Abstract
Soil salinity constitutes a major abiotic stress that contributes to soil degradation and crop yield reduction. Using arbuscular mycorrhizal fungi (AMF) inoculation can help to alleviate these deleterious effects. Most researches on AMF application are dealing with ecological restoration, whereas little consideration has been given to agriculture and legume production. The comparison of the efficacy of two AMF inoculums, one native originating from Algerian semiarid saline soils and one commercial inoculum, was carried out regarding their effects on the growth and the mineral nutrition of several legumes species, Medicago sativa, Medicago falcata, Trifolium repens and Trifolium alexandrinum, cultivated in semiarid Algerian saline soil under greenhouse conditions. Our results showed that native mycorrhizal inoculum enhanced shoot biomasses by 20%, mycorrhizal rate by 30%, shoot phosphorus content by 25% and K+/Na+ ratio by 45% for studied plants when compared with commercial inoculum. The best efficiency of the native AMF inoculum is probably due to the complementarity between the AMF strains which composed the inoculum. Funneliformis geosporum was the most abundant species recorded at the end of the experience in all plant roots especially with native inoculum. Our findings pointed out the effectiveness of native AMF inoculum application to promote agricultural production in semiarid saline soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic saline stresses seriously threaten agricultural productivity and food security [1] due to the accumulation of salts in soil horizons [2] especially under arid and semiarid climates [1]. In saline soils, NaCl dissolves in water and rapidly reach toxic levels of sodium (Na+) and chloride (Cl−) ions [3]. About 20% of cultivated lands are directly concerned by salinity [4] and this percentage is constantly increasing. It was estimated that 50% of arable land may be devastated by increased salinization through the year of 2050 [1]. Under arid and semiarid climates, salinity problem leads to major constraints on agricultural production [3]. These include nutritional disorder, increase of oxidative enzymes (catalase, peroxidase, among others) and proline content in plant tissues [5]. All these conditions can strongly disrupt the natural plant functioning through altering the reactive oxygen species (ROS) metabolism and consequently decrease crop yields [1, 5]. Thus, the development of some strategies to maintain the productivity and the quality of crop plants growing in these soils is a crucial step for food availability [2] and to ensure the sustainability of agricultural production worldwide [5].
Arbuscular mycorrhizal fungi (AMF), present in all terrestrial ecosystems, regardless of soil type, vegetation, or growing conditions, are known to colonize the majority of plant roots, to enhance plant physiological status, mineral acquisition and consequently plant growth [6]. AMF have also the ability to increase plant tolerance against biotic abiotic stress [7]. Begum et al. [8] reported that AMF have the potential to stimulate plant growth under extreme abiotic stress via several physiological modifications [8, 9]. They interfere in several mechanisms, such as morphological adaptations, osmotic adjustment, optimization of water resources [10], decrease in antioxidant enzymes such as peroxidase, glutathione reductase, superoxide dismutase, or catalase, which promote the elimination of ROS formed by abiotic stress conditions [8, 10]. The AMF salinity tolerant strains counteract osmotic stress by synthesizing organic (proline, glycine betaine) and inorganic (potassium cations) osmolytes, allowing them to maintain their cell turgor and metabolism [5] and consequently preserve balance of host plant metabolism [9]. Thus, AMF are primary biotic soil components which, when missing or impoverished, can lead to a disturbance of ecosystem functioning [6].
Investigation on AMF as new eco-friendly agent to restore degraded soils is increasing [7]. The process is based on the direct introduction of AMF propagules by plant inoculation into target soil [11]. However, the exploitation of these fungi in applied research programs requires the knowledge of how AMF adapt and react to the target ecosystem and soil [7]. Ortas et al. [1], reported that indigenous AMF may be a promising biological technology to improve plant performance and to alleviate salt stress damages. For that, one of the main challenges in AMF research is to understand how indigenous AMF soil diversity is related to their efficiency in promoting plant growth and tolerance to abiotic stresses [12].
In Mediterranean traditional agroecosystem, legume cultures contribute to socioeconomic development of local population [13]. White clover (Trifolum repens) and Berseem (Trifolium alexandrinum) are amongst the sixteen most cultivated Trifolium species in different agro-ecological Mediterranean regions [14]. They are used as plant fodder, green manure and valuable herbs in folk medicine [13]. In the same way, Alfalfa (Medicago sativa) and wild Lucerne (Medicago falcata) are perennial cosmopolitan species native to the Mediterranean areas [14, 15]. In Algerian arid and semiarid regions, these legumes are important in farming system while vulnerable to different biotic and abiotic stresses, such as soil salinity [13]. Hadj-Amor et al. [5] reported that legume species are sensitive to salinity stress and cannot withstand soil salinity > 1.6 dS m−1. Therefore, soil inoculation with AMF selected for their efficiency on plant production is a promising approach especially for legume crops, an approach that has been little used to date in semi-arid Algerian agricultural ecosystems.
A recent study underlined the importance of AMF symbiosis efficiency under saline stress environments when a significant improvement in plant biomass and in salt tolerance was recorded with application of indigenous AMF inoculum on native shrub (Tamarix articulata) [16]. Other studies also highlighted the importance of using indigenous AMF inoculum to improve nutrition and adaptation to saline constraints of different host plant species [7, 12]. However, a question remains: are AMF isolated from saline soils able to alleviate toxicity impact of soil saline elements and to improve mineral nutrition of a non-native agricultural legume plants while cultivated in saline soil? Therefore, the current study aims at (i) comparing the efficiency of two AMF inoculums, commercial and native, on the growth of four legumes (alfalfa, lucerne, clover and berseem) cultivated in semiarid Algerian saline soil under greenhouse conditions and (ii) evaluating their salt stress adaptability.
