Abstract
Zinc solubilizing rhizobacteria (ZSR) enhance the phyto-availability of Zn by converting its insoluble forms into usable forms that are essential for the growth and nutritional quality of crops. In the present study, a potential ZSR, hereafter referred to as strain N14, was isolated from the polyhouse rhizospheric soil of Punjab, India. The isolated rhizobacteria was found to be Gram-positive, aerobic, rod-shaped, and demonstrated a solubilization index of 63.75 on the Bunt Rovira (BR) medium. The 16S rRNA gene sequence analysis revealed that isolated strain N14 matches substantially with type strain Dietzia maris DSM 43672 T. In its ZnO broth assay, a significant amount of soluble Zn was detected along with a simultaneous decrease in pH of the broth. Ultra-performance liquid chromatography analysis revealed the release of organic acids, specifically, lactic acid and acetic acid by D. maris strain N14 which could be the reason for the decrease in broth pH. The production of indole acetic acid (29.91 µg/ml), gibberellic acid (4.72 µg/ml), ammonia (38.87 µg/ml), siderophore (0.89%), along with the release of HCN and appearance of phosphate solubilization zone (14.4 mm) with this strain suggested its possible plant growth-promoting (PGP) characteristics. Therefore, this strain was employed in the formulation of pellets which were applied for in vivo PGP studies using tomato plants. The developed bioformulated pellets showed a significant enhancement in plant growth as compared to control and vermicompost treated plants. To the best of our knowledge, this is the first report describing the Zn solubilizing and PGP characteristics of D. maris.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is an indispensable micronutrient for crop development and maturation because it regulates several enzyme reactions, biochemical functions, membrane permeability, and antioxidant properties in plants. Even though Zn is required in minimal quantity, a lack of it is known to hinder the growth quality and yield of many crops, including cereals, pulses, vegetables, and oil seeds. Zn-deficient plants have stunted growth, chlorosis, smaller leaves, and are more vulnerable to light, heat, and diseases. Zn deficiency also affects grain production, root development, pollen formation, and water transport [1]. Although Zn is abundant in soil, its phyto-accessibility is limited owing to its chelation with hydrated oxides of magnesium, calcium, iron, and manganese rendering soil Zn inaccessible to plants. However, many factors such as acidic soils, high content of organic matter, CaCO3, and phosphate could alleviate Zn phyto-availability [2].
The field application of ZnSO4 is one of the most prevalent ways of addressing plant Zn deficiency; nevertheless, about 99% of the applied Zn remains unutilized in the soil mass. This has been correlated with low fertilizer usage efficiency [3]. Therefore, the researchers have diverted their attention to find alternate methods, such as the exogenous use of plant growth-promoting rhizobacteria (PGPR), which appears to be an intriguing and environmentally acceptable method for improving Zn phyto-availability along with overall soil–plant nutrient availability.
The impact of ZSR (Zinc solubilizing rhizobacteria) on the development and productivity of crops is being researched extensively. Several studies have revealed that ZSR are potential contributors to organic and sustainable crop cultivation systems because they play essential roles in atmospheric nitrogen (N) fixation, solubilization as well as mobilization of nutrients such as phosphorus (P) and potassium (K), production of plant growth hormones, siderophore and various antimicrobial compounds [3, 4]. ZSR can also effectively use the natural Zn pools of soil by solubilizing the insoluble Zn and converting it into labile Zn, thereby augmenting its phytoavailability [5]. In vitro investigations on species of genera Pseudomonas, Thiobacillus, Gluconacetobacter, Acinetobacter, Burkholderia, Bacillus, Rhizobium, Enterobacter, Ralstonia, and Serratia have revealed their effectiveness in solubilizing the insoluble Zn forms such as ZnCO3, ZnO, and Zn3(PO4)2, thus, acting as potential Zn biofertilizers [3, 6, 7].
The genus Dietzia was proposed by Rainey et al., [8] and the members of this genus are Gram-positive, aerobic, short rod and coccoid-like, non-motile, non-endospore-forming, non-acid alcohol fast, and oxidase-catalase positive [9] belonging to the order Actinomycetales. Different strains of the genus Dietzia including D. maris, D. cinnamea, D. natronolimnaea, D. psychralcaliphila, D. schimae, D. kunjamensis, D. aerolata, D. papillomatosis, D. lutea, D. alimentaria, D. aurantiaca, D. timorensis, and D. massiliensis [10] have been diversely distributed in a variety of habitats such as soil, sea mud, or deep sea, human clinical specimens, and plant tissues. These strains and their metabolites have potential applications in foods, health supplements, pharmaceuticals, cosmetics, nutraceuticals, and bioremediation. To date, no significant research demonstrating the PGP potential of Dietzia maris has been documented. Keeping in view these lacunae, in the present study, we isolated efficient Zn solubilizing strain N14 from the rhizosphere of tomato plant (Solanum lycopersicum L.) grown in the polyhouse field of Mohali, Punjab, India. The strain produced a clear solubilizing zone on Bunt Rovira (BR) agar plates supplemented with ZnO. Therefore, further studies were carried out regarding the identification and characterization of the strain to establish the potential of Dietzia maris as a ZSR for the first time. D. maris strain N14 was further tested for the presence of plant growth-enhancing traits, including production of auxin, gibberellic acid, ammonia, and siderophore along with phosphate, and potassium solubilization. For in vivo analysis, an alginate-based bioformulation containing this strain was prepared, and its efficacy was tested on the vegetative growth of the tomato plants.
