Abstract
Salinity, a serious environmental pressure on crop production, might be counteracted by free-living and symbiotic inoculants entailing positive synergistic effects. Enhancement in nutrient uptake and/or production of antioxidants under the stress condition, can improve plant growth and yield. In this study, inoculation of Lallemantia iberica with Funneliformis mosseae and the sulfur solubilizing bacterium (Thiobacillus sp. T95 and T40) was evaluated under two salinity levels (6.72 dS/m and 0.91 dS/m as control). The root colonization, spore density, seed and biological yield, total soluble sugars, and nutrients were reduced by salt stress. Antioxidant enzyme activity (catalase, superoxide dismutase, peroxidase and ascorbate peroxidase), proline, contents of sodium and sulfur have increased under salt stress. The enzyme activities as well as the concentrations of nitrogen, phosphorus, potassium, sodium, and sulfur were dropped at the flowering stage (75 days after sowing). Seed and biological yield, antioxidant enzymes activity, proline content, and nutrients were significantly improved in mycorrhizal treatments. Inoculation of Thiobacillus exhibited the positive effect on root colonization, spore density, enzymes activity, and nutrients. Bacterial treatments (dual and single) significantly increased the sulfur and total soluble sugars. Totally, the mycorrhizal plants accumulated more enzymatically produced antioxidants, osmolytes, and showed improved nutrient uptake. Our results provide new insights into the relationship among arbuscular mycorrhizal fungi (AMF), biosulfur bacteria, and plant growth under saline conditions. In conclusion, the Lallemantia iberica inoculation with mycorrhizal fungi, either alone, or in combination with Thiobacillus, is indicated for optimum plant yield through alleviation of the salinity stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dragon's head (Lallemantia iberica (M. Bieb) Fisch. & C. A. Mey.) is an annual herb belonged to Lamiaceae family, spreads across Southwest of Asia and Europe and originates from the Caucasus and the Middle East [1]. Dragon's head is cultivated for its seeds oil (it contains up to 30% of a drying oil used in foods and industry), mucilage (treatment of nervous and hepatic diseases, and as a general tonic, aphrodisiac and expectorant remedies) [1, 2], and essential oil (effectiveness on breast cancer and antioxidant activities) [3].
In arid and semi- arid areas, steadily increasing salinization of soil has affected approximately 6% of the world’s land area [4]. The yield of most crop plants is reduced due to osmotic stress, ion toxicity, and nutrient deficiency along with elevation of salt concentration in the root environment [4]. Also, morphological and physiological changes have been observed in response to environmental stress leading to decrease the growth parameters in L. iberica [5]. The intensity of negative effects of soil salinity on growth, development, and morphophysiological characteristics of Lallemantia species is varied [6]. As a result of salinity stress, reactive oxygen species (ROS) is produced and damaging plant cells [7]. To prevent the production of ROS, plants use an effective scavenging system including non-enzymatic molecules (e.g., carotenoids, ascorbate, glutathione and tocopherols) as well as enzymatic antioxidants (superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR)) [7, 8].
Sulfur (S) and phosphorus (P), as two essential nutrients for plant growth, are usually applied through chemical fertilizers for achieving higher yields of crops, by leaving harmful effects on the environment and mostly with low quality [9]. While using bio-fertilizers like the AMF can provide extrinsic plants protection against salt stress along with improving nutritional status, water availability, and water use efficiency (WUE) [9, 10]. Mycorrhization enhances root hydraulic conductivity as well as adjusting the osmotic balance and enzyme activities [8], photosystem II efficiency, and photosynthetic product [9], antioxidant production, and finally through reducing the Na concentrations [11] of plants under the salt stress conditions.