Materials and Methods
AMF Inoculums
Two AMF inoculums were used in this experiment: commercial one (Symbivit, Inoculum plus, France) composed of six AMF species (Rhizophagus irregularis BEG140, Funneliformis mosseae BEG95, Claroideoglomus etunicatum BEG92, Claroideoglomus claroideum BEG96, Rhizoglomus microaggregatum BEG56, Funneliformis geosporum BEG199) and native one formed with eight AMF species, four species belonging to Funneliformis genus (F. geosporum, F. mosseae, F. coronatum, F. caledonium), one Oehlia species (O. diaphana), one Rhizoglomus species (R. fasiculatus) and two species belonging to the genera Septoglomus (S. constrictum) and Gigaspora (Gi. gigantea). The origin and taxonomic description and affiliation of each isolate have been described in details in Bencherif et al. [16]. Native AMF mixture was isolated from rhizosphere arid Algerian steppic saline soil (Boughzoul, 22° 16′ S, 166° 38′E). This native inoculum was produced in Unit of environmental chemistry and interaction between living organisms (UCEIV at ULCO, Calais, France) and was grown using container technique [17]; (https://invam.wvu.edu/methods/). Native inoculum production consisted of natural saline soil of mixture-species cultures with Allium ampeloprasum containing spores and root fragments.
Host Plants
The tested host plants were two Medicago species, alfalfa and lucerne (M. sativa. var. sativa and M. falcata var. falcata) and two species of Trifolium genus, clover and berseem (T. alexandrinum var Tigri and T. repens var hollandicum). The seeds of Medicago species were provided by University of Djelfa (Algeria) and the seeds of Trifolium species were provided by UCEIV (ULCO, France). M. falcata var. falacata are the only legume species cultivated in the region. Seeds were surface-disinfected in a 1.25% solution of sodium hypochlorite 12% for 5 min and then rinsed with distilled water in sterilized Petri dishes. The seeds were subsequently sown in sterilized vermiculite (autoclaved for 60 min; at 120 °C) before transfer to experimental pots.
Pot Culture Substrate
The soil used in greenhouse experiment is a steppic saline soils obtained from ploughed layer (0–40 cm) with 5 dS m−1 from Algerian semiarid region (34°40′00″N, 3°15′00″ E), with the following characteristics: sand 50%, silt 34% and 16% of clay with silty sandy-clayey texture; pHwater 7.5; total C 7.8 mg g−1; total N 0.3 mg g−1; available P 0.11 mg g−1; total Ca 12.4% and active Ca 6.2%; extractable Ni 0.3 mg g−1 and organic matter 1.4%.
Plant Inoculation and Growth Conditions
Seedlings were inoculated at their transfer to the pots (15 seedlings/ pot of 300 mL). Inoculum consisted of 50 g of mixture of rhizosphere soil containing homogenized spores, mycelium and colonized small root fragments. Fungal species mixes formulations were chosen according to the following considerations: (i) the technical difficulty that would have been involved in legume plantation in saline soils; (ii) a previous experiment on shrubs in saline soils that showed the better plant performance with native AMF inoculum [16].
Ten replicates for each AMF inoculum and ten non-mycorrhizal controls were set up, for a total of 120 pots arranged in fully randomized block design. Experimental design is schematized in supplementary material (Figs. S1). The pots were filled using natural saline non-sterilized soil. The mycorrhizal inoculums (50 g) were spread as a layer on the surface of the soil in the pots and covered with a thin layer of the soil. The cultures were kept in a greenhouse (temperature, 21–24 °C; relative humidity, 70%, with 8 h dark/16 h light) for 8 months and irrigated manually with tape water every 2 days. The experiment was carried out from September to April 2019.
Harvesting Procedure
For each plant, the height of the main stem was weekly measured, from the soil level to the tip of the stem. Plants were harvested after 8 months to secure sufficient mycorrhizal colonization [12]. Roots were removed from the soil by gentle washing, and shoots and roots separated. Approximately 500 mg per plant of fresh roots was retained for quantification of mycorrhizal colonization and approximately 50 mg of fresh roots of each plant were pooled per treatment, frozen in liquid nitrogen, and stored at − 80 °C for molecular analyses. The rest of plant tissues were weighed dried at 70 °C for 72 h and re-weighed.
Determination of AMF Root Colonization
A subsample of fresh roots was stained using trypan blue according to the method of Phillips and Hayman [18] modified by Dalpé and Séguin [19]. Tissue acidification was performed using 20% hydrochloric acid instead of 1%, and trypan blue concentration was 0.1% instead of 0.05%. AMF colonization of 45 root fragments (approximately 1-cm length for each fragment) per pot were calculated using magnified grid line intersect method according to McGonigle et al. [20], under a compound microscope (X100). The total mycorrhizal rate (including arbuscules, vesicles and hyphae) was estimated.
AMF Spore Extractions and Quantification
A subsample of substrate from each pot was air-dried to quantify spore abundance for AMF species. Spores were extracted using wet sieving method [21] from each pot culture, 10 g of dried substrate was wet sieved, and then the harvested material was suspended in 20 mL of water. The aliquots were immediately transferred to Petri dishes for spore counting and morphological identification under a stereomicroscope according to Blaskowski [22] and INVAM International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi (http://invam.wvu.edu/). Spore numbers were estimated per gram of soil for each pot.
Molecular Identification of AMF
Molecular approach was used to evaluate the presence or absence of AMF species in roots. Specific primers of each AMF species were designed and used to detect the presence or absence of each AMF isolate in roots. Genomic DNA extraction from root samples (100 mg), amplification by polymerase chain reaction (PCR) approach and visualization of the target region of rRNA were performed as described by Crossay et al. [12].