Materials and Methods
The rhizobacterial species was isolated from tomato crop grown in a polyhouse in the Mohali district (30.7046°N, 76.7179°E) of Punjab, India. Using 16S rRNA sequencing and various biochemical methods [11], the species was identified as Dietzia maris strain N14. After performing repeated subculturing, the pure culture was kept at − 20 °C in 20% glycerol in water (v/v) and further studied for its Zn solubilization potential and PGP traits.
The 16S rRNA gene sequence of Dietzia maris strain N14 was submitted to the GenBank nucleotide sequence database under the accession number MZ191749.
Evaluation of Zn Solubilizing Potential of D. maris Strain N14
To determine the inherent Zn solubilizing capability of D. maris, 0.01 ml of pure culture (24 h old) was stabbed to the center of petri plates containing 0.1% ZnO amended BR medium (10 g dextrose, 0.2 g KCl, 1 g (NH4)2SO4, 0.2 g MgSO4, 0.1 g K2HPO4, 15 g agar in 1000 ml double distilled H2O) [12] and incubated at 28 °C for 72, 120, and 168 h time intervals. The culture plates were analyzed for the formation of halo zones, and measurement of the Zn solubilization index (ZSI) was done as per the equation given below and elsewhere [5],
To further confirm the solubilization of ZnO, quantification of Zn was carried out using Atomic Absorption Spectroscopy (AAS) as per the method given in the literature [13]. For the same, sterilized 0.1% ZnO amended BR broth (50 ml) was inoculated with 1 ml of pure culture and incubated at 28 ℃ at 120 rpm. Subsequently, 2 ml of the sample was withdrawn at 72, 120, and 168 h time intervals followed by centrifugation at 10,000 rpm for 10 min at 4 ℃. Eventually, the pH of the supernatant was recorded. Finally, 0.5 ml of this culture filtrate was taken, followed by 200 times dilution with H2O, and subjected to AAS (Agilent 240FS AA) at 213.9 nm. To quantify soluble Zn, the following formula was used;
Furthermore, the production of organic acids by the isolated strain N14 was detected using UPLC to ascertain the relationship between pH and Zn quantification. For this, the same broth assay was used which was also employed for the estimation of soluble Zn and pH. Followed by centrifugation the cell-free supernatant was passed through a 0.22-micron membrane and 0.02 ml of culture filtrate was injected into a UPLC equipped with Hi-Plex H column (Agilent) of 300 mm length with 8 mm porosity. The chromatogram was recorded using 5 mM H2SO4 as a mobile phase at a flow rate of 0.6 ml/min and a refractive index detector which was maintained at 65 °C within a run time of 30 min. By using retention time and spiking the sample with organic acids viz. glycolic acid, acetic acid, lactic acid, and formic acids of HPLC-grade, elutes were identified and quantified.
In Vitro Plant Growth Traits of D. maris Strain N14
The inherent property of D. maris to generate indole acetic acid (IAA) was assessed qualitatively and quantitatively [14, 15], and for determining the gibberellic acid and siderophore production, a method reported by Borrow et al. [16] and Chrome-azurol S medium [17] were used respectively. The percent siderophore unit (psu) was used to calculate the amount of siderophore production using the following equation:
where Ar is the absorbance of the reference and As is the absorbance of the sample.
Ammonia quantification was done using Nessler’s reagent [11] and interpretation was made with the help of a standard curve of ammonium sulfate (0.8–5.0 µmol). The Pikovskaya’s agar [18], Aleksandrov medium [19], and Jessen’s agar medium [20], were used respectively for evaluating the P, K, and N traits of isolate N14.
Using alkaline picrate solution, the production of HCN was detected by observing the change in color from yellow to brown [21]. The activity of hydrolytic enzymes including cellulase, pectinase, and amylase production was also determined [6]. The respective medium was observed for clear distinct zones.
Pathogenicity of D. maris Strain N14
The pathogenicity of the isolate N14 was checked using the hemolytic test as per the method given elsewhere [22]. For this, 24 h old culture was streaked on the blood agar plate in triplicate, incubated at 28 ℃ for 168 h and examined for hemolysis.
Preparation of Pellets
The bio-formulated pellets were prepared using a method reported by Schoebitz et al. [23] with slight modification using N14 isolate, sodium alginate as a binder, ZnO as a source of Zn, and CaCl2 as a cross-linker. For this, a 1.5% solution of sodium alginate containing ZnO (0.1%) was mixed with broth containing N14 isolate (108 CFU/ml) in a 2:1 ratio. Subsequently, the mixture was extruded gently through a sterile syringe into a sterilized 0.1 M CaCl2 solution at room temperature to obtain beads.
