Abstract
A bacterial strain was isolated from an oil-contaminated site and on its’ further characterization, exhibited the potential of synthesising metabolites and the ability to degrade crude oil. Its’ morphological, biochemical and 16S rRNA analysis suggested that the bacterium belongs to Dietzia maris AURCCBT01. This strain rapidly grew in the medium supplemented with n-alkanes C14, C18, C20, C28 and C32 utilizing them as a sole carbon source and produced a maximum canthaxanthin pigment of 971.37 µg/L in the n-C14 supplemented medium and produced the lowest pigment yield of 389.48 µg/L in the n-C-32 supplemented medium. Moreover, the strain effectively degraded 91.87% of crude oil in 7 days. The emulsification activity of the strain was 25% with the highest cell surface hydrophobicity (70.26%) and it showed a decrease in surface tension, indicating that the biosurfactant production lowers the surface tension. This is the first report on the characterization of the strain, Dietzia maris AURCCBT01 and its’ novelty of alkane degradation and simultaneous production of canthaxanthin pigment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crude oil/hydrocarbon contamination of the land and aquatic sources is one of the major environmental problems that the world is currently confronted with. Crude oil contaminants cause high toxicity to the humans and environment and are quoted as one of the most alarming pollutants (Xu et al. 2018). Crude oils/fossil fuels are mixtures of hydrocarbon compounds (Saturates, Aromatics, Resins, Asphaltenes etc.). Ranging from smaller, volatile compounds to very large, non-volatile compounds, they have several distinctive physical characteristics, particularly dense, viscous and a strong tendency to adhere to the surfaces and to submerge beneath the water surface. Potential to obstinate into the soils and adhere to the water surfaces (Sieben et al. 2017), crude oils right from their extraction from oil reservoirs and aquatic beds of the earth and until they are refined into final petroleum products, undergo various production processes. During these processes and accidental oil spillages, our environment is exposed to various hazardous effects. All these hazardous effects pose a great threat to humans and ecology.
Safeguarding the health of humans and the environment from the hazardous effects of the toxic wastes of hydrocarbons is of utmost importance. Several oil pollution remedial measures like, thermal treatment, burying, evaporation, dispersion, advanced oxidation process, emulsion breakers, adsorption, chemical dispersants etc. are in vogue in this field, which could be employed depending upon the requirements (Ivshina et al. 2015). These are expensive technologies and encounter several drawbacks such as low solubility and non-polarity, besides leading to the incomplete treatment of oil contaminants (Dwivedi et al. 2019). Following the problems confronted in these expensive and drawback remediation processes, being the other choice methods, biological methods have evolved as recognized alternative ones that could be employed to remediate crude oil contaminated sites.
Employing bacterial sources as a key tool for remediation of contaminants is one such recognized biological approach. In this process, prospective bacterial sources are utilized, as a chief biological tool to degrade the pollutants of the petroleum hydrocarbons and oils, which is an appropriate, efficient, economic and eco-friendly remediation methodology (Lea-Smith et al. 2015). Such bacterial tools detoxify hazardous organic compounds by polymerization, mineralization and transformation (Sarma and Sarma 2010). Interestingly, many hydrocarbon-degrading bacteria are capable of producing bio-surfactants/bio-emulsifiers that will increase the solubility of insoluble substrates by utilizing them as a sole carbon source (Pacwa-Plociniczak et al. 2014; Ibrahim 2016).
Several studies reveal that the bacteria isolated from the oil-rich environments are associated with that type of hydrocarbons and to the surrounding environmental factors (Yang et al. 2015; Varjani and Gnansounou 2017). The studies of Ivshina et al. (2015) and Sakthipriya et al. (2015) testify that many bacteria isolated from oil-contaminated sites are capable of degrading hydrocarbons. Acinetobacter sp., Achromobacter sp., Flavobacterium sp., Rhodococcus sp., Geobacillus sp., Bacillus sp., Pseudomonas sp., Kocuria sp. and Dietzia sp., most effectively degrade hydrocarbons utilizing crude oil as their sole carbon source (Chandran and Das 2011; Sadighbayan et al. 2016; Thapa et al. 2012; Xia et al. 2015; Yudono et al. 2011; Lin et al. 2014; Hamme and Ward 2001; Nazina et al. 2005; Gharibzahedi et al. 2013a).
Naturally, bacterial strains native to oil-contaminated sites will have incredible degradation prospective, as their living environment itself would be a challenging one for them to adapt to that situation. Such indigenous bacteria of hydrocarbon-polluted sites utilize hydrocarbons for their carbon needs, grow and metabolize, and relieve themselves from physiological stress caused by hydrocarbons in the environment (Kleindienst et al. 2015). Some bacteria metabolize specific alkanes, while others break down aromatic fractions of hydrocarbons; these factors are relating to the type of hydrocarbon components and the potential of those native bacteria. These characteristics suggest that these indigenous bacteria are crucial for the degradation of petroleum hydrocarbons and they influence the transformation and the fate of the hydrocarbons in the environment and thus crediting the fact that they have different catalytic enzymes and their roles in oil-contaminated sites are varying (Xu et al. 2018). The study of Gurav et al. (2017) testifies that the bacterial consortium provides wider enzymatic activities and facilitates the biodegradation of recalcitrant compounds via co-metabolism and commensalism. Microbes use long-chain n-alkanes as carbon and energy sources and a higher rate of utilization of the hydrocarbons by bacterial consortium is reported. Among the hydrocarbon-degrading bacteria, Dietzia sp. is advantageous and more than 13 species have been reported (Gharibzahedi et al. 2013a). Dietzia sp. DQ12-45-1b utilized petroleum hydrocarbons whereas Dietzia sp. E1 utilized C6-C36 alkanes as carbon source (Bihari et al. 2011) and D. cinnamea P4 utilized C11–C36 alkanes (Weid et al. 2007). Dietzia sp. can degrade aromatic hydrocarbons like naphthalene, phenanthrene and fluoranthene (Kumar et al. 2011). Dietzia sp. DQ12-45-1b utilized C6-C40 n-alkanes, aromatic compounds and crude oils as carbon and energy sources. Yet there are not many reports on the degradation of hydrocarbons by Dietzia maris.
