance in Pseudomonas aeruginosa isolates from catheter associated urinary tract infection. Egyptian Journal of Medical Microbiology, 29, 2537–0979.
Acker, H. V., Dijck, P. V., & Coenye, T. (2014). Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal bio-films. Trends in Microbiology, 22(6), 326–333. https://doi.org/10.1016/j.tim.2014.02.001
Delcaru, C., Alexandru, I., Podgoreanu, P., Grosu, M., Stavropoulos, E., & Chifiriuc, M. C. (2016). Microbial bio-films in urinary tract infections and prostatitis: etiology, pathogenicity, and combating strategies. Pathogens, 5(4), 65, 1-12. https://doi.org/10.3390/pathogens5040065
Tasneem, U., Yasin, N., Nisa, I., Shah, F., Rasheed, U., Momin, F., Zaman, S., & Qasim, M. (2018). Bio-film producing bacteria: a serious threat to public health in developing countries. Journal of Food Science and Nutrition, 1(2), 25–31. https://doi.org/10.35841/food-science.1.2.25-31
Nazmeen, A., & Maiti, S. (2018). Prevalence, types and antibiotic sensitivity pattern in urinary tract infection (UTI) In Midnapore Town, India. Journal of Clinical and Molecular Pathology, 2, 1–16.
Soto, S. M. (2014). Importance of bio-films in urinary tract infections: new therapeutic approaches. Advances in Biology, 5, 1–13. https://doi.org/10.1155/2014/543974
Allam, N. G. (2017). Correlation between bio-film production and bacterial urinary tract infections: new therapeutic approach. Egyptian Journal of Microbiology, 52(1), 39–48. https://doi.org/10.21608/EJM.2017.1014.1021
McCarty, S., Woods, E., & Percival, S. L. (2014). Bio-films: from concept to Reality. Elsevier Inc, 143–163. https://doi.org/10.1016/B978-0-12-397043-5.00009-8
Ha, U. S., & Cho, Y. H. (2006). Catheter-associated urinary tract infections: new aspects of novel urinary catheters. International Journal of Antimicrobial Agents, 28(6), 485–490. https://doi.org/10.1016/j.ijantimicag.2006.08.020
Verderosa, A. D., Totsika, M., & Fairfull-Smith, K. E. (2019). Bacterial bio-film eradication agents: a current review. Medicinal and pharmaceutical chemistry, 7(824), 1–17. https://doi.org/10.3389/fchem.2019.00824
Ponnusamy, P., Natarajan, V., & Sevanan, M. (2012). In vitro Bio-film formation by uropathogenic Escherichia coli and their antimicrobial susceptibility pattern. Asian Pacific Journal of Tropical Medicine, 5(3), 210–213. https://doi.org/10.1016/S1995-7645(12)60026-1
Mann, E. E., & Wozniak, D. J. (2012). Pseudomonas bio-film matrix composition and niche biology. FEMS Microbiology Reviews, 36(4), 893–916. https://doi.org/10.1111/j.1574-6976.2011.00322.x
Fuente-Núñez, D. L., Reffuveille, C., Fairfull-Smith, K. E., & Hancock, R. E. W. (2013). Effect of nitroxides on swarming motility and bio-film formation, multicellular behaviors in Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 57, 4877–4881. https://doi.org/10.1128/AAC.01381-13
Khan, A., Farraj, D. A. A., Syeda, M. F., Muhammad, A. Y., Mohamed, S. E., Alkufeidy, R. M., Mustafa, A. Z. M. A., Bhasme, P., Alshammari, M. K., Alkubaisi, N. A., Abbasi, A. M., & Naqvi, T. A. (2020). Anti-bio-film activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. Journal of Infection and Public Health, 13(11), 1734–1741. https://doi.org/10.1016/j.jiph.2020.07.007
Cruz-Muniz, M. Y., Lopez-Jacome, L. E., Hernandez-Duran, M., FrancoCendejas, R., Licona-Limon, P., & Ramos-Balderas, J. L. (2017). Repurposing the anticancer drug mitomycin C for the treatment of persistent Acinetobacter baumannii infections. International Journal of Antimicrobial Agents, 49, 88–92. https://doi.org/10.1016/j.ijantimicag.2016.08.022
Rabin, N., Zheng, Y., Opoku-Temeng, C., Yixuan, D., Bonsu, E., & Sintim, H. O. (2015). Bio-film formation mechanisms and targets for developing antiBio-film agents. Future Medicinal Chemistry, 7(4), 493–512. https://doi.org/10.4155/fmc.15.6
Kalpana, B. J., Aarthy, S., & Pandian, S. K. (2012). Anti-bio-film activity of α-amylase from Bacillus subtilis S8-18 against bio-film forming human bacterial pathogens. ApplBiochemBiotechnol, 167(6), 1778–1794. https://doi.org/10.1007/s12010-011-9526-2
Catheter-associated urinary tract infections (CAUTI). (2015). Centers for Disease Control and Prevention. Available from: www.cdc.gov/hai/ca_uti/uti.html.