Spore extracts were produced for PCR by crushing spores with a pipette tip in 2 ml of 0.25 M NaOH, incubating them in a boiling water bath for 1 min, adding 1 ml of 0.5 M Tris HCl (pH 8.0) and 2 ml of 0.25 M HCl, boiling them again for 2 min. The samples were stored at − 20 °C until further analysis. The first round of PCR was used to amplify a fragment of the ITS using the universal primers ITS1 (TCCGTAGCTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC) [23, 24]. Extracts were used for PCR using each nucleotide at 50 µM, primers ITS1 and ITS4 at 0.2 µM. 9 µl of reaction mix was pipetted into each PCR tube. Specific primers (Table S1) were combined in two sets in the second amplification step to minimize the number of PCR reactions. First set comprised the reverse specific primers (GLOM5.8R, GIGA5.8R) in combination with ITS1F and in the second set the forward specific primers (ARCH1311, LETC1670) were combined with ITS4. 1 µl of T-DNA was added to each specific tube with 0.05 U of Tac polymerase (Appligene, Illkirch, France) per ml was used. The concentration of MgCl2 was adjusted to 1.5 mM. Cycling conditions after a host start at 60 °C, an initial denaturation of 3 min at 95 °C was followed by five cycles of 30 s at 95 °C, 30 s at 52 °C, and 1.5 min at 72 °C. Thereafter, 25 to 30 cycles with annealing at 51 °C were performed [24]. DNA fragments from successful reactions were concentrated and purified by precipitation with ice-cold ethanol in the presence of 0.3 M sodium acetate and 60 mg of glycogen per ml. Aliquots of the reaction mixture were examined by electrophoresis on 1.5% agarose gels + 0.5 × TBE buffer and 3 µl Ethidium bromide (ETBr) at running condition 60 V for 60 min and photographing under UV illumination. As molecular weight marker, a 100 bp ladder was used. Sequence similarities were compared with those in the GenBank databases using protein BLAST query. Only sequences belonging to Glomeromycota were selected for the subsequent analyses and the others were discarded.
Physiological Plant Analysis
Physiological and biochemical parameters were chosen to estimate the AMF inoculation efficiency on studied legume plants development. Mineral analyses of plants were performed on dry tissues. Total protein was evaluated using Bradford [25] method based on the observation that the absorbance maximum for an acidic solution of Coomassie Brilliant Blue G-250 shifts from 465 to 595 nm when binding to protein occurs. Proline amount in shoot and root was assessed using ninhydrine method of Sadasivam and Manickam [26], the absorbance was read at 520 nm using a spectrophotometer and the concentration of proline was determined using the standard curve and expressed as mg g−1. Shoot phosphorus content was analyzed by Tausky and Shorr [27] spectro-colorimetric method. Potassium shoot and root concentration was evaluated by flame photometry method [28]. Nitrogen content was determined according to Kjeldahl method. Mineral analyses (Na+, Cl−, Ca2+ and K+) were performed by mass spectrometry as reported by Pauwels et al. [29]. Chlorophyll was evaluated according to Holden [30] spectrometric method and the different chlorophyll values (Chla, Chlb and Chla + Chlb) were calculated according to Arnon [31] formula: (Chl.a = 12.7 (DO660) − 2.69 (DO750); Chl.b = 22.9 (DO660) − 4.86 (DO750); Chl.a + Chl.b = 8.02 (DO660) + 20.20 (DO750). Catalase activity was assayed by estimating the initial rate of disappearance of hydrogen peroxide using method of Aebi [32]. For that, 0.5 g of fresh plant samples were grinded and homogenized with 5.0 ml extraction buffer containing 50 mM phosphate buffered potassium pH 7.0, 0.4% polyvinylpoly pyrrolidone in a mortar and pestle on the ice. The homogenate was centrifuged at 12,000×g for 5 min. All operations were carried out at 4 °C. Peroxidase activities was measured for 3 min at 470 nm using the method described by Eterson et al. [33]. In brief, the reaction mixture was composed of K2HPO4/KH2PO4 (100 mM) buffer K2HPO4/KH2PO4 (pH 6.5), guaïacol (40 mM), H2O2 (10 mM) and enzyme extract (0.1 mL).
Statistical Analysis
All statistical analyses were performed using XLStat software version 2020.5. Plant height data (Table S2) were analyzed using the parametric tests with 10 levels and repeated measures ANOVA followed by Tukey’s HSD post hoc test. Effect of mycorrhizal variables (root colonization and sporulation) with native and commercial mixes on plants growth parameters was evaluated according to Pearson coefficient correlation test (Table 2). According to the three-factorial experimental design, a three-way analysis of variance was performed after checking the normality and homoscedasticity of the data sets. The mains sources of variation were the four legume species (4 levels), treatment of AMF inoculation (3 levels), soil salinity (one level) and the multiple interactions above the mains factors (Table 3). Detended Correspondance Analysis (DCA) using DECORANA program of PC-Ord 5.0 [34] with means of AMF data samples was carried out to evaluate AMF alfa biodiversity and to describe the relationship between the different AMF inoculation treatments and AMF species communities as well as the relationship between AMF species and the measured variables based on two factorial axes. The ecological attributes were calculated in the following manner: species richness (S), Shannon’s diversity index (H′) and equitability indices of Piélou (E). AMF spore as a discrete variable was analyzed by a model following a Poisson distribution (Skewness) (Table S4).
Results
Plant Growth, Mycorrhizal Colonization and Plant Responsiveness
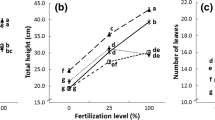
When comparing all treatments to non-inoculated control plants, the biomass of alfalfa, lucerne, clover and berseem were significantly enhanced at the end of the experiment while inoculated with the mixture of native AMF (P < 0.05) (Fig. 1). These increases were about 33, 38, 25 and 43% on shoot dry weights, respectively. No significant differences were recorded between shoot dry weights with commercial inoculum and control except for clover, where shoot biomass was increased by 11%. Root dry weights were significantly increased (16% for berseem to 26% for alfalfa) when inoculated with native AMF compared to control. Whereas, with commercial AMF inoculum plant biomass decreases (10% for berseem to 20% for alfalfa) as compared to control. The general AMF effect on plant height was significant 120 days after inoculation (P < 0.05). All native AMF-inoculated plants were significantly higher than controls from 180 days to the end of the experiment (Table S2, Fig. S2).