Dietzia sp. is one of the most promising strains for the production of carotenoid pigments, mainly canthaxanthin, an orange-red pigment comprising significant properties with keto-carotene. Canthaxanthin pigments have several health benefits like anti-oxidant, anti-inflammatory, anti-tumor, anti-cancer and as colouring mediators (Gharibzahedi et al. 2012); they are widely used in different industries such as cosmetics, nutraceuticals, food and feed industries (Goswami et al. 2012; Gharibzahedi et al. 2013b; Ravaghi et al. 2016). The global market for carotenoid (astaxanthin, beta-carotene, canthaxanthin, lutein, lycopene, zeaxanthin) is projected to be USD 1.53 billion by 2021 (Sathasivam and Ki 2018). Canthaxanthin has high market value thanks to the consumer demands owing to its’ strong antioxidant potential, besides its’ utility as nutraceuticals (Mezzomo and Ferreira 2016). Moreover, Dietzia sp. can be used both in agriculture and in environmental applications with easy and efficient precise methods. To the best of our knowledge, studies relating to alkane degradation and canthaxanthin pigment production from bacterial sources are very scarce.
The present study therefore was aimed to isolate a native bacterial strain from oil-contaminated sites, identify and characterize it, besides evaluating its prospective of pigment production and testing its crude oil degradation potential. Accordingly, in this study we have analyzed the characteristics and the efficiency of the strain Dietzia maris AURCCBT01, isolated from an oil-contaminated site, and now report the novel ability of its’ canthaxanthin pigment production and crude oil degradation prospective. We also propose it as a valuable tool for bioremediation of oil contaminant sites and pigment production.
Materials and methods
Isolation and selection of crude oil-degrading bacteria
The oil-contaminated soil samples were collected from the TecMec Engineering works, Coimbatore, Tamil Nadu, India (11o2′ N, 76o58′ E). The sampling site is located in an industrial hub, encompassing wastewater discharge outlets. Temperature, salt concentration and pH of the sampling site were 34 °C, 29.87 g/L and 8 respectively. The oil polluted sediment samples were collected using spatula in sterile containers, transported to laboratory and stored at 4 °C. Mineral salt medium (MSM) comprising NH4NO3 (1.0 g), CaCl2 (0.02 g), Mg2SO4 (0.05 g), K2HPO4 (1.0 g), and KH2PO4 (1.0 g) and trace element (5 mL) [Trace element: CaCl2 (2 mg/L), FeCl3·6H2O (50 mg/L), MnCl2·4H2O (0.5 mg/L), ZnSO4·7H2O (10 mg/L) and CuSO4 (0.5 mg/L)] in 1 L distilled water was prepared. For solid medium, agar–agar 20 g/L was added to MSM. The medium was autoclaved at 121 °C for 15 min.
10 g of oil-contaminated samples were soaked in 100 mL sterile distilled water in 250 mL conical flask. The flask was shaked vigorously and allowed to stand for 15 min and 10 mL of the supernatant was added to 100 mL of MSM containing 0.5% (w/v) crude oil as the sole carbon and energy source in the 250 mL conical flask and incubated at 30 °C, 150 rpm for 7 days. 100 µL were inoculated into MSM agar plates containing 0.5% (w/v) crude oil and incubated at 30 °C. The colonies obtained were sub-cultured on MSM plates and stored on Luria–Bertani agar slants at − 30 °C. The bacterial strain AURCCBT01 was selected for further study due to its ability to grow in the crude oil amended medium.
Identification of the strain AURCCBT01
Morphological characterization
The biochemical characteristics of the strain AURCCBT01 were studied by Gram staining, catalase, oxidase, methyl-red, Voges–Proskauer reactions, nitrate reduction, acid from glucose, indole production, nitrate reduction, hydrogen sulphide production, citrate utilization and urease production according to Bergey’s manual of determinative for bacteriology.
The strain AURCCBT01 was cultured on Bab3-8 medium, centrifuged at 8000 rpm for 15 min and the cell pellet was harvested. Phosphate buffer saline (PBS) was used to wash cell pellets three times and 2.5% glutaraldehyde was added for fixation and incubated at 4 °C for 6 h. After fixation, the cell pellets were again washed with PBS solution. Cell pellets were treated with increasing concentration of ethanol for dehydration and allowed to air dry. The sample was coated with platinum and examined under a JEOL-JSM-6390LV scanning electron microscope.
Analysis of 16S rRNA sequence and phylogenetic analysis
Standard methods were followed for the preparation of genomic DNA. The strain was cultured in LB broth overnight, centrifuged and pellets re-suspended with 1 X TE buffer containing 10% SDS and 120 µg/mL proteinase K and kept for incubation at 37 °C for 1 h. The phase separation was carried out and ethanol was used for precipitation of aqueous phase and the cells were re-suspended in TE buffer and stored at 4 °C. The DNA absorbance was measured at 260 nm, the purity was tested in 0.8% agarose gel, and ethidium bromide was used for staining. After DNA purification, the amplification of 16S rRNA genomic region was completed using universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1392R (5′-GGTTACCTTGTTACGACTT-3′).
Amplification of the 16S r RNA gene fragments were carried out in Palm 1870 cycler TM (Corbett, UK) under the standard conditions: initial denaturation at 94 °C for 5 min trailed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min and extension at 72 °C for 2 min and then an extension for 5 min at 72 °C. The reaction mixtures comprised of 2.5 U polymerase (Mango Taq, Bioline), 10 mL of buffer solution, 2.5 mL of 50 mM MgCl2, and 2.5 mL each of forward primer and reverse primer, genomic DNA, and water to 50 mL final volume and the PCR products were electrophoresed on 1% agarose gel.
DNA sequences obtained from the strain were aligned by CLUSTALW using MEGA. 16S rRNA gene sequences were transferred into BLAST to identify matches with existing characterized sequences (NCBI—http://www.ncbi.nlm.nih.gov). The nucleotide sequence was deposited in the NCBI GenBank database under Accession Number MN134494.