In Vivo Efficacy of D. maris Strain N14
To evaluate the plant growth-enhancing traits of D. maris, in vivo bioassay in tomato plants was conducted using prepared pellets. For this, tomato seeds were procured and their surface was sterilized by immersing them in mercuric chloride (0.1%) solution for about 2–3 min followed by rinsing them twice with double distilled water. Afterward, these were soaked in water overnight. Subsequently, such seeds were planted in pro-trays with autoclaved soil. After 21 days of growth, seedlings were washed and transplanted into plastic pots containing sterilized soils. Only one seedling per pot was retained and followed by a week of transplantation, bio-formulated pellets and vermicompost were applied thoroughly to soils along with a control experiment as per the details given in Table 1.
Pots were incubated in direct sunlight at room temperature and 100 ml of water was added every 48 h. All plant growth variables including length of shoot and root, number of leaves and branches, and weight of fresh plants were recorded after 45 days of transplantation. The soil was removed from the roots in all containers to collect root specimens. For each treatment, experiments were carried out in triplicates.
Statistical Analysis
The plant growth statistical analysis was done using Duncan’s multiple range test (DMRT) with a statistical package for the social sciences (SPSS-24) software. DMRT demonstrated a significant difference between treatments with P < 0.005. All the findings were presented as an average of three replicates with standard errors.
Results
Biochemical and Molecular Identification of D. maris Strain N14
The preliminary identification of the isolated strain N14 was done according to Bergey’s Manual of Determinative Bacteriology [11] and outcomes are listed in Table 2. The bacteria were found to be Gram-positive, tiny rod-shaped, and appeared as slight orange-colored colonies. The strain N14 tested positive for catalase, urease, citrate utilization, and nitrate reduction while Voges–Proskauer, methyl red, H2S production and starch hydrolysis were found to be negative. Also, glucose and dextrose as carbon sources supported the growth of the bacterial strain N14. However, strain N14 was not found to utilize lactose and sucrose.
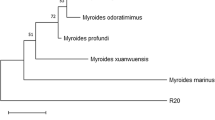
Phylogenetic analysis was performed to identify the N14 isolate. The phylogenetic tree was constructed by aligning 16S rRNA gene sequences of species belonging to the genus Dietzia with validly published names along with 16S rRNA gene sequence of N14 isolate. The 16S rRNA gene was analyzed by performing the multiple sequence alignment and analysis of the data was performed by the maximum likelihood method (Fig. 1) using the software MEGA11 [24]. The genetic distance was calculated according to the Kimura-2 model. Bootstrap analyses were based on 1000 replications. The 16S rRNA gene sequence of strain N14 was a continuous stretch of 1053 bp. The strains belonging to Dietzia genus used to construct the phylogenetic tree were D. maris, D. aurantiaca, D. alimentaria, D. cercidiphylli, D. natronolimnaea, D. aerolata, D. papillomatosis, D. timorensis, D. psychroalcalophilus, D. cinnamea, and Tsukamurella pulmonis [25,26,27]. The 16S rRNA gene sequence of N14 isolate showed similarity (99.53%) with gene sequence of type strain Dietzia maris (DSM 43,672)T and was designated as D. maris strain N14.
Phylogenetic tree showing the position of D. maris strain N14 relative to the different species of the genus Dietzia based on 16S rRNA gene sequence data using the Maximum Likelihood method. Bootstrap values (expressed as percentages of 1000 replications) of > 50% are given at the nodes. Scale bar represents 0.01 substitutions per nucleotide position
Zn Solubilization Capability and Organic Acid Production
The first investigation for solubilization of ZnO by D. maris was performed by measuring the halozone diameters in plate assay using BR media supplemented with 0.1% ZnO. The bacteria demonstrated a gradual increase in the solubilizing zone with an increase in incubation time. The ZSI were 39.15 ± 1.15, 42.5 ± 2.5, and 63.75 ± 1.25 at 72, 120, and 168 h of time intervals respectively when incubated at 28 ℃. Furthermore, the quantification using AAS revealed that D. maris was able to solubilize significant amounts of Zn with values of 21.72, 75.84, and 98.79 ppm at time intervals of 72, 120, and 168 h respectively in ZnO liquid broth (Fig. 2). Alongside, decrease in pH of broth was also observed which suggest the inverse relationship between pH and Zn solubilization as indicated in Fig. 2. The decrease in pH was due to the release of organic acids which were identified and estimated using UPLC. Mainly, two acids viz., lactic acid and acetic acids were detected at different time intervals as shown in Fig. 3 wherein lactic acid was found in higher amounts. Surprisingly, the release of glycolic acid and formic acid has not been detected.
In Vitro Plant Growth Traits of D. maris Strain N14
None of the various reported ZSR has the innate capability to promote plant growth and development via. all possible pathways (direct and indirect) such as the production of siderophore, solubilization of Zn, P, K. Hence, it is necessary to investigate individual PGP traits of the D. maris to establish its potentiality as a PGPR. The various PGP properties of D. maris are demonstrated in Table 3.