Jacobsen, S. M., & Shirtliff, M. E. (2011). Proteus mirabilis bio-films and catheter-associated urinary tract infections. National Center for Biotechnology Information., 2(5), 460–465. https://doi.org/10.4161/viru.2.5.17783
Bahadur, L., Ratna, S., & &Khana B. B. (2019). Comparative study of antimicrobial resistance and bio-film formation among Gram-positive uropathogens isolated from community-acquired urinary tract infections and catheter-associated urinary tract infections. Infection and Drug Resistance, 12, 957–963. https://doi.org/10.2147/IDR.S200988
Francolini, I., & Donelli, G. (2010). Prevention and control of bio-filmbased medical-device-related infections. FEMS Immunology and Medical Microbiology, 59, 227–238. https://doi.org/10.1111/j.1574-695X.2010.00665.x
Namasivayam, S. K. R., Beninton, B., Christo, B., Karthigai, S. M., Kumar, K. A. M., & Deepak, K. (2013). Anti-bio-film effect of biogenic silver nanoparticles coated medical devices against bio-film of clinical isolate of Staphylococcus aureus.Global. Journal of Medical Research, 13(3), 1–7.
Trautner, B. W., & Darouiche, R. O. (2004). Role of biofilm in catheter-associated urinary tract infection. American Journal of Infection Control, 32(3), 177–183. https://doi.org/10.1016/j.ajic.2003.08.005
Bose, S., & Ghosh, A. K. (2015). Diagnosis of biofilm- associated infections in medical devices. Biomaterials and Medical Device - Associated Infections, 71–80. https://doi.org/10.1533/9780857097224.1.71
Kafil, H. S., & Mobarez, A. M. (2015). Assessment of biofifilm formation by enterococci isolates from urinary tract infections with difffferent virulence profiles. Journal of King Saud University – Science, 27, 312–331. https://doi.org/10.1016/j.jksus.2014.12.007
Lassek, C., Burghartz, M., Chaves-Moreno, D., Otto, A., Hentschker, C., Fuchs, S., Bernhardt, J., Jauregui, R., Neubauer, R., Becher, D., Pieper, D. H., Jahn, M., Jahn, D., & Riedel, K. (2015). A metaproteomics approach to elucidate host and pathogen protein expression during catheter-associated urinary tract infections (CAUTIs). Molecular & Cellular Proteomics, 14(4), 989–1008. https://doi.org/10.1074/mcp.M114.043463
Murugan, K., Selvanayaki, K., & Al-Sohaibani, S. (2016). Urinary catheter indwelling clinical pathogen biofilm formation, exopolysaccharide characterization and their growth influencing parameters. Saudi Journal of Biological Sciences, 23, 150–159. https://doi.org/10.1016/j.sjbs.2015.04.016
Sabir, N., Ikram, A., Zaman, G., Satti, L., Gardezi, A., Ahmed, A., & Ahmed, P. (2017). Bacterial biofilm-based catheter-associated urinary tract infections: causative pathogens and antibiotic resistance. American Journal of Infection Control, 45(10), 1101–1105. https://doi.org/10.1016/j.ajic.2017.05.009
Jamal, M., Ahmad, W., Andleeb, S., Jalil, F., Muhammad, I., Muhammad, A. N., Hussain, T., Muhammad, A., Muhammad, R., & Muhammad, A. K. (2018). Bacterial biofilm and associated infections. Journal of the Chinese Medical Association, 81, 7–11. https://doi.org/10.1016/j.jcma.2017.07.012
Bai, F., Cai, Z., & Yang, L. (2019). Recent progress in experimental and human disease-associated multi species biofilms. Computational and Structural Biotechnology Journal, 17, 1234–1244. https://doi.org/10.1016/j.csbj.2019.09.010
Melton, C. N., & Anderson, G. G. (2019). Biofilms and disease: a persistent threat. Encyclopedia of Microbiology (Fourth Edition), 510–519. https://doi.org/10.1016/B978-0-12-801238-3.66119-6