Utilization of carbon source by the strain AURCCBT01
The ability of the strain D. maris AURCCBT01 to consume hydrocarbons as the sole carbon source was tested by culturing 5 mL of the 72 h old culture (OD600 value 1) in Reasoner’s 2 medium comprising in peptone 0.5 g, yeast extract 0.5 g, casamino acid 0.5 g, glucose 0.5 g, soluble starch 0.5 g, K2HPO4 0.3 g, MgSO4.7H2O 0.05 g, sodium pyruvate 0.3 g in lL of distilled water. After 48 h, the culture was centrifuged at 8000 rpm for 5 min, cells harvested were washed three times with sterile saline solution to eliminate residual organic compounds. The cells were inoculated into 100 mL of MSM medium supplemented with different alkanes (C14, C18, C20, C28, C32, C36, C40 and C44), branched alkanes (pristine) and aromatic hydrocarbons (phenantherene) in 250 mL Erlenmeyer flasks. The concentrations in flasks for n-alkanes and aromatic hydrocarbons were 1000 mg/L and 400 mg/L respectively. Two controls were maintained, control 1 had only cells with no hydrocarbon whereas control 2 had only hydrocarbons with no cells. The cultures were incubated at 30 °C, 150 rpm for 7 days and the cell growth was recorded by measuring OD600 in the medium.
Pigment synthesis
In addition, the pigment extraction was carried out following the method of Rezaeeyan et al. (2017) with few modifications. The culture (10 mL aliquot) was centrifuged using cooling centrifuge at 7000 g for 30 min (Remi C-24 plus). The cells were re-suspended in double-distilled water and cell lysis occurred. The pellets were washed with NaCl solution and centrifuged to obtain yellow color pellets. The pellets were then suspended in ethanol, vortexed for 5 min and centrifuged to obtain the pigments. The pigment extracts were filtered and analysed by scanning the absorbance in the region of 350–650 nm using UV–spectrophotometer (LI-UV-7000). The maximum absorbance was determined at a wavelength of 472 nm.
Degradation of crude oil by D. maris AURCCBT01
5 mL of the 72 h old culture (OD600 value 1) in Reasoner’s 2 medium were harvested by centrifugation at 5000 rpm for 15 min, washed three times with sterile saline solution and inoculated into 100 mL of MSM in 250 mL Erlenmeyer flasks amended with crude oil (0.75% w/v) at pH 6. The culture medium was incubated at 30 °C, 150 rpm for 14 days. Control was incubated without bacterial cells and the experiments were conducted in triplicate. After 14 days of incubation colour change in the medium was observed and the medium was filtered to separate the biomass. The filtrate was centrifuged at 8000 rpm for 15 min, the supernatant was examined by UV–VIS spectrophotometer at 609 nm, and the degradation percentage was calculated as follows:
The residual oil in the culture medium was extracted using the equivalent volume of n-hexane and dried using vacuum rotary evaporator. The crude oil components were separated using column chromatography and analysed using Gas chromatography. The hydrocarbons were analysed using 7890 A gas chromatography-mass spectrometer (Agilent, USA) using nitrogen as carrier gas at a flow rate of 2 mL/min; injector temperature 280 °C; detector temperature 300 °C; initial column temperature 80 °C for 2 min and raised to 280 °C and maintained for 20 min at a rate of 5 °C/min.
Effect of pH and NaCl on crude oil degradation
5 mL of the 72 h old culture (OD600 value 1) in Reasoner’s 2 medium were harvested by centrifugation at 5000 rpm for 15 min, washed three times with sterile saline solution and inoculated into 100 mL of MSM in 250 mL Erlenmeyer flasks. To study the effect of pH on crude oil degradation, MSM medium supplemented with crude oil (0.75% w/v) at the initial pH 5–9 adjusted using 1 mol/L NaOH or 1 mol/L HCl solution respectively in each flask and the control was maintained without cells. The cultures were incubated at 30 °C, 150 rpm for 7 days and the cell growth was recorded by measuring OD600 in the medium.
Salt content is an important factor in the degradation of petroleum hydrocarbons in the soil because the high osmotic potential directly influences the metabolic activity of bacteria (Ahamed et al. 2014). So, the influence of sodium chloride on crude oil degradation was assessed using various NaCl concentrations ranging from 10, 20, 40, 80 g/L. The effect of NaCl on oil degradation was tested as described in the earlier section and the saturated hydrocarbon degradation efficiency was measured using GC–MS. The control sample without inoculum was used to detect abiotic loss of saturated hydrocarbons. The experiment was performed in triplicate and the results are expressed as mean and standard deviations.
Emulsification, cell adhesion and surface tension
The cell-free culture filtrate and crude oil in the equal ratio were mixed and vortexed for 5 min and incubated for 24 h at 28 °C. The height of the emulsified layer by the total length of the column is the emulsification activity (Bosch et al. 1988). The bacterial cell adhesion to hydrocarbon was measured by following the method of Plaza et al. (2005). The 14 days old cell culture was used to measure surface tension and expressed in mN/m.
Statistical analysis
All experiments were conducted in triplicate and the values were expressed as means. The means were compared using one-way ANOVA and the Tukey test (SPSS software, 14.0) to indicate any significant differences among the parameters and variables. Results were considered significant if p < 0.05.
Results and discussion
Isolation and characterization of the strain AURCCBT01
Microbes that can degrade crude oils are usually isolated from oil-polluted environments such as oil spill area and oil reservoirs (Xu et al. 2018). According to Tremblay et al. (2017) more than 79 genera including Dietzia sp., were isolated from the oil-polluted environments that are capable of degrading petroleum hydrocarbons. Liu et al. (2017) in their study have testified that oil-polluted sites were dominated by Gram-negative bacteria and predominant hydrocarbon degraders are Gram-positive bacteria comprising Rhodococcus, Mycobacterium, Dietzia etc. and proposed that they are suitable for degradation of oil pollutants. In this study, twelve bacterial strains were isolated from the oil-contaminated soil samples collected from TecMec Engineering work, Coimbatore, Tamil Nadu, India. Of them, only the strain, Dietzia maris AURCCBT01 showed the highest growth on crude oil amended medium and so it was selected for further study.
The cells of the strain AURCCBT01 tested in the present study are Gram-positive cocci, flat, smooth, non-sporing, halotolerant and catalase positive. The colonies are circular, convex, glistening and expressing orange pigment on agar medium (Fig. 1a). The surface morphology of the bacterial cells studied using scanning electron microscopy (SEM) and Fig. 1b showed that the cells are spherical in shape. The strain was initially identified by biochemical tests and then molecular identification followed. The biochemical characterization of the strain identified in our study is illustrated in Table 1.