Root and shoot biomasses (mg/pot) of inoculated with commercial and native inoculum and non-inoculated plants: a alfalfa, b lucerne, c clover and d berseem cultivated on saline soil. Error bars represent the standard error of 10 replicates. Means for shoot and root followed by the same letter in each panel do not differ significantly (P ≤ 0.05)
Mycorrhizal rate and percentage of arbuscules are provided in Fig. 2. Full data are accessible in Table S2. In non-inoculated soils, mycorrhizal colonization was between 3% for berseem to 8% for alfalfa. Mycorrhizal colonization was superior to 50% for all plants inoculated with native inoculum. Lower colonization levels were found with the commercial inoculum (30–35%) (Fig. 2). Arbuscules were clearly visible and well-developed in all AMF treatments with arbuscule colonization values higher than 40% for native AMF inoculum (Table S3). Commercial AMF inoculation provides lower arbuscular values for the four studied plants than control. It was between 9% for alfalfa and 11% for berseem and about 10% for the two remaining plant species. Positive correlation was revealed between mycorrhizal rates and shoot and root biomasses (Table 2).
Total mycorrhizal rates of inoculated with commercial and native inoculum and non-inoculated: a alfalfa, b lucerne, c clover and d berseem cultivated on saline soil. Error bars represent the standard error of 10 replicates. Means followed by the same letter in each panel do not differ significantly (P ≤ 0.05)
AMF Spore Production and Molecular Identification
Isolation and quantification of the AMF spores associated to the rhizosphere soil of each plant species indicates more abundance with native inoculum (Table S4). After quantification 43, 46, 65 and 54 spores g−1 of dry soil, were recorded for alfalfa, lucerne, clover and berseem, respectively.
Specific primers of each AMF species indicated presence of all native AMF species in roots of the four studied legume species inoculated by native inoculum with different frequencies. F. geosporum was the dominant species (Fig. 3). Only three AMF species were detected from the commercial inoculum treatment: F. geosporum, F. mosseae and G. etunicatum in roots of clover, lucerne and alfalfa, respectively. Interestingly, the two AMF species F. geosporum and F. mosseae were recorded in roots of non-inoculated alfalfa and clover (Table S4).
Plant Mineral Nutrition Analyses
The major elements needed for plant nutrition (i.e., P, K+, N, Na+, Ca2+) were analyzed in shoots and roots of inoculated and control plants (Fig. 4).
Phosphorus, potassium and nitrogen shoot and root contents (%) of inoculated with commercial and native inoculum and non-inoculated: a alfalfa, b lucerne, c clover and d berseem cultivated on saline soil after 8 months of culture. Error bars represent the standard error of 10 replicates. Means followed by the same letter in each panel do not differ significantly (P ≤ 0.05). A. Phosphorus content; B. potassium content; C. Nitrogen content
Mineral Elements Analysis in Aerial Parts
Phosphorus concentration (Fig. 4a) was enhanced in shoot tissues exclusively by native AMF inoculum (by 46% for alfalfa to 64% for clover). The phosphorus concentration of lucerne and berseem was enhanced by the both inoculation treatments (Fig. 4a). Potassium concentration in shoots (Fig. 4b) was significantly enhanced with native AMF inoculum by about 20% for the four studied legume plants. With commercial AMF inoculum Potassium concentration was not significantly different when compared with control except for clover when it was enhanced by 15% than control. Nitrogen concentration in shoot tissues (Fig. 4c) was generally increased by AMF inoculation (11% with commercial inoculum to 17% with native inoculum). For berseem, no significant difference was recorded between the two inoculation treatment and control on shoot nitrogen content. Pearson coefficient revealed positive correlation between phosphorus, potassium and nitrogen concentration in aerial parts of the four studied plants and mycorrhizal parameters under both inoculation treatments (Table 2).
The content of K+, Ca2+ was higher in shoot of AMF inoculated plants with a decrease of Na+ and Cl− content (Table 1). It was observed that under salinity significant increase of the K+/Na+ shoot ratio (50, 43, 41 and 30%) was induced by native AMF inoculum respectively for alfalfa, clover, berseem and lucerne. Ca+2/Na+ ratio was enhanced by native AMF inoculum for all studied shoot (by 22, 18, 15 and 12%) of berseem, lucerne, clover and alfalfa tissues (Table 1). For AMF commercial inoculum Ca2+/Na+ ratio was not significantly different when compared with controls except for clover and alfalfa (14 and 20%, respectively).
Mineral Elements in Roots Parts
A significant enhancement of phosphorus concentration in root tissues with native inoculum was observed as compared with control (21, 56 and 67%) for clover, alfalfa and Lucerne, respectively (Fig. 4a). For berseem phosphorus concentration in roots tissues was not significantly different when compared with control. In addition, no significant difference on phosphorus root concentration was recorded with commercial inoculum except for Lucerne (40%) comparatively to control.
Potassium concentration was increased (21 to 56%) for all studied legumes species with native AMF inoculum, while no significant difference was recorded with commercial inoculum as compared to control (Fig. 4b). Nitrogen root content was significantly stimulated by native AMF inoculum than commercial one except for clover (Fig. 4c).
In root tissues K+/Na+ ratio was enhanced in the presence of native AMF inoculum (by 34, 26, 23 and 19%) respectively for berseem, alfalfa, lucerne and clover (Table 1). Similar to shoot content, K+/Na+ root ratio was enhanced for clover and alfalfa by 15% in the presence of commercial inoculum. Ca2+/Na+ ratio was enhanced by native AMF inoculum for all studied root (between 13 and 19%) legumes plants tissues (Table 1). AMF commercial inoculum showed no significant effect on Ca2+/Na+ ration in plants root tissues. Native inoculation treatment showed positive correlation between shoot mineral elements and mycorrhizal parameters, while non-significant correlation was observed under commercial inoculation treatment (Table 2).
Physiological and Biochemical Plant Responses
Physiological and Biochemical Shoot Responses
AMF treatments differently affected plant water content in shoots tissues (Figs. S3). It was significantly higher in both AMF inoculated plants shoot tissues compared to controls by about 32% more with native AMF mix and by 26% with commercial AMF inoculum.
Plant chlorophyll content was stimulated by native AMF inoculum (20%) and by commercial AMF inoculum (14%) comparatively to control (Table 1). This result is consolidating by positive correlation between plant chlorophyll and total mycorrhizal parameters under native inoculation treatment (Table 2).