Solis-Velazquez, O. A., Gutiérrez-Lomelí, M., Guerreo-Medina, P. J., Rosas-García, M. L., Iñiguez-Moreno, M., & Avila-Novoa, M. G. (2020). Nosocomial pathogen biofilms on biomaterials: different growth medium conditions and components of biofilms produced in vitro. Journal of Microbiology, Immunology, and Infection. https://doi.org/10.1016/j.jmii.2020.07.002
Francolini, I., Hall-Stoodley, L., & Stoodley, P. (2020). 2.2.8 - Biofilms, biomaterials, and device-related infections. Biomaterials Science (Fourth Edition), 823–840. https://doi.org/10.1016/B978-0-12-816137-1.00054-4
Regev-Shoshani, G., Ko, M., Crowe, A., & Av-Gay, Y. (2011). Comparative efficacy of commercially available and emerging antimicrobial urinary catheters against bacteriuria caused by E. coli in vitro. Urology, 78(2), 334–339. https://doi.org/10.1016/j.urology.2011.02.063
Mulani, M. S., Kamble, E. E., Kumkar, S. N., Tawre, M. S., & Pardesi, K. R. (2019). Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Frontiers in Microbiology, 539, 1–24. https://doi.org/10.3389/fmicb.2019.00539
Baral, B., & Mozafari, M. R. (2020). Strategic moves of “Superbugs” against available chemical scaffolds: signaling, regulation, and challenges. American Chemical Society, 3(3), 373–400. https://doi.org/10.1021/acsptsci.0c00005
Almalki, M. A., & Varghese, R. (2020). Prevalence of catheter associated bio-film producing bacteria and their antibiotic sensitivity pattern. Journal of King Saud University – Science, 32, 1427–1433. https://doi.org/10.1016/j.jksus.2019.11.037
Maharjan, G., Khadka, P., Shilpakar, G. S., Chapagain, G., & Dhungana, G. R. (2018). Catheter-associated urinary tract infection and obstinate bio-film producers. Canadian Journal of Infectious Diseases and Medical Microbiology, 1–7. https://doi.org/10.1155/2018/7624857
Aygun, F., Aygun, F. D., Varol, F., Durak, C., Çokugras, H., Camcıoglu, Y., & Çam, H. (2019). Infections with carbapenem-resistant Gram-negative bacteria are a serious problem among critically ill children: a single-centre retrospective study. Pathogens, 8(2), 69,1-13. https://doi.org/10.3390/pathogens8020069
Umema, A., Muhammad, Z. M., & Malik, A. (2020). Rising prevalence of multidrug-resistant uropathogenic bacteria from urinary tract infections in pregnant women. Journal of Taibah University Medical Sciences, 16(1), 102–111. https://doi.org/10.1016/j.jtumed.2020.10.010
Hagos, D. G., Mezgebo, T. A., Berhane, S., & Medhanyie, A. A. (2019). Bio-film and hemagglutinin formation: a hallmark for drug resistant uropathogenic Escherichia coli. BMC Research Notes, 12, 358. https://doi.org/10.1186/s13104-019-4382-1
Gunardi, W. D., Karuniawati, A., Umbas, R., Bardosono, S., Lydia, A., Soebandrio, A., & Safari, D. (2021). Bio-film-producing bacteria and risk factors (gender and duration of catheterization) characterized as catheter-associated bio-film formation. International Journal of Microbiology., 8869275, 1–10. https://doi.org/10.1155/2021/8869275
Snopkova, K., Dufkova, K., Klimesova, P., Vanerkova, M., Ruzicka, F., & Hola, V. (2020). Prevalence of bacteriocins and their co-association with virulence factors within Pseudomonas aeruginosa catheter isolates. International Journal of Medical Microbiology, 310, 151454. https://doi.org/10.1016/j.ijmm.2020.151454