The complete 16S rRNA gene sequence (1483 bp) of the strain obtained was matched with GenBank databases by BLAST searches. The outcome of the BLAST indicated that the strain AURCCBT01 exhibited maximum similarity to Dietzia maris within the genus Dietzia. The similarity index was 99.86% and so the sequence was submitted into the GenBank under the accession number MN134494. The phylogenetic analysis of the strain suggests that it belongs to the genus Dietzia and shows a highest similarity with Dietzia maris (Fig. 1c). All these characteristics suggest that the strain AURCCBT01 could be a prospective one in the line of recognised oil-degrading bacteria.
Optimization of carbon source by the strain AURCCBT01
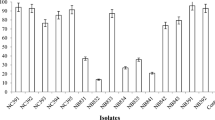
The strain D. maris AURCCBT01 tested for its ability to degrade n-alkanes and utilize aromatic hydrocarbons as a sole carbon source. This strain utilizes n-alkanes in the medium and degraded C14, C18, C20, C28, C32 effectively after 7 days, whereas degradation was not observed for higher alkanes such as C36, C40, C44, pristine and phenantherene (Table 2). Similarly, the canthaxanthin production was higher in the medium supplemented with C14, C18, C20, C28, C32 and drastically lowered in C36, C40 and C44 suggesting that the growth of D. maris AURCCBT01 reduced in higher n-alkanes. This strain effectively utilizes n-alkanes as carbon source and produces canthaxanthin, which was higher (971.37 µg/L) in n-C14, lowers gradually in C18, C20, C28 and the lowest production of 389.48 µg/L was in C32. There was no production of canthaxanthin in higher alknanes such as C36, C40, C44, pristine and phenantherene (Table 2). The Gram-positive bacteria play an important role in the degradation of oil-polluted sites because of their enzymatic and metabolic activities (Brzeszcz and Kaszycki 2018). In this study, the tested strain D.maris AURCCBT01 degraded medium chain length n-alkanes by enhancing the hydrogenase and catalase activities and has thus testified its potential as other Gram-positive bacterial hydrocarbon degraders.
Owing to the outer permeability membrane made of mycolic acids (C30-C90 α-alkyl, β-hydroxy fatty acids), the Dietzia sp. exhibited high hydrophobicity favouring enhanced degradation (Gutierrez et al. 2011). When the cells are exposed to n-alkanes, the hydrophobicity increases in the exponential phase and becomes hydrophilic in the stationary phase. The hydrophobic factors are responsible for cell-surface hydrophobicity in Dietzia sp., which suggest the presence of hydrophobic bioemulsifier (Wang et al. 2013). Once the n-alkane becomes available to the strains, it penetrates the cell membrane and so oxidation occurs, and degradation starts in the early stationary phase and their surface hydrophobicity decreases (Bihari et al. 2010). The strain D. maris AURCCBT01 has exhibited high hydrophobicity and produced bio-emulsifier, leading to better degradation.
Canthaxanthin, a ketocarotenoid has eight isoprenoid groups and 11 bonds as conjugated polyene called chromophore together with carbonyl group substitution. The canthaxanthin production is stimulated in the existence of medium chain n-alkanes, which are associated with the uptake system and intracellular binding sites, thereby enhancing the pigmentation (Gharibzahedi et al. 2013b). Rostami et al. (2014) have reported that canthaxanthin production is possible due to the stimulation of precursors of the carotenoid pathway and mevalonic acid pathway in the presence of carbon source in the medium thereby enhancing the synthesis and accumulation of canthaxanthin. Canthaxanthin is synthesized from isopentenyl pyrophosphate (IPP) and dimethylallyldiphosphate via the mevalonic acid (MVA) pathway and methylerythritol phosphate (MEP) to achieve the carbon precursors. Canthaxanthin plays an important role in membrane stabilization by Dietzia sp. and the decreased production in higher n-alkanes supplemented medium is due to the decreased functionality of the biomembranes in higher alkanes. Canthaxanthin, being a unique carotenoid, is gaining industrial importance owing to its broad-ranging applications such as food, nutraceuticals, cosmetic industries etc.
Crude oil degradation by D. maris AURCCBT01
To investigate the crude oil degradation potential of the strain AURCCBT01, it was cultivated in crude oil (0.75% w/v) and the culture started to become turbid at day 4. The turbidity increased sharply until the end of the experiment (day 14). After 7 days of cultivation, the hydrocarbons (< C28) started to degrade and the hydrocarbons with chain length > 32 remained similar to control. The gas chromatogram (Fig. 2) revealed that the strain could degrade a wide range of hydrocarbons (C14-C28). About 68.7% of the hydrocarbons were utilized in the first 4 days and about 23.17% in the next 2 days and totally 91.87% in 7 days; in the remaining days the degradation reduced gradually. Overall, the strain tested in this study has exhibited its’capability to use crude oil as the key carbon source and showed the highest degradation (91.87%) of the n-alkanes (C14–C28) in a short time of 7 days and has thus exhibited its’ prospective as a bio-tool to degrade oil-contaminated sites (Fig. 3).
Al-Wasify and Hamed (2014) have reported that the Pseudomonas aeruginosa, Bacillus subtilis and Acinetobacter lwoffi degrade crude oils to a maximum rate of 77.8%, 76.7% and 74.3% respectively and the mixture of this bacterial consortium degraded a maximum of 88.5%. Another study testifies that the isolate Streptomyces parvus B7 showed biodegradation efficiency up to 82% aided by the high bio-surfactant production (Parthipan et al. 2018). Bayat et al. (2015) have testified that the strains Micrococcus luteus isolate BHA7, Pseudoalteromonas sp. isolate BHA8 and Shewanella haliotis isolate BHA35 can degrade 88.08%, 27.13% and 69.17% of crude oils respectively. The bacterial consortium (Serratia proteamaculans S1BD1, Alcaligenes sp., R. erythropolis) achieved the highest degradation of 85.25% in 15 days than that of individual strains (S. proteamaculans S1BD1: 68.0%, Alcaligenes sp.: 63.7% and R. erythropolis: 54.9%) (Xia et al. 2019). The study of Abdulla et al. (2019) shows that the strain, Bacillus cereus degraded 98% of the hydrocarbons in 15 days. The bacterial strain Dietzia sp. CN-3 isolated from petroleum contaminated soils by Chen et al. (2017) utilized a wide range of alkanes C14-C32 and crude oil as the sole carbon sources. Dietzia cinnamea KA1 isolated by Kavynifard et al. (2015) was able to utilize and degrade 95.7% of the crude oil after 5 days under optimal conditions. Wang et al. (2011) have testified that Dietzia sp. DQ12-45-1b utilized n-alkanes (C6-C40) as a sole carbon source. The strain Dietzia cercidiphylli C-1 isolated from the Venezuelan oil contaminated soils degraded 25.58% of extra heavy oil in 14 days (Dai et al. 2017). In our study, the strain D. maris AURCCBT01 showed the maximum degradation rate of n-alkanes (91.87%) in 7 days which is positively comparable to the other oil-degrading bacteria.