Salt stress increased significantly proline content in shoots tissues of the four studied control plants (Table1), while AMF inoculation decreased shoot proline content with the two AMF inoculums. However, proline amount in shoot tissues was reduced by native AMF inoculum (12, 26, 36 and 80%) than control and by 38, 46, 72 and 18% than commercial inoculum for alfala, lucerne, berseem and clover. Proline decrease in shoot tissues was positively correlated with mycorrhizal parameters under both inoculation treatments (Table 2).
Shoot protein content was significantly increased by native AMF inoculum than control and commercial inoculum (Figs. S4). It was about 15% for alfalfa and 13% for the remaining studied legume species as compared to control and about 17% for alfalfa, 14% for berseem and 11% for both lucerne and clover comparatively to commercial AMF treatment (Figs S4). Commercial AMF mix enhanced shoot protein content (13%) only for lucerne. Total mycorrhizal rate under native treatment recorded positive correlation with protein content, while under commercial treatment no significant correlation was recorded (Table 2).
AMF inoculation treatments influenced significantly plant catalase and peroxidase enzyme activities (Table 1). Significant reduction of catalase activity was observed in shoot tissues with native AMF inoculation (42, 30, 27, and 17%) respectively for alfalfa, clover, berseem and lucerne compared to control. Interestingly reduction of catalase activity was highest with native AMF inoculum than with commercial AMF inoculum. It was between (22 and 7%) for alfalfa and lucerne shoot tissues. Peroxidase activity was decreased in plant shoot tissues with native AMF inoculum (8%) and with commercial AMF inoculum (2%) as compared to controls. Mycorhizal parameters recorded a positive correlation with enzymatic activities of the four studied plants under both inoculation treatments (Table 2).
Physiological and Biochemical Root Responses
In root tissues water content was increased by native AMF inoculation (about 20%) and by commercial AMF inoculation (14%) in the four studied plants (Figs. S3).
Proline content was increased by salt stress in roots tissues of the four studied control plants (Table 1), and reduced by AMF inoculation treatments. With native AMF inoculum proline root content decreased (by 61, 37, 23 and 21%) as compared to control, and (by 36, 20, 18 and 15%) as compared to commercial AMF inoculum for alfalfa, Lucerne, clover and berseem. That is consolidating by positive correlation between mycorrhizal parameters and root proline content under native inoculation treatment (Table 2).
Root protein content was significantly increased by native AMF inoculum than control (22%) for lucerne and (15%) for the remaining studied roots tissues (Figs. S4). Commercial AMF inoculum enhanced root protein content (11 and 14%) for lucerne and clover.
In root tissues, a catalase activity was reduced with native AMF inoculum (88, 77, 71 and 50%) and with commercial AMF inoculum (60, 44, 34 and 33%) as compared to control for Alfalfa, clover, berseem and lucerne, respectively (Table 1). For root tissues peroxidase activity was significantly reduced by native AMF inoculum more than by commercial AMF inoculum by 40% for the four studied legumes species (Table 1). Pearson coefficient showed significant positive correlations between enzymatic activities in root tissues and mycorrhizal parameters (Table 2).
Global Statistical Analyses
Pearson correlation coefficient showed significant total correlations between analyzed variables (Table 2). Mycorrhizal parameters (spores production, total mycorrhizal rate and percentage of arbuscules) recorded a significant positive correlation with root biomass; shoot biomass, phosphorus and potassium concentration in shoots and with K+/Na+ ratio in shoots. Total mycorrhizal rate was negatively correlated with the salt concentrations in shoots.
α-diversity was determined using the richness (S) and the evenness (E) of the AMF species identified after host plant growth. Analyze of variance (ANOVA) reveals that AMF richness was affected by soil salinity (Table 3). Significant impact of plant species on AMF richness was also recorded (Table 3). When considering the evenness index, a very different trend was observed (Table 3). A decrease on AMF evenness and loss diversity were induced by soil salinity. The impact of native and commercial AMF inoculation on evenness is apparent in increased AMF diversity. A significant effect of the combined impact of plant species and AMF inoculation was evidenced. Alfalfa and berseem appeared to host the most diverse AMF community, whereas clover and lucerne present the lowest value for this index (Fig. 3).
Two clusters were clearly differentiated by DCA ordination (Fig. 5). Cluster 1 was composed by commercial AMF inoculum. This cluster was characterized by a lower biomass, significant nitrogen and phosphorus contents in roots and shoots, higher Na+ and Cl− concentrations in shoots, when compared with the second cluster. Cluster 2 was represented by native AMF inoculation. This cluster was characterized by a higher biomass, a higher mycorrhizal rate, a greater arbuscular abundance in the root system, a higher Ca2+/Na+ and K+/Na+ ratios in roots and shoots, and higher phosphorus, potassium and nitrogen concentrations in root and shoot tissues. The results also indicated that the different AMF species are dispersed in the second cluster and showed different abilities.