Nagvekar, V., Sawant, S., & Supriya, A. (2020). Prevalence of multidrug-resistant Gram-negative bacteria cases at admission in a multi speciality hospital. Journal of Global Antimicrobial Resistance, 22, 457–461. https://doi.org/10.1016/j.jgar.2020.02.030
Quana, J., Dai, H., Liaoe, W., Zhao, D., Shif, Q., Zhang, L., Shi, K., Akova, M., & Yua, Y. (2021). Etiology and prevalence of ESBLs in adult community-onset urinary tract infections in East China: a prospective multicenter study. The Journal of Infection, 83, 175–181. https://doi.org/10.1016/j.jinf.2021.06.004
Sabir, N., Ikram, A., & Zaman, G. (2017). Bacterial bio-film-based catheter-associated urinary tract infections: causative pathogens and antibiotic resistance. American Journal of Infection Control, 45(10), 1101–1105. https://doi.org/10.1016/j.ajic.2017.05.009
Davis, N. F., & Flood, H. D. (2011). The pathogenesis of urinary tract infections. Intech. https://doi.org/10.5772/22308
Biswas, B. A., Naik, S., Nagarajan, D., & Iqubal, M. Z. (2020). Assessment of bio-film formation among the clinical isolates of Escherichia coli in a tertiary care hospital. Microbiology Research Journal International, 30(1), 26–32. https://doi.org/10.9734/mrji/2020/v30i130187
Wabe, Y. A., Reda, D. Y., Abreham, E. T., Gobene, D. B., & Ali, M. M. (2020). Prevalence of asymptomatic bacteriuria, associated factors and antimicrobial susceptibility profile of bacteria among pregnant women attending Saint Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. Therapeutics and Clinical Risk Management, 16, 923–932.
Ahmed, S. S., Shariq, A., Alsalloom, A. A., Babikir, I. H., & Alhomoud, B. N. (2019). Uropathogens and their antimicrobial resistance pattern: relationship with urinary tract infections. International Journal of Health Sciences, 13(2), 48–55.
Wang, S., Zhang, Y., Zhang, X., & Li, J. (2019). An evaluation of multidrug-resistant (MDR) bacteria in patients with urinary stone disease: data from a high-volume stone management center. World Journal of Urology, 38(2), 425–432. https://doi.org/10.1007/s00345-019-02772-0
Rajivgandhi, G. N., Alharbi, N. S., Kadaikunnan, S., Khaled, J. M., Kanisha, C. C., Ramachandran, G., Manoharan, N., & Alanzi, K. F. (2021). Identification of carbapenems resistant genes on bio-film forming K. pneumoniae from urinary tract infection. Saudi Journal of Biological Sciences, 28, 1750–1756. https://doi.org/10.1016/j.sjbs.2020.12.016
Niveditha, S., Pramodhini, S., Umadevi, S., Kumar, S., & Stephen, S. (2012). The isolation and the biofilm formation of uropathogens in the patients with catheter associated urinary tract infections (UTIs). Journal of Clinical and Diagnostic Research, 6, 1478–1482. https://doi.org/10.7860/JCDR/2012/4367.2537
Anusha, S. U., & Sundar, S. K. (2014). Esbl& Bio-film-producing uropathogenic pathogens and their antibiotic susceptibility patterns from urinary tract infection of patients at Namakkal, Tamilnadu: a case study. Journal of Natural Remedies, 8(1), 381–386. https://doi.org/10.7324/JAPS.2014.40905
Mak, R. H., & Kuo, H. J. (2006). Pathogenesis of urinary tract infection: an update. Current Opinion in Pediatrics, 18(2), 148–152. https://doi.org/10.1097/01.mop.0000193276.39495.0d
Khandelwal, P., Abraham, S. N., & Apodaca, G. (2009). Cell biology and physiology of the uroepithelium. American Journal of Physiology. Renal Physiology, 297, F1477–F1501. https://doi.org/10.1152/ajprenal.00327.2009