Degradation mechanism
Being a complex mixture of different alkanes, crude oil encompasses dissimilar numbers of carbons in the chain. When the crude oil spills on the earth, it quickly becomes obstinate into the soil and the native microbes utilise them as their carbon source through a step-by-step oxidative degradation process (Wang et al. 2012). During the oxidation process, the number of carbon in the chain reduced sequentially (either one or multiple) is depending upon the number of active carbon sites in the chain that took part in the oxidation process. The mechanism involved in the oxidative degradation of linear alkanes by the tested strain is interesting. Accordingly, two types of mechanisms are proposed viz. terminal carbon oxidation and grabbed internal carbon oxidation and these are presented in Fig. 4.
In the first mechanism (terminal carbon oxidation), the oxidative active site is terminal carbon, usually a methyl group in a parent alkane chain. Initially, the methyl group is oxidised to methyl alcohol. Following the continuation of microbial degradation, the alcohol is converted into aldehyde and on further oxidation yields acid. A simple self-condensation of these acids liberate the daughter alkane chain with one carbon less in number. If the same methyl oxidation process takes place at both the terminal carbon of the parent alkane chain, the daughter product of this first cycle is the alkane chain with two carbon atoms less in number. Consequently, the same oxidative degradation is repeated on the first cycle product daughter molecule-1 under the microbial medium and produces alkane with less number of carbon atom or atoms depending upon the number of terminal carbon active sites. In all the cycles, the reduced carbon liberates in the form of its oxides specifically as carbon dioxide (Wang et al. 2012). The cycle is repeated extensively under the microbial condition until it reached to very small alkanes.
The terminal carbon oxidation mechanism is also possible in the inner carbon of the alkane chain. The only difference of the inner carbon oxidation from terminal carbon is the formation of ketone instead of aldehyde and causes alkane chain breaking. The activation of inner carbon is random in nature and rupture of the alkane chain is always asymmetric. The most denoted point in the inner carbon oxidation is the rate of degradation that is very high in the inner case whereas in the terminal mode the number of carbon is reduced gradually.
To find the formation of the alcohol, aldehyde and acid, functional group spot tests were carried out. Ceric ammonium nitrate test was performed on microbial medium and sample extracted after 7 days. The alcohol present in the medium reacted with ceric ammonium nitrate resulting in a red-brown solution, which confirmed the formation of alkoxy cerium(IV) compound. The observed colour change supports the presence of aliphatic alcohol formation during the oxidative degradation of the aliphatic chain (Scheme 1).
Fehling’s Test, which is specific for aliphatic aldehyde functional groups, was carried out on microbial medium (sample collected after 10 days) to confirm the aliphatic aldehyde formation. To the extract medium, Fehling’s Cu2+ solution was added. Reduction of copper ion by the aliphatic aldehyde resulted in a red precipitate known as copper(I) oxide. The observed precipitate formation confirmed the presence of aliphatic aldehyde in the microbial medium (Scheme 2).
Sodium bicarbonate test was carried out on the microbial medium to confirm the presence of aliphatic acid functionals. The sample solution was extracted from the crude microbial medium after 12 days of degradation process. The extract was first condensed by slow evaporation and the crude was dissolved in methanol. To this alcoholic solution, a small amount of sodium bicarbonate was added and brisk effervescence observed due to the CO2 gas liberation, which confirmed the aliphatic acid formation in the microbial medium (Scheme 3).
The overall mechanism of degradation of hydrocarbon by the strain is also represented in Fig. 5.
Effect of pH and NaCl on crude oil degradation
The optimum pH range for crude oil degradation by the strain was investigated by growing its’ cells at 30 °C, 150 rpm. The degradation rate of crude oil changed from different pH range 5–9. The degradation percentage increased gradually to 93.7% at pH 6 and 7 (Fig. 6a). pH plays a pivotal role in bacteria as it influences the utilization of nutrients, adsorption, enzyme secretion etc. However, the degradation rate decreased significantly when the pH increased to 8 and 9. This strain requires neutral pH for optimum growth and acidic or alkali pH does not favour the growth of D. maris AURCCBT01. Luo et al. (2013) have studied the effect of pH and reported that at pH 7, Pseudomonas strain F4 showed maximum degradation. Several studies testify that bacterial growth and oil degradation occur at neutral pH (Whang et al. 2009; Xia et al. 2012). The degradation prospect of crude oil by individual bacterium and a mixed consortia in a study was found to be at pH 7 (Sathishkumar et al. 2008) and the present finding on the degradation potential of D. maris AURCCBT01 at pH 6 and 7 too is corresponding comparatively with earlier studies.
Inorganic salts are important for bacterial growth as it is necessary for enzyme reaction, maintaining cell equilibrium and osmotic pressure (Shen et al. 2015). NaCl at different concentrations (10, 20, 40, 80 g/L) were used to study the influence of NaCl on crude oil degradation. The degradation rate decreased with increasing concentrations of NaCl. This strain exhibited the highest degradation capacity of 86.5% at NaCl concentrations of 20 g/L and 83.4% at 40 g/L (Fig. 6b). The salinity had little impact on the growth of D. maris AURCCBT01 and the degradation rate was above 70% even at 80 g/L of NaCl concentrations. This result demonstrates that the maximum NaCl concentration does not affect the degradation rate of this strain and it exhibited its potential to degrade crude oil at high salinity too.