According to our investigation, D. maris produced a considerable amount of IAA with values of 15.182 ± 9.00 µg/ml, 16.136 ± 1.35 µg/ml, and 29.909 ± 5.14 µg/ml after an incubation of 72, 120, and 168 h at 28 ℃, respectively. However, the production of GA was comparatively lower with the values of 4.50 ± 4.01 μg/ml, 4.19 ± 4.01 μg/ml, and 4.72 ± 1.18 μg/ml at 72, 120, and 168 h intervals of incubation at 28 ℃, respectively. Other two important PGP traits are the production of siderophore with a marked amount of 89.10 ± 0.42% after 72 h of incubation and the release of ammonia with the amounts of 17.45 ± 1.32 µg/ml, 38.87 ± 3.15 µg/ml and 16.50 ± 1.76 µg/ml at time intervals of 72, 120, and 168 h were also observed respectively. Apart from that, D. maris was found to be positive for P solubilizing trait with halazone diameter of 12.2 ± 1.2, 14.3 ± 1.12, and 14.4 ± 1.15 mm at 72, 120, and 168 h time intervals of inoculation, respectively. However, it tested negative for K solubilization and N fixation.
Pathogenicity of D. maris Strain N14
To evaluate the pathogenicity of D. maris, the hemolysis zone on blood amended agar plates was studied up to 168 h. The blood plates revealed no indications of hemolysis suggesting that the isolated D. maris strain N14 could not be harmful to people, thus it may be employed for further studies for different plants as well as making formulations of biofertilizers.
In Vivo Plant Growth Traits of D. maris Strain N14
As previously discussed, the in vitro studies on the isolate N14 showed multiple traits of PGPR such as the production of IAA, gibberellic acid, ammonia, siderophore, and solubilization of phosphate. Furthermore, in vivo experiments were also conducted to test and confirm the efficacy of isolate N14 as a PGPR. Four experiments in triplicates were carried out under similar environmental conditions, however, with different treatments. In one of the groups, 10 pellets, and in another one 20 pellets were applied which are hereafter referred to as P1 and P2. The other two groups were a non-treated control and a treated one with vermicompost, designated as C and V, respectively. After 45 days of transplantation, vegetative growth was recorded in the all-subject plants, and the results are shown Figs. 4 and 5.
Effect of D. maris strain N14 on various growth parameters in tomato after 45 days of different treatments; a The number of leaves and branches. b Root and shoot length. c Weight of full plant, shoot, and root. Values represent the mean ± SD of three experimental replicates. C—Plants with no treatment, V—Plants treated with vermicompost, P1—Plants treated with 10 bioformulated pellets, and P2—Plants treated with 20 bioformulated pellets
D. maris solubilizes P from fixed organic/inorganic reserve enhancing its phytoavailability and hence, promoting root development. Because of the expanded surface area, roots can colonize more soil, enhancing the quality and quantity of nutrients [28]. Statistical analysis using DMRT test (Supplementary Table S1) revealed that the number of leaves and branches was maximum in the P2 group in comparison to the V and C treated plant groups. The same group exhibited a similar trend for the shoot and root length with values of 37.67 ± 0.76 cm and 18.87 ± 0.32 cm, respectively. The mean weight of the fresh whole plant, shoot, and root was also found to be significantly higher in P2 as compared to other treatments.
Discussion
Most often, exogenous Zn-based fertilizers, as well as sufficient natural Zn reserves, could not avail the required amount of Zn to many plants for sufficient growth owing to their less solubility in water. In such circumstances, soil microbial communities, specifically, ZSR can play key roles via producing organic acids and/or chelating agents and/or protein extrusion which solubilize Zn with different mechanisms. Therefore, ZSR could be an excellent supplement to chemical fertilizers. Keeping in view this approach, the isolation and identification of a potential ZSR from the tomato rhizosphere of a polyhouse of Mohali, Punjab was carried out. Based on the different biochemical and molecular characterizations, the strain was characterized as gram-positive, small rod-shaped, non-pathogenic ZSR, and identified as Dietzia maris strain N14. However, most of the ZSR belonging to the genus Bacillus, Pseudomonas, Azospirillum, Xanthomonas, Gluconacetobacter, Acinetobacter, and Enterobacter have been documented [28,29,30,31,32]. Noticeably, none of the research focusing on the Zn solubilization trait of the genus Dietzia has been reported. However, in the present study, the same genus with N14 strain has been identified as a potential solubilizer and showcased high solubility index of 63.75 ± 1.25 on the 0.1% ZnO-amended solid medium after 168 h of incubation which is remarkably higher than those of the earlier reported ZSR [5, 33]. Further, our findings are comparable with the previous studies wherein a higher solubilizing zone was observed for the bacterial isolates when a ZnO-amended solid medium was used [3, 33,34,35]. A previous study on Bacillus megaterium showed a solubilization zone of 50 ± 0.03 mm [36] and B. altitudinis demonstrated a solubilization index of 5.11 ± 1.0 cm [5] on ZnO supplemented medium. On the same line, an investigation of the Zn solubilizing capability of 70 bacterial isolates from the rhizosphere of wild pepper and cardamom growing in the forest areas highlights B. megaterium for the clear zone diameter of 15.3 mm on ZnO supplemented medium [6]. In another study, Acinetobacter sp. AGM3 and AGM9 showcased a solubilization zone diameter of 13.21 and 11.74 mm respectively in ZnO amended medium after 12 days [32].