Lee, G. (2011). Uroplakins in the lower urinary tract. International Neurourology Journal, 15, 4–12. https://doi.org/10.5213/inj.2011.15.1.4
Eto, D. S., Jones, T. A., Sundsbak, J. L., & Mulvey, M. A. (2007). Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathogens, 3, e100. https://doi.org/10.1371/journal.ppat.0030100
Jacobsen, S. M., & Shirtliff, M. E. (2011). Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence, 2, 460–465. https://doi.org/10.4161/viru.2.5.17783
Sharma, A. K., Dhasmana, N., Dubey, N., Kumar, N., Gangwal, A., Gupta, M., & Singh, Y. (2017). Bacterial virulence factors: secreted for survival. Indian Journal of Microbiology, 57, 1–10. https://doi.org/10.1007/s12088-016-0625-1
Flores-Mireles, A., Hreha, T. N., & Hunstad, D. A. (2019). Pathophysiology, treatment, and prevention of catheter-associated urinary tract infection. Topics in Spinal Cord Injury Rehabilitation, 25(3), 228–240. https://doi.org/10.1310/sci2503-228
Bien, J., Sokolova, O., & Bozko, P. (2012). Role of uropathogenic Escherichia coli virulence factors in development of urinary tract infection and kidney damage. International Journal of Nephrology. https://doi.org/10.1155/2012/681473
Boyd, E. F. (2012). Bacteriophages- encoded bacterial virulence factors and phage pathogenicity island interaction. Advances in Virus Research, 82, 91–118. https://doi.org/10.1016/B978-0-12-394621-8.00014-5
Trautner, B. W., & Darouiche, R. O. (2004). Role of bio-film in catheter-associated urinary tract infection. American Journal of Infection Control, 32, 177–183. https://doi.org/10.1016/j.ajic.2003.08.005
Warren, J. (2001). Catheter-associated urinary tract infections. International Journal of Antimicrobial Agents, 17, 299–303. https://doi.org/10.1016/s0924-8579(00)00359-9
Kurosaka, Y., Ishida, Y., Yamamura, E., Takase, H., Otani, T., & Kumon, H. (2001). A non-surgical rat model of foreign body-associated urinary tract infection with Pseudomonas aeruginosa. Microbiology and Immunology, 45, 9–15. https://doi.org/10.1111/j.1348-0421.2001.tb01268.x
Warren, J. (1997). Catheter-associated urinary tract infections. Infectious Disease Clinics of North America, 11, 609–622. https://doi.org/10.1016/s0891-5520(05)70376-7
Breitenbucher, R. (1984). Bacterial changes in the urine samples of patients with long-term indwelling catheters. Archives of Internal Medicine, 144, 1585–1588.
Huigens III, R. W., Abouelhassan, Y., & Yang, H. (2019). Phenazine antibiotic inspired discovery of bacterial bio-film-eradicating agents. ChemMedChem, 20, 1–19. https://doi.org/10.1002/cbic.201900116
Qureshi, S., Naveed, A. B., Yousafzai, M. T., Ahmad, K., Ansari, S., Lohana, H., Mukhtar, A., & Qamar, F. N. (2020). PLoS Neglected Tropical Diseases, 14(10), 1–10. https://doi.org/10.1371/journal.pntd.0008682
Ahmed, N., Ali, Z., Riaz, M., & Zeshan, B. (2020). Evaluation of antibiotic resistance and virulence genes among clinical isolates of Pseudomonas aeruginosa from cancer patients. Asian Pacific Journal of Cancer Prevention, 21(5), 1333–1338. https://doi.org/10.31557/apjcp.2020.21.5.1333
Pallett, A., & Hand, K. (2010). Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. Antibiotics and Chemotherapy, 65, 25–33. https://doi.org/10.1093/jac/dkq298
Geta, K. (2019). Factors, impacts and possible solutions of antibiotic resistance: review article. World Scientific News, 138(2), 225–247.