The Thiobacillus bacteria make sulfate available, through its oxidation process with the formation of thiosulfate and tetrathionate. These bacteria increase the cationic exchange rate of soil by activating beneficial soil microbes and facilitation of biological oxidation in soil, resulting in improved nutrients uptake, thereby increasing the crop yield by 30–60% [12, 13]. Also, the production of sulfuric acid reduces soil pH, therefore isolated H+ can displace the Na from clay particles, and then increase sodium subsequent leaching and decline in soil salinity [14]. The interaction between Thiobacillus and mycorrhiza may have positive, neutral, and negative effects on plant growth through mycorrhizal root association [15]. The positive effect of combined inoculation of AMF and Thiobacillus species has been reported on Allium cepa [9], as well as combined usage of mycorrhiza with other beneficial bacteria [8]. The reports on the interaction between sulfur oxidizing bacteria and AMF under salinity conditions have been scarce. Therefore, inoculation with Funneliformis mosseae and Thiobacillus sp. (individually and dual) is necessary, due to possible positive and/or negative effects on nutrient uptake and antioxidant enzymes activity of plants. These effects may be varied in soil salinity stress. Thus, the main purpose of this work was to investigate the impact of earlier mentioned soil microorganisms individual or dual impact of Funneliformis mosseae and Thiobacillus sp. on plant (Lallemantia iberica) productivity under salinity stress in comparison with non-saline condition.
Materials and Methods
Site Explanation and Climatic Particularity
A 2-year (2017–2018) trial was conducted at Agricultural Research Farm of Urmia University (latitude 37°33′09″ N, 45°05′53″ E and altitude 1320 m), considered as a semi-arid regime. Meteorological data for these two years are presented in Fig. 1.
Experimental design was a split plot arrangement based on the randomized complete block design with three replications. Treatments were soil salinity (0.91 dS/m as control and 6.72 dS/m) as main plots, and bio-fertilizer (untreated as control, Funneliformis mosseae + Thiobacillus sp., Funneliformis mosseae and Thiobacillus sp.) as sub plots. Lallemantia iberica seeds (Kurdistan-2 landrace), supplied by the Dryland Agricultural Research Institute (DARI), were sown at a depth of 3 cm in plots of 250-by-200 cm size on 15 March 2017 and 16 March 2018. Seeds were planted with 2 cm spaces in rows, forming 6 rows and the spacing between the rows was 30 cm. The mycorrhizal inoculum was a mixture of sterile sand, mycorrhizal hyphae, and spores (20 spores/g inoculum) and colonized root pieces, which were produced on Zea mays L. host plants provided by Dr. Y. Rezaee Danesh (Department of Plant Protection, Urmia University). The AMF inoculum (250 g/m2) was banded in the rows at a depth of 10 cm under the seeds, and lightly covered with soil. The sulfur was in the form of pelletized sulfur-bentonite mixture and Thiobacillus (7 kg/ha Thiobacillus sp. bacteria + 250 kg/ha organic bentonite sulfur). For bacterial treatments, the seeds were inoculated with Thiobacillus sp. T95 and T40 (9.3 × 107). A biological fertilizer (SulfoBarvar-1) was prepared from GreenBiotech, Tehran, Iran, before being immediately planted. The activity of Thiobacillus bacteria was measured and confirmed before applying the fertilizer. For this purpose, sample of biosulfur fertilizer was inoculated into 100 ml of Thiobacillus enrichment medium, and uninoculated medium was used as a control. Total sulfate and pH [16] and bacteria population [17] were determined at the end of incubation period. For the non-fertilizer (control treatment), seeds were sown without inoculation. Characteristics of saline soil in subgroup typic Endoaquepts and non-saline soil in subgroup typic Calcixerepts [18] are reported in Table 1.
Measurements
Seed and Biological Yields
To measure seed and biological yield, 30 plants were randomly harvested from each plot and transferred to the laboratory in separate bags. Then, seed and biological yield of each plant were measured using precision scales.
Root Colonization and Spore Density
After harvesting of the plants (110 days after sowing, DAS), to determine the percentage of root colonization, 1 cm long segments of roots were carefully washed, cleared in 10% KOH for 1 h at 90 °C, and were then stained with 0.05% lactic acid-glycerol-Trypan Blue [19]. AMF colonization was estimated using the grind-line intersect method [20].
The method of wet sieving and decanting without using sugar and centrifuge solution was applied to extract soil spores. Specifically, 100 g of soil was poured into conical flask, and mixed with 1 L of water, and the mixture brought to suspension. The soil mixture was agitated to free the AMF spores from soil and allowed to be settled for several minutes where the supernatant was decanted through standard sieves [21], and was counted by microscope (Model CX22, Japan).
Nutrients
Samples of leaves and roots were taken from 50 DAS (vegetative stage) and 75 DAS (70% flowering stage), washed in distilled water, and were then placed into paper bags and were oven dried (at 75 °C) for 48 h. The dried samples were ground in a knife mill, and aliquots were separated to determine K, N, P, S, and Na. The values were expressed on a percentage basis.