DCA ordination between AMF species distribution according to the different AMF mixes (commercial and native) and control with studied parameter for the four studied plants on saline soil. Data were based on means of 10 replicates. Red triangles indicate the identified AMF species. Blue dots represent the studied parameters. The proximity of red triangle to the parameters means that the corresponding AMF species influenced the parameter. Red triangles close to the center indicate that the AMF species are common to all the studied parameters. NI non-inoculated, CI commercial inoculum, NM native mix, POD peroxydase, CAT catalase. N nitrogen, P phosphorus, K potassium. Ovals represent two groups: first for native inoculum effect and the second for commercial inoculum
Discussion
Previous work of Bencherif et al. [16] highlighted the efficiency of native inoculum on Tamarix articulata development in the revegetation program under saline soil conditions. In the present study the mixture of native AMF inoculum showed more ability to colonize root systems of the four studied legume species than commercial inoculum. Indeed, highest root colonization rates were clearly recorded with native AMF inoculum, with a highly significant positive correlation between plant biomasses and mycorrhizal rates. This finding is in accordance with several previous studies carried out with many plant species (M. sativa, Chrysanthemum morifolium, Metrosideros laurifolia, Sorghum) grown under different abiotic stresses [11, 12, 35, 36]. All these studies explained that when plants faced abiotic stress, AMF inoculant, belonging to native species, were more efficient than exotic AMF ones. The taxonomic differentiation of the AMF spores contained in the native inoculation treatment indicated that these AMF species completed their life cycle under saline soil condition. These results indicated the adaptation of AMF strains that make up the native inoculum to saline soil [12]. The results also show that after the two inoculation treatments, only a few AMF species colonized plants. This may be due to some competition between inoculated species during root colonization process or to an incompatibility between these species and the legume plants tested, as reported by previous studies [12, 37, 38]. Overall, the commercial inoculant tested shows relatively low efficacy both in terms of plant growth and root colonization rates. Only two AMF species (F. mosseae and F. geosporum) were detected in the four root legume species at the end of the experiment when the commercial inoculum was used. This result is in agreement with the study of Neji Mahmoudi et al. [35] on alfalfa species. The predominance of F. geosporum and its presence in non-inoculated alfalfa cultivated in Algerian semiarid saline soils suggest its tolerance to harsh conditions. It should be noted that F. geosporum seems to develop stress resistance strategies (r-strategies) [39]. It was reported that F. geosporum is able to develop extraradical mycelium ‘common mycelial networks’, which play key roles in ecosystems [40, 41]. These networks may also result from the interconnection of hyphae via anastomosis mechanism known as ‘hyphae healing mechanism’, a major process that allows the fungus to maintain its integrity following physical injury and their survival to environmental stresses [40, 42]. In addition, He et al. [43] reported that F. geosporum differentiates spores with thick walls and dark pigmentation, which could give them a better resistance to abiotic stresses. Moreover, their sporulation rate seems to be higher than in other AMF species. Moreover, study of Okon et al. [44] works on Telfairia occidentalis grown under high salinity conditions shows that F. geosporum develops a strong mycelial network in the rhizosphere of the plants and thus increases their water and nutrient supply. These behavioral characteristics give F. geosporum added value as a bioinoculant for cultivation in saline and semi-arid environments.
The high rate of arbuscules in the root tissue was positively correlated with legume species growth, which reflects the functional status of the symbiosis [1, 9]. This can be attributed to hyphae healing mechanism which requires three steps to complete fusion with cytoplasmic/protoplasmic under physical stress conditions: (a) Septum formation to stop cytoplasmic flow, (b) initiation, elongation and contact to other hyphae (c) (d) hyphal tips fusion and cytoplasmic/protoplasmic flow re-establishment [40]. This is part of r-strategies developed by native AMF species, which highlights that combination of an appropriate AMF species is crucial to stimulate plant growth, as reported by Yang et al. [45], in their meta-analysis review.
Given the higher mycorrhizal rate recorded with native inoculum, shoot and root phosphorus concentration are significantly higher in legume plants with native AMF treatment, except for berseem whose shoot phosphorus concentration has not been significantly improved by any of the inoculation treatments. Thonar et al. [46] found that AMF inoculation improved phosphorus concentration in tissues of M. truncatula and Tuo et al. [47] recorded the same enhancement for clover. However, our results are not in accordance with those of Shokri and Maadi [48] for berseem species, where they recorded a significant increase on shoot phosphorus content with AMF inoculation. The observed response of berseem to inoculation suggests that despite the positive correlation recorded between percentage of arbuscules and shoot and root phosphorus concentration, AMF did not have enough time to be efficient in transferring nutrients to the host plant. The results obtained showed clearly the effectiveness of both inoculation treatment in the transfer of potassium to the aerial part of alfalfa, indicating the fragility of this species in the saline soil as described by Neji Mahmoudi et al. [35]. In fact, the photosynthetic capacity is enhanced by adequate potassium concentration in plant tissues, which also reduce the oxidative salt stress [46]. In addition, our results suggested positive efficiency of the native AMF inoculum on nitrogen shoot concentration, which was in agreement with the study of Namdari et al. [14] on alfalfa. The enhancements of mineral elements (N, P, K) by native AMF species recorded in present study suggested that a good mixture of AMF improved photosynthesis, which automatically enriched nutritional balance [10, 11, 47]. The positive correlation between percentage of arbuscules and plant mineral nutrients, indicates efficiency of native AMF inoculum by the relative allocation of nitrogen to photosynthesis and it’s dilution in a larger volume of tissues due to the high increase of plant biomasses. Indeed, Tuo et al. [47] noted that the mineral improvement increased stomata conductance, leaf water potential, and photosynthetic efficiency, thus, enhancing abiotic stress tolerance of host plants [8, 49], which are in total agreement with present results.
In the same context, the native AMF inoculum synergistically enhance K+/Na+ ratio and decrease proline concentration in shoots tissues as abiotic stress measure of adaptation. That gives a better osmoregulation capacity [48], which is beneficial for plant health and growth performance under salinity stress [1, 2, 9, 49]. Legume species have a poor ability to exclude salt, if Na+ accumulates in amounts exceeding the ability of the cells to compartmentalize this ion in the vacuole, enzyme activity may be inhibited or cells may be dehydrated [9, 49]. In turn, Shokri and Maddi [48] explained that AMF inoculation maintain high concentration of K+ and low concentration of Na+ in the cytosol by regulating the expression and activity of Na+ and K+ transporters and of H+ pumps that generate the driving force for transport. In addition, Santander et al. [49] have shown with lettuce that the a good combination AMF/plant species gives a higher biomass production, increased synthesis of proline, increased N uptake, and noticeable changes in ionic relations, particularly reduced accumulation of Na+, under stress conditions. Therefore, prevention of Na+ accumulation in the plant and enhancement of K+ concentrations in shoots and roots observed in this work highlighted the powerful effect of AMF native inoculation as antioxidant defenses [50].