Lahiri, D., Nag, M., Banerjee, R., Mukherjee, D., Garai, S., Sarkar, T., Dey, A., Sheikh, H. I., Pathak, S. K., Edinur, H. A., Pati, S., & Ray, R. R. (2021). Amylases: biofilm inducer or biofilm inhibitor? Frontiers in Cellular and Infection Microbiology, 11, 660048. https://doi.org/10.3389/fcimb.2021.660048
Wu, H., Moser, C., Wang, H. Z., Høiby, N., & Song, Z. J. (2015). Strategies for combating bacterial bio-film infections. International Journal of Oral Science, 7(1), 1–7. https://doi.org/10.1038/ijos.2014.65
Mariana, C. C., Alexandru, M. G., Veronica, L., Alexandra, B., Stefanos, T., Raluca, G., & Serban, B. (2014). Contribution of antimicrobial peptides to the development of new and efficient antimicrobial strategies. Current Proteomics, 11, 98–107. https://doi.org/10.2174/157016461102140917121943
Tenke, P., Köves, B., Nagy, K., Hultgren, S. J., Mendling, W., Wullt, B., Grabe, B., Wagenlehner, F. M. E., Cek, M., Pickard, R., Botto, H., Naber, K. G., & Johansen, T. E. B. (2012). Update on bio-film infections in the urinary tract. World Journal of Urology, 30(1), 51–57. https://doi.org/10.1007/s00345-011-0689-9
Lahiri, D., Dash, S., Dutta, R., & Nag, M. (2019). Elucidating the effect of anti-biofilm activity of bioactive compounds extracted from plants. Journal of Biosciences, 44, 52. https://doi.org/10.1007/s12038-019-9868-4
Iskandar, K., Molinier, L., Hallit, S., Sartelli, M., Catena, F., Coccolini, F., Craig Hardcastle, T., Roques, C., & Salameh, P. (2020). Drivers of antibiotic resistance transmission in low- and middle-income countries from a “One Health” perspective-a review. Antibiotics, 9(7), 372. https://doi.org/10.3390/antibiotics9070372
Lahiri, D., Nag, M., Sheikh, H. I., Sarkar, T., Edinur, H. A., Pati, S., & Ray, R. R. (2021). Microbiologically-synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Frontiers in Microbiology, 12, 636588. https://doi.org/10.3389/fmicb.2021.636588
Bernardes, E. V. T., Lewenza, S., & Reckseidler-Zenteno, S. (2015). Current research approaches to target bio-film infections. National Center for Biotechnology Information, 3(6), 36–49. https://doi.org/10.14304/surya.jpr.v3n6.5
Sharma, G., Sharma, S., Sharma, P., Charma, D., Chandola, D., Dang, S., Gupta, S., & &Gabrani R. (2016). Escherichia coli bio-film: development and therapeutic strategies. Journal of Applied Microbiology, 121, 309–319. https://doi.org/10.1111/jam.13078
Junker, L. M., & Clardy, J. (2007). High-throughput screens for small-molecule inhibitors of Pseudomonas aeruginosa bio-film development. Antimicrobial Agents and Chemotherapy, 51(10), 35, 82–90. https://doi.org/10.1128/AAC.00506-07
Davies, D. G., & Marques, C. N. (2009). A fatty acid messenger is responsible for inducing dispersion in microbial bio-films. Journal of Bacteriology, 191(5), 1393–1403. https://doi.org/10.1128/JB.01214-08
Soothill, J. (2013). Use of bacteriophages in the treatment of Pseudomonas aeruginosa infections. Expert Review of Anti-Infective Therapy, 11(9), 909–915. https://doi.org/10.1586/14787210.2013.826990
Mohanta, Y. K., Biswas, K., Jena, S. K., Hashem, A., Abd-Allah, E. F., & Mohanta, T. K. (2020). Abutilon indicum (L.) Sweet leaf extracts assisted bio-inspired synthesis of electronically charged silver nano-particles with potential antimicrobial, antioxidant and cytotoxic properties. American Scientific Publishers, 7(1), 94–100. https://doi.org/10.1166/mat.2018.1484
Dubey, P., Tiwari, A., Tiwari, P., Awasthi, S., Rai, A. K., & Watal, G. (2019). Phytochemical and phyto elemental profile of J. officinale. International Journal of Pharmacognosy and Phytochemical Research, 11(1), 5–9.
Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D. G., & Lightfoot, D. A. (2017). Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants, 6, 42. https://doi.org/10.3390/plants6040042
Anchez, E., Morales, C. R., Castillo, S., Leos-Rivas, C., García-Becerra, L., & Martínez, D. M. O. (2016). Antibacterial and anti bio-film activity of methanolic plant extracts against nosocomial microorganisms. Evidence-based Complementary and Alternative Medicine. https://doi.org/10.1155/2016/1572697
Famuyide, I. M., Aro, A. O., Fasina, F. O., Eloff, J. N., & McGaw, L. J. (2019). Antibacterial and antiBio-film activity of acetone leaf extracts of nine under-investigated South African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complementary and Alternative Medicine, 19(1), 141. https://doi.org/10.1186/s12906-019-2547-z
Taid, T. C., Rajkhowa, R. C., & Kalita, J. C. (2014). A study on the medicinal plants used by the local traditional healers of Dhemaji district, Assam, India for curing reproductive health related disorders. Advances in Applied Science Research, 5(1), 296–301.