In the present work, AAS measurements indicated that D. maris strain N14 exhibited maximum solubilization of Zn at 168 h of incubation with a value of 98.79 ppm. The observed value was found to be significantly higher than those of 20.33 ppm, 36.54 μg/ml, and 33.14 μg/ml solubilized by B. megaterium, Acinetobacter sp. AGM3 and AGM9, respectively [32, 36]. Another study on Pseudomonas sp. VBZ4 and P. stutzeri VBZ17 revealed the solubilization of Zn with the amount of 26.8 mg/l and 22.2 mg/l on ZnO-enriched media [37]. Previous studies on ZSR showed that isolates could easily solubilize considerable amounts of Zn in the liquid medium, but large halo zones have not been observed on solid media [29, 38]. However, based on our findings, it must be pointed out that D. maris showed significant ZnO solubilization in both BR plates and broth assay. Furthermore, an increase in the Zn availability could be correlated to decrease in pH of the medium. This is in line with previous studies that documented similar observations [3, 34].
Different mechanisms could be involved in Zn solubilization, the most common being the acidification of the medium by exudation of inorganic/organic acids [30, 34] and siderophore production [21]. The rhizobacterial population on the root surface is 10 times higher than in bulk soil. These rhizobacteria utilize sugars that are present in root exudates via metabolic activities and produce organic acids that aid in the solubilization of Zn. Moreover, biochemical analysis of D. maris strain N14 showed that it can utilize sugars such as glucose, dextrose, and sucrose, therefore production of organic acids can be justified. Earlier studies have shown that gluconic acid and its keto-derivatives are the most common acid among different organic acids produced by ZSR for solubilizing inorganic Zn compounds [6, 34, 39, 40]. However, few studies have also highlighted the production of other organic acids such as oxalic acid, tartaric acid, formic acid, acetic acid, pentanoic acid, fumaric acid, and maleic acid [33, 35, 41]. In the present study, only two organic acids viz. lactic acid and acetic acid were produced by D. maris and our findings can be justified based on those of Costerousse et al., [30] and Mumtaz et al. [33] demonstrated that ZSR could have different solubilization mechanisms.
The PGP characteristics of any rhizobacteria can be attributed to the production of diverse metabolites including IAA, siderophores, ammonia, and activities such as solubilization of N, P, and K [6, 7, 42]. Siderophores are compounds that can chelate iron and cause iron deficiency for growing pathogens in the plant rhizosphere, indirectly suppressing the growth of these pathogens. The importance of siderophore production in species viz., Bacillus, Pseudomonas, Enterobacter, and Acinetobacter have been studied earlier [42,43,44]. Interestingly, the bacterial strains producing siderophores possess antifungal activity. For instance, Acinetobacter sp., a potential Zn solubilizer inhibits the growth of Fusarium oxysporum [45]. Siderophore production was also observed in D. maris strain N14 which can be linked to the high amount of Zn solubilization in the medium [42]. Based upon the characteristics of siderophore production, strain N14 may aid in suppressing phytopathogen in the rhizosphere.
IAA and gibberellic acid are important determinants of plant growth and are produced by most of the ZSR belonging to genera Burkholderia, Serratia, Acinetobacter, Gluconacebacter, Azotobacter, Rhizobia, Bacillus, Pseudomonas, and Enterobacter [5, 28, 33]. Strains belonging to Bacillus spp. were found to produce IAA in the range of 15.8–25.6 μg/ml [46]. The ZSR Enterobacter cloacae (EPS-14) and Pantoea agglomerans (EPS-17) produced 12.125 μg/ml and 8.449 μg/ml of IAA, respectively [21]. For the Pseudomonas spp., production of IAA was found to be in the range of 22.2 to 40.6 μg/ml [47]. In the present study, strain N14 produced 9.90 µg/ml and 4.72 μg/ml of IAA and gibberellic acid, respectively at 168 h.
The essential nutrients N, P, and K are abundantly present in the soil; however, they remain inaccessible to plants. The ZSR bacterial species belonging to Bacillus, Pseudomonas, Enterobacter, Rhizobium, Burkholderia, and Agrobacterium have the potential to sequester P or K from soil [7, 21]. Our findings also highlighted the P solubilization by D. maris, however, it was found to be negative for K solubilization and N fixation.
Ammonia production is another important trait of PGPR where an organism can break down complex nitrogenous materials like peptones and release ammonia into the soil. The released ammonia is taken up by the plant as a nutrient source. There can be an accumulation of ammonia in soils which enhances the alkalinity, consequently, suppressing the growth of certain fungi and benefiting crops indirectly. According to our studies, isolate N14 was found to be positive for ammonia production and thus can be effective against phytopathogens.
The HCN production enhances the antagonistic capability of bacteria against several phytopathogens and is produced by various ZSR including Pseudomonas sp., Burkholderia sp., Enterobacter sp., and Bacillus sp., [6, 21, 33]. In the present study, the D. maris strain N14 also confirms the production of HCN, therefore, it can act as a potential biocontrol agent. Furthermore, as per our investigation, the D. maris strain N14 was found to be deficient in defense-related hydrolytic enzyme activities such as amylase, pectinase, and cellulose, however, the production of siderophore could suppress the growth of pathogens.