Emulsification, cell adhesion and surface tension
Most of the oil-degrading bacteria produce biosurfactants to facilitate oil uptake and degradation by emulsifying the hydrocarbons (Karlapudi et al. 2018). The n-alkane degradation co-existed with a substrate adhesion mechanism. The Dietzia sp. and Rhodococcus sp. possess three distinct levels of cell-surface hydrophobicity during cell growth. When cells grown on water–soluble substrates, moderate hydrophobicity can be observed whereas extreme, hydrophobicity can be observed when the cells are exposed to n-alkanes in the exponential phase while the cells suddenly become hydrophilic when reaching the stationary phase (Bihari et al. 2010). The presence of hydrophobic bioemulsifier is very likely in Dietzia sp. because the rapid loss of hydrophobicity coincided with alkane growth substrates. The effect of crude oil on emulsification, cell surface hydrophobicity and surface tension of D. maris AURCCBT01 are shown in Table 3. Emulsification potential is an important index for screening the efficiency of bio-surfactant producing microbes (Al-Hawash et al. 2019). Emulsification activity for this strain, D. maris AURCCBT01 was 35% which was reported for other oil-degrading bacteria as well (Al-Hawash et al. 2018).
The strain D. maris AURCCBT01 showed the highest cell surface hydrophobicity (70.26%). Hassanshahian et al. (2012) have reported that cell surface hydrophobicity is an important characteristic of crude oil degradation and the D. maris AURCCBT01, tested in this study satisfies this hydrophobicity characteristics. Hydrophobicity decreases gradually during degradation and this decrease is attributed to the depletion of carbon source caused by the substrate degradation. Also the decrease in hydrophobicity is related to the production of bio-surfactants which alters the hydrophobicity of the surface and affects the adhesion of microbes over the surface (Vijayakumar and Saravanan 2015). Bio-surfactants improve hydrocarbon degradation by inducing cell surface hydrophobicity and thereby increasing the direct contact between cells and substrates (Al-Hawash et al. 2019).
There is a decrease in surface tension (52 mN/m) of the strain D. maris AURCCBT01, which indicates that the bio-surfactant production lowers the surface tension (Zhang et al. 2012). Rahbari-Sisakht et al. (2017) have also reported that the bio-surfactant production is proportional to hydrocarbon degradation and the bio-surfactant production by the tested strain D. maris AURCCBT01 is matching with earlier studies. Also, the bio-surfactants have acted as emulsifying agents and thereby decreased the surface tension. The outcomes compared above indicate that this strain produces bio-surfactants and has an acceptable level of emulsification activity. Bio-surfactants with high surface activity and environmental compatibility are generally used for the degradation of oil-contaminated sites (Hassanshahian et al. 2012).
Conclusions
Previous studies on diverse bacterial species indigenous to crude oil/hydrocarbon-contaminated sites have validated that such bacterial strains utilize hydrocarbons as their sole carbon source and thrive well in that unfriendly environment metabolizing valuable compounds like pigments, prospective for industrial applications. By that way, they are remarkable natural sources to remediate hazardous toxic effects of the hydrocarbon/crude oil contaminated sites besides synthesizing valuable bio-products. Therefore, identification of the prospective bacterial sources from such a challenging environment and employing them as a key bio-tool to remediate hydrocarbon/crude oil contaminated sites should be the future strategy to deal with such environmental issues. This study is one such one wherein a novel strain AURCCBT01, belonging to Dietzia sp. was isolated from an oil-contaminated site and its biochemical characteristics and 16S rRNA sequences testified that the cells are Gram-positive cocci, flat, smooth, non-sporing, halotolerant, catalase positive and expressing orange pigment on agar medium. This strain was able to grow on n-alkanes utilizing them as the sole carbon source and simultaneously produced canthaxanthin pigment. Being an initial study on Dietzia maris, this study of ours demonstrates the potential of the novel strain AURCCBT01 for alkane degradation with simultaneous production of canthaxanthin pigment, a valuable compound for nutraceuticals and pharma applications.
References
Abdulla KJ, Ali SA, Gatea IH, Hameed NA, Maied SK (2019) Biodegradation of crude oil using local bacterial isolates. Ear Env Sci 388:012081
Ahamed F, Dhar K, Ferdouse J, Anwar MN (2014) Effect of salinity on the growth of petroleum hydrocarbons degrading Bacillus sp. isolated from chronically polluted ship breaking yards. Elixir Biotech 68:22280–22283
Al-Hawash AB, Alkooranee JT, Abbood HA, Zhang J, Sun J, Zhang X, Ma F (2018) Isolation and characterization of two crude oil degrading fungal strains from Rumaila oil field. Iraq Biotechnol Rep 17:104–109
Al-Hawash AB, Zhang X, Ma F (2019) Removal and biodegradation of different petroleum hydrocarbons using the filamentous fungus Aspergillus sp. RFC-1. Microbiol 8:e619
Al-Wasify RS, Hamed SR (2014) Bacterial degradation of crude oil using local isolate. Int J Bacteriol. https://doi.org/10.1155/2016/6013871
Bayat Z, Hassanshahian M, Hesni MA (2015) Enrichment and isolation of crude oil degrading bacteria from some mussels collected from Persian Gulf. Mar Pollu Bulle 101:85–91
Bihari Z, Szabo Z, Szvetnik A, Balazs M, Bartos P, Tolmacsov P, Zombori Z, Kiss I (2010) Characterization of a novel long chain n-alkane degrading strain, Dietzia sp. E1. Z Naturforsch 65:693–700
Bihari Z, Szvetnik A, Szabo Z, Blastyak A, Zombori Z, Balazs M (2011) Functional analysis of long-chain n-alkane degradation by Dietzia spp. FEMS Microbio Lett 316:100–107