The multivariate analyses clearly highlighted the efficiency of the native AMF saline strains to improve nutritional balance and enhanced alleviation of salt stress effect more than introduced ones under Algerian semiarid saline conditions [51]. The DCA analysis for AMF species distribution through inoculation treatments and growth studied parameters indicated a significant effect of some native AMF species, such as F. geosporum, F. mosseae and F. coronatum on different plant development parameters confirming the significant effectiveness of native AMF mixture on legume species development in saline abiotic stress conditions [36, 50]
Conclusion
A synergy between native AMF strains and legumes species (alfalfa, clover, wild alfalfa and berseem) poorly adapted to the semi-arid environmental conditions of Algerian saline soils has been demonstrated. In fact, native inoculums were able to significantly reduce salt accumulation in all four legumes tested. In addition, native AMF strains also show similar beneficial effects for both non-native (Trifolium species) and native legumes (Medicago species). These results highlight the effectiveness of native arbuscular symbioses for the development and adaptation of legumes in saline soil under semi-arid conditions. F. geosporum strains were found to be the most efficient biofertilizing agent for legume production in saline soil in semi-arid regions of Algeria. Thus, our results open new perspectives for the use of indigenous AMF in phytorestoration.
References
Ortas I, Rafique M, Çekiç FÖ (2021) Do mycorrhizal fungi enable plants to cope with abiotic stresses by overcoming the detrimental effects of salinity and improving drought tolerance? In: Shrivastava N, Mahajan S, Varma A (eds) Symbiotic soil microorganisms. Soil biology 60. Springer, New York
Garg N, Pandey R (2015) Effectiveness of native and exotic arbuscular mycorrhizal fungi on nutrient uptake and ion homeostasis in salt-stressed Cajanus cajan L. (Millsp.) genotypes. Mycorrhiza 25:165–180
Djanaguiraman M, Prasad PVV (2013) Effects of salinity on ions transport, water relations and oxidative damage. In: Ahmad P, Azooz MM, Prasad MNV (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 89–113
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics. https://doi.org/10.1155/2014/701596
Hadj-Amor Z, Araya T, Kim DG, Bouri S, Lee J, Ghiloufi W, Yang Y, Kang H, Jharia MK, Banerjee A, Lal R (2022) Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: a review. Sci Total Environ 843:156946. https://doi.org/10.1016/j.scitotenv.2022.156946
Smith SE, Read DJ (2008) Mineral nutrition, toxic element accumulation and water relations of arbuscular mycorrhizal plants. In: Smith SE, Read DJ (eds) Mycorrhizal symbiosis, 3rd edn. Academic Press, London
Begum N, Ahangeer MA, Su Y, Lei Y, Mustafa NSA, Ahmad P, Zhang L (2019) Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 8:579. https://doi.org/10.3390/plants8120579
Tereucán G, Javiera AR, Oyarzún NP, Santander C, Winterhalter P, Ferreira PAA, Cornejo P (2021) Shifts in biochemical and physiological responses by the inoculation of arbuscular mycorrhizal fungi in Triticum aestivum growing under drought conditions. Sci Food Agric 102(5):1927–1938. https://doi.org/10.1002/jsfa.11530
Xiao X, Chen J, Liao X, Yan Q, Liang G, Liu J, Wang D, Guan R (2022) Different arbuscular mycorrhizal fungi established by two inoculation methods improve growth and drought resistance of cinnamomum migao seedlings differently. Biology 11:220. https://doi.org/10.3390/biology11020220
Meddich A, Oufdou K, Boutasknit A (2020) Use of organic and biological fertilizers as strategies to improve crop biomass, yields and physicochemical parameters of soil. In: Meena RS (ed) Nutrient dynamics for sustainable crop production. Springer, New York
Crossay T, Cavaloc Y, Majorel C, Redecker D, Medevielle V, Amir H (2020) Combinations of different arbuscular mycorrhizal fungi improve fitness and metal tolerance of sorghum in ultramafic soil. Rhizosphere 14:100204. https://doi.org/10.1016/j.rhisph.2020.100204
Crossay T, Majorel C, Redecker D, Gensous S, Medevielle V, Durrieu G, Cavaloc Y, Amir H (2019) Is a mixture of arbuscular mycorrhizal fungi better for plant growth than single-species inoculants? Mycorrhiza 29(4):325–339. https://doi.org/10.1007/s00572-019-00898-y
Mouradi M, Farissi M, Bouizgaren A, Makoudi B, Kabbadj A, Very AA, Sentenac H, Qaddourya A, Ghoulam C (2016) Effects of water deficit on growth, nodulation and physiological and biochemical processes in Medicago sativa-rhizobia symbiotic association. Arid Land Res Manag 30(2):193–208
Namdari A, Arani AB, Moradi A (2018) Arbuscular mycorrhizal (Funneliformis mosseae) improves alfalfa (Medicago sativa L.) re-growth ability in saline soil through enhanced nitrogen remobilization and improved nutritional balance. J Cent Eur Agric 19(1):166–183
Mohamed AB, Mansour H, Ali F (2009) Diversity of lucerne (Medicago sativa l.) populations in south Tunisia. Pak J Bot 41(6):2851–2861
Bencherif K, Dalpé Y, Lounès-Hadj Sahraoui A (2019) Influence of native arbuscular mycorrhizal fungi and Pseudomonas fluorescens on Tamarix shrubs under different salinity levels. In: Giri B, Varma A (eds) Microorganisms in saline environments: strategies and functions. Soil biology, vol 56. Springer, New York
Koske RE, Gemma JN (1997) Mycorrhizae and succession in plantings of Beachgrass in sand dunes. Ann J Bot 84:118–130
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular rnycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc 55:158–161
Dalpé Y, Séguin SM (2013) Microwave-assisted technology for the clearing and staining of arbuscular mycorrhizal fungi in roots. Mycorrhiza 23:333–340
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol 115:495–501
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244
Blaszkowski J (2012) Glomeromycota. IB Publisher Polish Academy of sciences, Poland
White TJ, Bruns T, Lee S, Taylors J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. Academic press, Cambridge, pp 315–322