Even though immuno-compromised individuals and experimental non-human primates have been documented to be susceptible to few opportunistic Dietzia infections, our results revealed that D. maris strain N14 isolated from the polyhouse rhizospheric soil is non-pathogenic and to the best of our knowledge, no human infection has been observed with it.
Several published results indicate that if an organism exhibits in vitro PGPR features such as IAA generation, phosphate solubilization, ammonia, and siderophore production, then it must also exhibit plant growth promotion in vivo. Our study also confirms this hypothesis as a significant enhancement in the vegetative growth of tomato plants has been observed when plants are inoculated with bioformulated D. maris. Moreover, these results are directly in line with the previous findings where the production of IAA, gibberellic acid, ammonia, and siderophore by ZSR enhances the Zn2+ accessibility and also results in better nutritional absorption in tomato plants [37]. Similarly, some of ZSRs with sufficient PGP traits for other crops such as capsicum, rice, wheat, chickpea and maize [3, 33, 36, 44, 47] have also been reported. Likewise, D. maris strain N14 isolated from tomato rhizospheric soil possesses Zn solubilization potential as well as significant PGP traits for tomato plants.
Conclusion
Though the soil in many places has enough reserves of Zn, nonetheless, its deficiency in plants has been identified as a significant reason for reduced growth in vegetables and fruits. At times, direct application of Zn-based fertilizers could not result in the expected outcomes owing to various reasons such as salinity, pH, textures of soil. One of the reasons for it could be the presence of Zn in the inaccessible form. Therefore, to combat this complication, there is a dire need of identifying and developing ZSR bacteria which can be employed in formulations to develop biofertilizers. Such an approach could be eco-friendly and effective to develop efficient Zn-based biofertilizers.
In the present study, the D. maris has shown prominent Zn and P solubilization potential along with the production of HCN, ammonia, IAA, gibberellic acid, and siderophore. Moreover, they have shown excellent in vivo efficacy for the vegetative growth of tomato plants in comparison to vermicompost and control treatments. Given the fact that various rhizobacteria are yet to be thoroughly investigated for plant growth-boosting activities and that known PGPR strains have either lost their Zn solubilizing potentiality or succumbed to excessive use of chemical fertilizers, our findings, establish the potentiality of D. maris strain N14 as an effective Zn solubilizer and a promising candidate with PGP characteristics. The application of D. maris strain N14 can remarkably enhance the bioavailability of Zn in tomato plants, however, further studies need to be done to confirm the efficacy of D. maris strain N14 on commercial crops of tomato plants and evaluate the feasibility of this approach using field trials. Apart from that, future research may include the application of the D. maris on different crops and as well as to evaluate its effects on soil behavior.
Moreover, there should be more research into the possible connections between D. maris and other PGPRs/ZSRs to develop more effective consortia for enhancing Zn phyto-availability. The mechanisms and the production of specific metabolites by D. maris that contributes to plant growth enhancement could be thoroughly investigated. Compared to other PGPR, ZSR lacks molecular knowledge which must be investigated further in the future.
Data Availability
The sequencing data of Dietzia maris strain N14 was deposited in NCBI with GenBank accession no. MZ191749.
Code Availability
Not applicable.
References
Tavallali V, Rahemi M, Eshghi S et al (2010) Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L. ’Badami’) seedlings. Turkish J Agric For 34:349–359. https://doi.org/10.3906/tar-0905-10
Rani N, Kaur R, Kaur S (2020) Zinc solubilizing bacteria to augment soil fertility-a comprehensive review. Int J Agric Sci Vet Med 8:38–44
Gontia-Mishra I, Sapre S, Tiwari S (2017) Zinc solubilizing bacteria from the rhizosphere of rice as prospective modulator of zinc biofortification in rice. Rhizosphere 3:185–190. https://doi.org/10.1016/j.rhisph.2017.04.013
Kalam S, Basu A, Podile AR (2020) Functional and molecular characterization of plant growth promoting Bacillus isolates from tomato rhizosphere. Heliyon 6:e04734. https://doi.org/10.1016/j.heliyon.2020.e04734
Kushwaha P, Srivastava R, Pandiyan K et al (2021) Enhancement in Plant Growth and zinc biofortification of chickpea (Cicer arietinum L.) by Bacillus altitudinis. J Soil Sci Plant Nutr 21:922–935. https://doi.org/10.1007/s42729-021-00411-5