Bosch MP, Robert M, Mercade ME, Espuny MJ, Parra JL, Guinea J (1988) Surface active compounds on microbial cultures. Tenside Sur Deterg 25:208–212
Brzeszcz J, Kaszycki P (2018) Aerobic bacteria degrading both n-alkanes and aromatic hydrocarbons: an undervalued strategy for metabolic diversity and flexibility. Biodegrad 29:359–407
Chandran P, Das N (2011) Degradation of diesel oil by immobilized Candida tropicalis and bioflm formed on gravels. Biodegradation 22:1181–1189
Chen W, Li J, Sun X, Min J, Hu X (2017) High efficiency degradation of alkanes and crude oil by a salt tolerant bacterium Dietzia species CN-2. Int Biodet Biodegr 118:110–118
Dai X, Yan G, Guo S (2017) Characterization of Dietzia cercidiphylli C-1 isolated from extra heavy oil contaminated soil. RSC ADV 7:19486–19491
Dwivedi A, Chitranshi S, Gupta A, Kumar A, Bhat JL (2019) Assessment of the petroleum oil degradation capacity of indigenous bacterial species isolated from petroleum oil-contaminated soil. Int J Environ Res 13:735–746
Gharibzahedi SMT, Razavi SH, Mousavi SM (2012) Developing an emulsion model system containing canthaxanthin biosynthesized by Dietzia natronolimnaea HS-1. Int J Biol Macromol 51:618–626
Gharibzahedi SMT, Razavi SH, Mousavi M (2013a) Potential applications and emerging trends of species of the genus Dietzia: a review. Ann Microbiol 64:421–429
Gharibzahedi SMT, Razavi SH, Mousavi SM (2013b) Microbial canthaxanthin: perspectives on biochemistry and biotechnological production. Eng Life Sci 13:408–417
Goswami G, Chakraborty S, Chaudhuri S, Dutta D (2012) Optimization of process parameters by response surface methodology and kinetic modeling for batch production of canthaxanthin by Dietzia maris NIT-D (accession number: HM151403). Bioprocess Biosyst Eng 35:1375–1388
Gurav R, Lyu H, Ma J, Tang J, Liu Q, Zhang H (2017) Degradation of n-alkanes and PAHs from the heavy crude oil using salt tolerant bacterial consortia and analysis of their catabolic genes. Environ Sci Pollut Res 24:11392–11403
Gutierrez JA, Teramoto M, Yamazoe A, Harayama S, Figueras A, Novoa, (2011) Alkane degrading properties of Dietzia sp.HB08, a key player in the prestige oil spill biodegradation. J Appl Microbiol 111:800–810
Hamme VJD, Ward OP (2001) Physical and metabolic interactions of Pseudomonas sp. Strain JA5-B45 and Rhodococcus sp. Strain F9–D79 during growth on crude oil and effect of a chemical surfactant on them. Appl Environ Microbiol 67:4874–4879
Hassanshahian M, Tebyanian H, Cappello S (2012) Isolation and characterization of two crude oil degrading yeast strains, Yarrowia lipolytica PG-20 and PG 32, from the Persian Gulf. Mar Pollut Bull 64(7):1386–1391
Ibrahim HMM (2016) Biodegradation of used oil by novel strains of Ochrobactrum anthropic HM-1 and Citrobacter freundii HM-2 isolated from oil-contaminated site. 3Biotech 6:226
Ivshina IB, Kuyukina MS, Krivoruchko AV, Elkin AA, Makarov SO, Cunningham CJ, Peshkur TA, Atlas RM, Philp JC (2015) Oil spill problems and sustainable response strategies through new technologies. Environ Sci Process Impacts 17:1201–1219
Karlapudi AP, Venkateswarulu TC, Tammineedi J, Kanumuri L, Ravuru BK, Dirisala VR, Kodali VP (2018) Role of biosurfactants in bioremediation of oil pollution—a review. Petroleum 4(3):241–249
Kavynifard A, Ebrahimipour G, Ghasempour A (2015) Optimization of crude oil degradation by Dietzia cinnamea KA1, capable of biosurfactant production. J Basic Microbiol 56(5):566–575
Kleindienst S, Paul JH, Joye SB (2015) Using dispersants after oil spills: impacts on the composition and activity of microbial communities. Nat Rev Microbiol 13:388–396
Kumar S, Upadhayay SK, Kumari B, Tiwari S, Singh SN, Singh PK (2011) In vitro degradation of fluoranthene by bacteria isolated from petroleum sludge. Bioresour Technol 102:3709–3715
Lea-Smith DJ, Biller SJ, Davey MP, Cotton CA, Sepulveda BMP, Turchyn AV (2015) Contribution of cyanobacterial alkane production to the ocean hydrocarbon cycle. Proc Natl Acad Sci USA 112:13591–13596
Lin M, Liu YH, Chen WW, Wang H, Hu XK (2014) Use of bacteria-immobilized cotton fibers to absorb and degrade crude oil. Int Biodeter Biodegr 88:8–12
Liu Q, Tang J, Gao K, Gurav R, Giesy JP (2017) Aerobic degradation of crude oil by microorganisms in soils from four geographic regions of China. Sci Rep 7:14856
Luo Q, Zhang JG, Shen XR, Sui X, Fan ZQ (2013) Characterization of a novel diesel oil-degrading Pseudomonas sp. strain F4. Fresenius Environ Bullet 22:689–697
Mezzomo N, Ferreira RS (2016) Carotenoids functionality, sources and processing by supercritical technology: a review. J Chem. https://doi.org/10.1155/2016/3164312
Nazina TN, Sokolova DS, Grigoryan AA, Shestakova NM, Mikhailova EM, Poltaraus AB, Tourova TP, Lysenko AM, Osipov GA, Belyaev SS (2005) Geobacillus jurassicus sp. nov., a new thermophilic bacterium isolated from a high-temperature petroleum reservoir, and the validation of the Geobacillus species. Syst Appl Microbiol 28:43–53
Pacwa-Plociniczak M, Plaza GA, Poliwoda A, Piotrowska-Segat Z (2014) Characterization of hydrocarbon degrading and biosurfactant producing Pseudomonas sp. P-1 strain as a potential tool for bioremediation of petroleum contaminated soil. Enviorn Sci Pollut Res Int 21(15):9385–9395
Parthipan P, Elumalai P, Ting YP, Rahman PKSM, Rajasekar A (2018) Characterization of hydrocarbon degrading bacteria isolated from Indian crude oil reservoir and their influence on biocorrosion of carbon steel API 5LX. Int Biodet Biodeg 129:67–80
Plaza GA, Ulfig K, Brigmon RL (2005) Surface active properties of bacterial strains isolated from petroleum hydrocarbon bioremediated soil. Pol J Microbiol 54(2):161–167
Rahbari-Sisakht M, Pouranfard A, Darvishi P, Ismail AF (2017) Biosurfactant production for enhancing the treatment of produced water and bioremediation of oily sludge under the conditions of Gachsaran oil field. J Chem Technol Biotechnol 92(5):1053–1064