Redecker D (2000) Specific PCR primers to identify arbuscular mycorrhizal fungi within colonized roots. Mycorrhiza 10:73–80
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem 72:248–254
Sadasivam S, Manickam A (1996) Biochemical methods, 2nd edn. Int. Publ. Ltd., New Delhi
Tausky HH, Shorr E (1963) A micro-colorimetric method for the determination inorganic phosphorus. J Biol Chem 202:675–685
Saint Arnand JD (1966) Dosages des éléments minéraux majeurs chez les végétaux. Méthodes utilisées au laboratoire de diagnostic Foliaire de l 'ORSTOM
Pauwels J M, Van Ranst E, Verloo M, Mvondo ZA (1992) Manuel de laboratoire de pédologie. Publications Agricoles 28. Agence Générale de la Coopération au Développement. Bruxelles
Holden M (1965) Chlorophyll. In: Goodwin TW (ed) Chemistry and biochemistry of plant pigments. Academic Press, Cambridge, pp 461–488
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenol-oxydase in Beta vulgaris L. Plant Physiol 24:1–15
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Eterson MD, Prasad TK, Stewart CR (1995) Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol 109:1247–1257
McCune B, Mefford MJ (2011) Pc-Ord. Multivariate analysis of ecological data. MjM Software, Gleneden Beach
Neji Mahmoudi N, Mosbah M, Abdedaiem R, Bessadok K, Mars M (2017) Effects of inoculation with Arbuscular Mycorrhizal Fungi on growth and water stress tolerance of Medicago sativa in arid region of Tunisia. Int J Biol Sci 2(2):10–29
Wang Y-H, Wang M, Li Y, Wu A, Huang J (2018) Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 13:e0196408
Sutlovic D, Gamulin S, Definis-Gojanovic M, Gugic D, Andjelinovic S (2008) Interaction of humic acids with human DNA. Electrophoresis 29(7):1467–1472
Opel KL, Chung D, McCord BR (2010) A study of PCR inhibition mechanisms using real time PCR. J Forens Sci 55:25–33
Ramirez-Viga T, Guadarrama P, Castillo-Argüero S, Estrada-Medina H, García-Sánchez R, Hernández-Cuevas L, Sánchez-Gallén I, Ramos-Zapata J (2019) Relationship between arbuscular mycorrhizal association and edaphic variables in Mangroves of the Coast of Yucatán, Mexico. Wetlands. https://doi.org/10.1007/s13157-019-01196-1
de la Providencia IE, Fernandez F, Declerck S (2007) Hyphal healing mechanism in the arbuscular mycorrhizal fungi Scutellospora reticulata and Glomus clarum differs in response to severe physical stress. FEMS Microbiol Lett 268:120–125. https://doi.org/10.1111/j.1574-6968.2006.00572.x
Giovannetti M, Avio L, Sbrana C (2015) Functional significance of anastomosis in arbuscular mycorrhizal networks. In: Horton T (ed) Mycorrhizal networks. Ecological studies (analysis and synthesis) 224. Springer, New York
de la Providencia IE, de Souza FA, Fernandez F, Séjalon-Delmas N, Declerck S (2005) Arbuscular mycorrhizal fungi exhibit distinct pattern of anastomoses formation and hyphal healing mechanism between different phylogenic groups. New Phytol 165:261–271
He F, Tanga M, Zhong SL, Yang R, Huang L, Zhang HQ (2016) Effects of soil and climatic factors on arbuscular mycorrhizal fungi in rhizosphere soil under Robinia pseudoacacia in the Loess Plateau, China. Eur J Soil Sci 67:847–856
Okon OG, Eneh GDO, Uboh GD, Uyon PP (2020) Enhancement of salt tolerance via Glomus geosporum Inoculation in Telfairia occidentalis Hook. F Seedlings. Int Lett Nat Sci 76:13–22
Yang H, Zhang Q, Koide RT, Hoeksema JD, Tang J, Bian X, Hu S, Chen X, Cahill J (2016) Taxonomic resolution is a determinant of biodiversity effects in arbuscular mycorrhizal fungal communities. J Ecol 105:219–228. https://doi.org/10.1111/1365-2745.12655
Thonar C, Frossard E, Šmilauer P, Jansa J (2014) Competition and facilitation in synthetic communities of arbuscular mycorrhizal fungi. Mol Ecol 23:733–746
Tuo X-Q, Li HE, Ying-Ning Z (2017) Alleviation of drought stress in white clover after Inoculation with arbuscular mycorrhizal fungi. Not Bot Hortic Agrobot 45(1):220–224
Shokri S, Maadi B (2009) Effects of arbuscular mycorrhizal fungus on the mineral nutrition and yield of Trifolium alexandrinum plants under salinity stress. J Agron 8:79–83
Santander C, Sanhueza M, Olave J, Borie F, Valentine C, Cornejo P (2019) Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J Soil Sci Plant Nutr 19(2):321–331. https://doi.org/10.1007/s42729-019-00032-z
Basiru S, Hirji M (2022) Does commercial inoculation promote arbuscular mycorrhizal fungi invasion? Microorganisms 10:404. https://doi.org/10.3390/microorganisms10020404
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identi¢cation of arbuscular mycorrhizal fungi. FEMS. https://doi.org/10.1111/j.1574-6941.2008.00531.x
Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984. https://doi.org/10.1111/j.1469-8137.2011.03962.x
Acknowledgements
This work is part of the academic project of the Ministry of Scientific Research and University of Djelfa (Algeria) PRFU (Code: D04N01UN170120200006). This work has also been carried out in the framework of the Alibiotech project which is financed by the European Union, the French State and the French Region of Hauts-de-France. We are grateful to every one which helps us to establish this work, especially Adel Djouklafi for the statistical analysis. The authors thank the two anonymous reviewers for their useful comments.
Funding
This work does not received any specific funding.
Author information
Authors and Affiliations
Contributions
KB designed the research, provided AMF cultures, conducted the greenhouse experiment, analyzed data and wrote the manuscript; AH, MA and ZB contributed to the greenhouse conductance and to molecular analysis; BT Provide plant seed and contribute to native inoculum production; YD contributed to AMF taxonomy and to the research supervision; ALH supervised and designed the research and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karima, B., Amima, H., Ahlam, M. et al. Native Arbuscular Mycorrhizal Inoculum Modulates Growth, Oxidative Metabolism and Alleviates Salinity Stresses in Legume Species. Curr Microbiol 80, 66 (2023). https://doi.org/10.1007/s00284-022-03145-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03145-4