To measure the percentage of nitrogen, dried plant was used for wet digestion (Digestion in volumetric flask with sulfuric acid—salicylic acid and oxygenated water). Total nitrogen was determined by Kjeldahl (Model Vapodest 20 s, Germany) digestion, distillation, and in terms of titration procedures by McGill and Figueiredo [22].
The percentages of Na and K in samples were determined using flame photometer (Model Jenway PFP7, UK) according to Waling et al. [23]. The ratio of K/Na was also calculated. Leaf phosphorus was determined using the molybdenum blue procedure by Chapman and Pratt [24]. The absorbance was taken at 420 nm using a spectrophotometer (Spectrophotometer, Model UV/Vis 2100 PC, USA). The sulfur concentration of dried tissues was determined through di-acid digestion by applying turbidity method using a spectrophotometer at 420 nm wavelength [25].
Enzyme Extraction
Leaf samples were collected in 50 DAS (vegetative stage) and 75 DAS (70% flowering stage). To extract the specimens, 0.5 g of the plant tissues, stored in the—80 °C, was thoroughly crushed with liquid nitrogen, received 1.5 mL of extraction buffer, was completely homogenized, and eventually centrifuged in an Eppendorf tube at 10.000×g for 20 min at 4 °C. The upper phase was separated to read the protein level and activity of the antioxidant enzymes [26].
To measure the activity of superoxide dismutase (SOD), 50 μL of extract was mixed with 1 mL of superoxide dismutase solution, containing 0.05 mol potassium phosphate buffer (pH 7.8), 75 μmol NBT, 13 mmol of l-methionine 0.1 mol EDTA, and 2 μmol riboflavin. To establish the reaction, the mixture was placed in a light chamber for 15 min, and after that the absorbance of solution was read using a spectrophotometer at 560 nm [27].
The quantitative concentration of ascorbate peroxidase was measured by Nakano and Asada [28] method. With respect to this method, 50 μL of extract was mixed with 50 mmol of potassium phosphate buffer (pH 7) and 0.1 mmol H2O2 in an ice bath, and immediately 50 μL of the enzyme extract was added to that. Finally, by addition of 0.5 mmol ascorbate (ascorbic acid), the reduction in absorbance at 290 nm for 1 min was recorded using a spectrophotometer.
To measure the quantitative activity of peroxidase (POX), 50 μL of the enzyme extract was mixed with 3 mL of 0.1 mol potassium phosphate buffer and 50 μL of pure giacole liquid (C7H8O2) as an electron donor). Next, 50 μL of 3% hydrogen peroxide was added (as an electron receptor), and immediately changes in optical absorption at 436 nm were recorded using a spectrophotometer at intervals of 15 s to 3 min [29].
Catalase activity was measured in terms of Dhindsa et al. [30], by which 50 μL of extraction was mixed with 1 mL of catalase measurement solution, containing 50 mL of potassium phosphate buffer (pH 7) and 15 mmol of hydrogen peroxide (H2O2). Then, the absorbance was read using a spectrophotometer at 240 nm for 1 min.
Osmolytes
In 50 DAS (vegetative stage) and 75 DAS (85% flowering stage), according to the method of Bates et al. [31], 0.5 g of leaf tissue was placed in 10 mL of 3% sulfosalicylic acid and was then homogenized. The homogeneous mixture was filtered, and 2 mL of this solution was mixed with 2 mL of ninhydrinic acid. Furthermore, 2 mL of acetic acid was added to each tube, and then incubated for 1 h at 100 °C. The reaction mixture was added in cold water and 4 mL of toluene. The upper phase was removed and the absorbance was determined at 520 nm. Proline concentration (μmol/g FW) was calculated as follows:
where FP: Proline content (μmol/g FW); R number read from the spectrophotometer; T The amount of toluene consumed (in all samples is the same as 4 mL); W The sample weight used (for this test is 0.5).
In 50 and 75 DAS, according to the method of Irigoyen et al. [32], the concentration of total soluble sugars was estimated by applying the anthron. In this method, 0.5 g of fresh leaves were placed in about 5 mL of 95% ethanol and were then centrifuged at 3500 × g for 15 min. The absorbance of supernatant (mixed with anthron + sulfuric acid, and then placed in a boiling water bath for 10 min) was read at 620 nm.