Ravaghi M, Razavi SH, Mousavi SM, Sinico C, Fadda AM (2016) Stabilization of natural canthaxanthin produced by Dietzia natronolimnaea HS-1 by encapsulation of niosomes. LWT 73:498–504
Rezaeeyan Z, Safarpour A, Amoozegar MA, Babavalian H, Tebyanian H, Shakeri F (2017) High carotenoid production by a halotolerant bacterium, Kocuria sp. strain QWT-12 and anticancer activity of its carotenoid. EXCLI J 16:840–851
Rostami F, Razavi SH, Sepahi AA, Gharibzahedi SMT (2014) Canthaxanthin biosynthesis by Dietzia natronolimnaea HS-1: effects of inoculation and aeration rate. Braz J Microbiol 45(2):447–456
Sadighbayan K, Mazaheri Assadi M, Farazmand A, Monadi A, Aliasgharzad N, Mobaiyen H (2016) Biodegradation potential of soils in Tabriz Petroleum Refnery for removing solid polycyclic hydrocarbons Khosrow. Adv Biores 7:57–63
Sakthipriya N, Doble M, Sangwai JS (2015) Fast degradation and viscosity reduction of waxy crude oil and model waxy crude oil using Bacillus subtilis. J Pet Sci Eng 134:158–166
Sarma A, Sarma H (2010) Enhanced biodegradation of oil products by some microbial isolate supplemented with heavy metals. Int J Bot 6:411–448
Sathasivam R, Ki JS (2018) A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar Drugs 16:26
Sathishkumar M, Binupriya AR, Baik SH, Yun SE (2008) Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon contaminated areas. Clean Soil Air Water 36:92–96
Shen T, Pi Y, Bao M, Xu N, Li Y, Lu J (2015) Biodegradation of different petroleum hydrocarbons by free and immobilized microbial consortia. Environ Sci Proc Impact 17(12):2022–2033
Sieben VJ, Stickel AJ, Obiosa-Maife C, Rowbotham J, Memon A, Hamed N, Ratulowski J, Mostowfi F (2017) Optical measurement of saturates, aromatics, resins and asphaltenes in crude oil. Energy Fuel 31(4):3684–3697
Thapa B, Kc AK, Ghimire A (2012) A review on bioremediation of petroleum hydrocarbon contaminants in soil. Kathmandu Univ J Sci Eng Technol 8:164–170
Tremblay J, Yergeau E, Fortin N, Cobanli S, Elias M, King TL, Lee K, Greer CW (2017) Chemical dispersants enhance the activity of oil-and gas condensate-degrading marine bacteria. ISME J 11:2793–2808
Varjani SJ, Gnansounou E (2017) Microbial dynamics in petroleum oilfields and their relationship with physiological properties of petroleum oil reservoirs. Bioresour Technol 245:1258–1265
Vijayakumar S, Saravanan V (2015) Biosurfactants-types, sources and applications. Res J Microbiol 10(5):181–192
Wang XB, Chi CQ, Nie Y, Tang YQ, Tan Y, Wu G (2011) Degradation of petroleum hydrocarbons (C6–C40) and crude oil by novel Dietzia strain. Biores Technol 102:7755–7761
Wang Z, Xu Y, Wang HY, Zhao J, Gao DM, Li FM, Xing B (2012) Biodegradation of crude oil in contaminated soils by free and immobilized microorganisms. Pedosphere 22(5):717–725
Wang XB, Nie Y, Tang YQ, Wu G, Wu XL (2013) N-alkane chain length alters Dietzia sp. strain 12–45-1b biosurfactant production and cell surface activity. Appl Environ Microbiol 79:400–402
Weid I, Marques JM, Cunha CD, Lippi RK, Santos S, Rosado AS, Lins U, Seldin L (2007) Identification and biodegradation potential of a novel strain of Dietzia cinnamea isolated from a petroleum contaminated tropical soil. Syst Appl Microbiol 30:331–339
Whang LM, Liu PWG, Ma CC, Cheng SS (2009) Application of rhamnolipid and surfactin for enhanced diesel biodegradation—effects of pH and ammonium addition. J Hazard Mater 164:1045–1050
Xia W, Li J, Xia Y, Song Z, Zhou J (2012) Optimization of diesel oil biodegradation in seawater using statistical experimental methodology. Water Sci Technol 66:1301–1309
Xia W, Dong H, Zheng C, Cui Q, He P, Tang Y (2015) Hydrocarbon degradation by a newly isolated thermophilic Anoxybacillus sp. with bioemulsifer production and new alkB genes. RSC Adv 5:102367–102377
Xia M, Fu D, Chakraborty R, Singh RP, Terry N (2019) Enhanced crude oil depletion by constructed bacterial consortium comprising bioemulsifier producer and petroleum hydrocarbon degraders. Biores Technol 282:456–463
Xu X, Liu W, Tian S, Wang W, Qi Q, Jiang P, Gao X, Li F, Li H, Yu H (2018) Petroleum hydrocarbon degrading bacteria for the remediation of oil pollution under aerobic conditions: a perspective analysis. Front Microbiol 9:2885
Yang Y, Wang J, Liao J, Xie S, Huang Y (2015) Abundance and diversity of soil petroleum hydrocarbon-degrading microbial communities in oil exploring areas. Appl Microbiol Biotechnol 99:1935–1946
Yudono B, Said M, Sabaruddin Napoleon A, Fanani Z (2011) Kinetics approach of biodegradation of petroleum contaminated soil by using indigenous isolated bacteria. J Trop Soils 16:33–38
Zhang J, Zhang X, Liu J, Li R, Shen B (2012) Isolation of a thermophilic bacterium Geobacillus sp. SH-1 capable of degrading aliphatic hydrocarbons and naphthalene simultaneously and identification of its naphthalene degrading pathway. Bioresour Technol 124:83–89
Acknowledgements
Dr. C.K. Venil thanks the UGC for awarding the Dr. D.S. Kothari Postdoctoral Fellowship (BL/17-18/0479). In addition, the authors thank Anna University, Regional Campus—Coimbatore for providing the necessary facilities to carry out the project work.
Funding
This work was supported and funded by the University Grants Commission (UGC), New Delhi under Dr. D.S. Kothari Post-doctoral Fellowship (BL/17-18/0479) dated 25 September 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.

13205_2021_2807_MOESM1_ESM.jpg
Supplementary file1 (JPG 52 KB) (a) Ceric ammonium nitrate test for alcohol. (b) Fehling’s test for aldehyde. (c) Bicarbonate test for acid.
Rights and permissions
About this article
Cite this article
Venil, C.K., Malathi, M. & Devi, P.R. Characterization of Dietzia maris AURCCBT01 from oil-contaminated soil for biodegradation of crude oil. 3 Biotech 11, 291 (2021). https://doi.org/10.1007/s13205-021-02807-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02807-7