Das, S. (2020). Natural therapeutics for urinary tract infections—a review. Future Journal of Pharmaceutical Sciences., 6, 64. https://doi.org/10.1186/s43094-020-00086-2
Lagha, R., Abdallah, F. B., & AL-Sarhan, B. O. & Al-Sodany, Y. (2019). Antibacterial and bio-film inhibitory activity of medicinal plant essential oils against Escherichia coli Isolated from UTI patients. Molecules, 24(6), 1161. https://doi.org/10.3390/molecules24061161
Tenke, P., Koves, B., & Johansen, T. E. B. (2014). An update on prevention and treatment of catheter-associated urinary tract infections. Current Opinion in Infectious Diseases, 27(1), 102–107. https://doi.org/10.1097/QCO.0000000000000031
Danese, P. N. (2002). Antibiofilm approaches: prevention of catheter colonization. Chemistry & Biology, 9, 873–880.
Nowatzki, P. J., Koepsel, R. R., Stoodley, P., Min, K., Harper, A., Murata, H., Donfack, J., Hortelano, E. R., Ehrlich, G. D., & Russell, A. J. (2012). Salicylic acid-releasing polyurethane acrylate polymers as anti-biofilm urological catheter coatings. Acta Biomaterialia, 8, 1869–1880. https://doi.org/10.1016/j.actbio.2012.01.032
Sabharwal, N., Chhibber, S., & Harjai, K. (2014). New possibility for providing protection against urinary tract infection caused by Pseudomonas aeruginosa by non-adjuvanted flagellin ‘b’ induced immunity. Immunology Letters, 162(2), 229–238. https://doi.org/10.1016/j.imlet.2014.10.018
Fisher, L. E., Hook, A. L., Ashraf, W., Yousef, A., Barrett, D. A., Scurr, D. J., Chen, X., Smith, E. F., Fay, D. M., Parmenter, C. D. J., Parkinson, R., & Bayston, R. (2015). Biomaterial modification of urinary catheters with antimicrobials to give long-term broad spectrum anti-biofilm activity. Journal of Controlled Release, 202, 57. https://doi.org/10.1016/j.jconrel.2015.01.037
Singha, P., Locklin, J., & Handa, H. (2017). A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomaterialia, 50, 20–40. https://doi.org/10.1016/j.actbio.2016.11.070
Andrade, M., Malheiro, J., Borges, F., Saavedra, M. J., & Simo˜ es, M. (2020). The potential of phytochemical products in biofilm control. Recent Trends in Biofilm Science and Technology, 273–293. https://doi.org/10.1016/B978-0-12-819497-3.00012-X
Lahiri, D., Nag, M., Dutta, B., Dey, S., Mukherjee, D., Joshi, S. J., & Ray, R. R. (2021). Antibiofilm and anti-quorum sensing activities of eugenol and linalool from Ocimumtenuiflorum against Pseudomonas aeruginosa biofilm. Journal of Applied Microbiology. https://doi.org/10.1111/jam.15171
Low, J. L., Kao, P. H. N., Tambyah, P. A., Koh, Hua Ling, G. L. E., Kline, K. A., Cheow, W. S., & Leong, S. S. J. (2021). Development of a polymer-based antimicrobial coating for efficacious urinary catheter protection. Biotechnology Notes., 2, 1–10. https://doi.org/10.1016/j.biotno.2020.12.001
Barbieri, R., Coppo, E., Marchese, A., Daglia, M., Sobarzo-Sánchez, E., Seyed, F. N., & Sayed, M. N. (2017). Phytochemicals for human disease: an update on plant-derived compounds antibacterial activity. Microbiological Research, 196, 44–68. https://doi.org/10.1016/j.micres.2016.12.003
Acknowledgements
The kind support of the Senior Management team, Vice-Chancellor of Centurion University Prof. Supriya Pattanayak; Dean School of Paramedics and Allied Health Sciences (SoPAHS) Prof. S.K. Jha; Head of the Department, SoPAHS, Dr. Soumya Jal is highly acknowledged.
Author information
Authors and Affiliations
Contributions
Conceptualization and writing — original draft preparation: Susmita Chakrabarty (PhD scholar); supervision, helping in framing and editing — Monali Priyadarsini Mishra (supervisor) and Dipankar Bhattacharyay (co-supervisor).
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chakrabarty, S., Mishra, M.P. & Bhattacharyay, D. Targeting Microbial Bio-film: an Update on MDR Gram-Negative Bio-film Producers Causing Catheter-Associated Urinary Tract Infections. Appl Biochem Biotechnol 194, 2796–2830 (2022). https://doi.org/10.1007/s12010-021-03711-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03711-9