Dinesh R, Srinivasan V, Hamza S et al (2018) Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma 321:173–186. https://doi.org/10.1016/j.geoderma.2018.02.013
Bhakat K, Chakraborty A, Islam E (2021) Characterization of zinc solubilization potential of arsenic tolerant Burkholderia spp. isolated from rice rhizospheric soil. World J Microbiol Biotechnol 37:1–13. https://doi.org/10.1007/s11274-021-03003-8
Rainey FA, Klatte S, Kroppenstedt RM, Stackebrandt E (1995) Dietzia, new genus including Dietzia maris comb. nov., formerly Rhodococcus maris. Int J Syst Bacteriol 45:32–36. https://doi.org/10.1099/00207713-45-1-32
Koerner RJ, Goodfellow M, Jones AL (2009) The genus Dietzia: a new home for some known and emerging opportunist pathogens. FEMS Immunol Med Microbiol 55:296–305. https://doi.org/10.1111/j.1574-695X.2008.00513.x
Kämpfer P, Falsen E, Frischmann A, Busse HJ (2012) Dietzia aurantiaca sp. nov., isolated from a human clinical specimen. Int J Syst Evol Microbiol 62:484–488. https://doi.org/10.1099/ijs.0.032557-0
Cappuccino JG, Sherman N (1992) Microbiology; A Laboratory Manual, 3rd edn. Rockland Community College, NY
Bunt JS, Rovira AD (1955) Microbiological studies of some subantarctic soils. J Soil Sci 6:119–128. https://doi.org/10.1111/j.1365-2389.1955.tb00836.x
Yasmin R, Hussain S, Rasool MH et al (2021) Isolation, Characterization of Zn solubilizing bacterium (Pseudomonas protegens RY2) and its Contribution in growth of chickpea (Cicer arietinum L) as deciphered by improved growth parameters and Zn content. Dose-Response 19:1–12. https://doi.org/10.1177/15593258211036791
Shao J, Xu Z, Zhang N et al (2015) Contribution of indole-3-acetic acid in the plant growth promotion by the rhizospheric strain Bacillus amyloliquefaciens SQR9. Biol Fertil Soils 51:321–330. https://doi.org/10.1007/s00374-014-0978-8
Palaniappan P, Chauhan PS, Saravanan VS et al (2010) Isolation and characterization of plant growth promoting endophytic bacterial isolates from root nodule of Lespedeza sp. Biol Fertil Soils 46:807–816. https://doi.org/10.1007/s00374-010-0485-5
Borrow A, Brian PW, Chester VE et al (1955) Gibberellic acid, a metabolic product of the fungus Gibberella fujikuroi: some observations on its production and isolation. J Sci Food Agric 6:340–348. https://doi.org/10.1002/jsfa.2740060609
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
RI P, (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Hu X, Chen J, Guo J (2006) Two phosphate- and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J Microbiol Biotechnol 22:983–990. https://doi.org/10.1007/s11274-006-9144-2
HL J, (1942) Nitrogen fixation in leguminous plants. II. Is symbiotic nitrogen fixation influenced by Azotobacter? Pro Line Soc 67:205–212
Kamran S, Shahid I, Baig DN et al (2017) Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front Microbiol. https://doi.org/10.3389/fmicb.2017.02593
Russell FM, Biribo SSN, Selvaraj G et al (2006) As a bacterial culture medium, citrated sheep blood agar is a practical alternative to citrated human blood agar in laboratories of developing countries. J Clin Microbiol 44:3346–3351. https://doi.org/10.1128/JCM.02631-05
Schoebitz M, Simonin H, Poncelet D (2012) Starch filler and osmoprotectants improve the survival of rhizobacteria in dried alginate beads. J Microencapsul 29:532–538. https://doi.org/10.3109/02652048.2012.665090
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. https://doi.org/10.1093/molbev/msab120
Li J, Zhao GZ, Zhang YQ et al (2008) Dietzia schimae sp. nov. and Dietzia cercidiphylli sp. nov., from surface-sterilized plant tissues. Int J Syst Evol Microbiol 58:2549–2554. https://doi.org/10.1099/ijs.0.2008/000919-0
Gharibzahedi SMT, Razavi SH, Mousavi SM (2014) Characterization of bacteria of the genus Dietzia: an updated review. Ann Microbiol 64:1–11. https://doi.org/10.1007/s13213-013-0603-3
Venil CK, Malathi M, Devi PR (2021) Characterization of Dietzia maris AURCCBT01 from oil-contaminated soil for biodegradation of crude oil. 3 Biotech 11:1–13. https://doi.org/10.1007/s13205-021-02807-7
Karnwal A, Dohroo A (2018) Effect of maize root exudates on indole-3-acetic acid production by rice endophytic bacteria under influence of L-tryptophan. F1000Research 7:1–11. https://doi.org/10.12688/f1000research.13644.1
Sunithakumari K, Padma Devi SN, Vasandha S (2016) Zinc solubilizing bacterial isolates from the agricultural fields of Coimbatore, Tamil Nadu, India. Curr Sci 110:196–205. https://doi.org/10.18520/cs/v110/i2/196-205
Costerousse B, Schönholzer-Mauclaire L, Frossard E, Thonar C (2018) Identification of heterotrophic zinc mobilization processes among bacterial strains isolated from wheat rhizosphere (Triticum aestivum L.). Appl Environ Microbiol. https://doi.org/10.1128/AEM.01715-17
Bapiri A, Asgharzadesh A, Mujallali H et al (2012) Evaluation of Zinc solubilization potential by different strains of fluorescent pseudomonads. J Appl Sci Environ Manag 16:295–298
Gandhi A, Muralidharan G (2016) Assessment of zinc solubilizing potentiality of Acinetobacter sp. isolated from rice rhizosphere. Eur J Soil Biol 76:1–8. https://doi.org/10.1016/j.ejsobi.2016.06.006
Mumtaz MZ, Ahmad M, Jamil M, Hussain T (2017) Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol Res 202:51–60. https://doi.org/10.1016/j.micres.2017.06.001
Saravanan VS, Kalaiarasan P, Madhaiyan M, Thangaraju M (2007) Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn2+) and zinc chelates on root knot nematode Meloidogyne incognita. Lett Appl Microbiol 44:235–241. https://doi.org/10.1111/j.1472-765X.2006.02079.x
Upadhyay H, Gangola S, Sharma A et al (2021) Contribution of zinc solubilizing bacterial isolates on enhanced zinc uptake and growth promotion of maize (Zea mays L.). Folia Microbiol (Praha) 66:543–553. https://doi.org/10.1007/s12223-021-00863-3
Bhatt K, Maheshwari DK (2020) Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech. https://doi.org/10.1007/s13205-019-2033-9
Karnwal A (2021) Pseudomonas spp., a zinc-solubilizing vermicompost bacteria with plant growth-promoting activity moderates zinc biofortification in tomato. Int J Veg Sci 27:398–412. https://doi.org/10.1080/19315260.2020.1812143
Khanghahi MY, Ricciuti P, Allegretta I et al (2018) Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environ Sci Pollut Res 25:25862–25868. https://doi.org/10.1007/s11356-018-2638-2
Di Simine CD, Sayer JA, Gadd GM (1998) Solubilization of zinc phosphate by a strain of Pseudomonas fluorescens isolated from a forest soil. Biol Fertil Soils 28:87–94. https://doi.org/10.1007/s003740050467
Fasim F, Ahmed N, Parsons R, Gadd GM (2002) Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol Lett 213:1–6. https://doi.org/10.1016/S0378-1097(02)00725-5
Vidyashree DN, Muthuraju R, Panneerselvam P, Mitra D (2018) Organic acids production by zinc solubilizing bacterial isolates. Int J Curr Microbiol Appl Sci 7:626–633. https://doi.org/10.20546/ijcmas.2018.710.070
Eshaghi E, Nosrati R, Owlia P et al (2019) Zinc solubilization characteristics of efficient siderophore-producing soil bacteria. Iran J Microbiol 11:419–430. https://doi.org/10.18502/ijm.v11i5.1961
Mumtaz MZ, Barry KM, Baker AL et al (2019) Production of lactic and acetic acids by Bacillus sp. ZM20 and Bacillus cereus following exposure to zinc oxide: a possible mechanism for Zn solubilization. Rhizosphere 12:100170. https://doi.org/10.1016/j.rhisph.2019.100170
Fahsi N, Mahdi I, Mesfioui A et al (2021) Plant growth-promoting rhizobacteria isolated from the jujube (Ziziphus lotus) plant enhance wheat growth, zn uptake, and heavy metal tolerance. Agriculture. https://doi.org/10.3390/agriculture11040316
Farokh RZ, Sachdev D, Pour NK et al (2011) Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J Microbiol Biotechnol 21:556–566. https://doi.org/10.4014/jmb.1012.12006
Mumtaz MZ, Ahmad M, Jamil M et al (2018) Bacillus strains as potential alternate for zinc biofortification of maize grains. Int J Agric Biol 20:1779–1786. https://doi.org/10.17957/IJAB/15.0690
Malik DK, Sindhu SS (2011) Production of indole acetic acid by Pseudomonas sp.: effect of coinoculation with Mesorhizobium sp. Cicer on nodulation and plant growth of chickpea (Cicer arietinum). Physiol Mol Biol Plants 17:25–32. https://doi.org/10.1007/s12298-010-0041-7
Gusain P, Paliwal R, Singh V (2017) Rhizoremediation of cadmium-contaminated soil associated with hydroxamate siderophores isolated from Cd-resistant plant growth–promoting Dietzia maris and Lysinibacillus strains. Int J Phytoremediation 19:290–299. https://doi.org/10.1080/15226514.2016.1225281
Acknowledgements
All authors acknowledge the support of the Center of Innovative and Applied Bioprocessing, Mohali, India for providing access to Ultra-performance liquid chromatography (UPLC). All the authors are thankful to Chandigarh University for providing financial support, lab, and other infrastructure.
Funding
The study was carried out using the internal funds of Chandigarh University.
Author information
Authors and Affiliations
Contributions
The research proposal was designed by SK. NR performed the experimental work. Data collection, analysis, and paper writing were done by NR and GK. The UPLC analysis and its interpretation was done by NP. VM contributed in preparing high-resolution figures and the final editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval
The research was approved by the Departmental Research Committee.
Consent to Participations
Not applicable.
Consent for Publications
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rani, N., Kaur, G., Kaur, S. et al. Plant Growth-Promoting Attributes of Zinc Solubilizing Dietzia maris Isolated from Polyhouse Rhizospheric Soil of Punjab. Curr Microbiol 80, 48 (2023). https://doi.org/10.1007/s00284-022-03147-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03147-2