Statistical Analysis
The analysis of variance (ANOVA) for both years was performed using GLM procedure (SAS 9.1.3, SAS Institute Inc., Cary, NC, USA), with the means compared by Duncan multiple range test at P ≤ 0.05.
Results
Analysis of variance of 2-year data indicated the significant interaction effect of ″Salinity × Inoculants″ on the root colonization, spore density, seed and biological yields, nitrogen, phosphorus, sodium, and potassium of either roots or leaves, on the concentrations of leaf CAT, POX, SOD, APX and proline in 50 DAS (vegetative stage), and also on the phosphorus, sulfur, sodium and potassium of roots and leaves, K/Na (roots and leaves), POX, SOD, proline and TSS in 75 DAS (flowering stage) (Supplementary Tables 1, 2 and 3). There was no significant effect of salinity and/or inoculants on the TSS at the vegetative stage (50 DAS). The inoculants indicated non-significant effect on APX at both vegetative and flowering stages (Supplementary Table 3). The effect of year on some measured traits including spore density, seed and biological yields, Kleaf and Kroot (75 DAS), SOD, APX (50 DAS), CAT and POX (75 DAS), proline (50 and 75 DAS) were significant and higher values were observed in second year (2018) (Supplementary Tables 1, 2 and 3).
Root Colonization and Spore Density
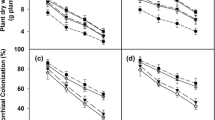
Root colonization and spore population had significant reduction for single and dual inoculations under the saline conditions. The highest root colonization (66.72%) and spore density (101.3 spores/g soil) were belonged to inoculation with F. mosseae under non-saline condition followed by ″F. mosseae + Thiobacillus″ (60.54% and 88.5 spores/g soil, respectively). Root colonization of those plants inoculated with F. mosseae was reduced to 23.23% under saline condition. Under salinity stress, the spore density had no significantly differences between single and dual inoculation (Fig. 2a and b). The lowest root colonization under both saline and non-saline conditions was similar in the absence of mycorrhizal inoculant for non-inoculated plants and bacterial treatment (Fig. 2a).
Means comparison of root colonization (a), spore density (b), seed yield (c) and biological (d) affected by AMF and/or bacterial inoculants under two salinity levels (non-saline and saline conditions). Different letters show significant differences at 5% probability level (Duncan's multiple range test). 0 = non-inoculated, B = Thiobacillus sp., M = Funneliformis mosseae, M + B = Funneliformis mosseae + Thiobacillus sp
Seed and Biological Yields
Seed and biological yields were decreased under salinity stress and also mycorrhizal plants (dual and/or single) had significant effect on yields under these conditions. Also, in non-saline conditions mycorrhizal plants (F. mosseae + Thiobacillus and F. mosseae) had the highest biological yield (0.85 and 0.81 g per plant, respectively) and plants with ″F. mosseae + Thiobacillus″ treatment had the highest seed yield (0.39 g per plant) (Fig. 2 and d).
Nutrients
Leaf nitrogen (Nleaf) and leaf phosphorus (Pleaf) were significantly declined under the saline condition, at both vegetative (50 DAS) and flowering (75 DAS) stages. Under salinity stress, treatments involving AMF resulted in a greater increment in Nleaf and Pleaf compared to those of bacterial inoculation. The highest nitrogen and phosphorus percentage were belonged to 50 DAS and use of inoculants under non-saline, while the minimum was observed by the untreated control plants in 75 DAS under salinity stress (Fig. 3a and b).
Means comparison of leaf nitrogen (a), phosphorus (b) and sulfur (c) affected by AMF and/or bacterial inoculants under two salinity levels (non-saline and saline conditions). Different letters show significant differences at 5% probability level (Duncan's multiple range test). 0 = non-inoculated, B = Thiobacillus sp., M = Funneliformis mosseae, M + B = Funneliformis mosseae + Thiobacillus sp
Thiobacillus (single or with fungi) resulted in increasing the sulfur accumulation under both of saline conditions. Therefore, at flowering stage (75 DAS), it was higher for dual inoculation in saline condition. The maximum leaf S was obtained from dual inoculated plants in both salinity stresses especially at flowering stage. A positive synergistic effect of organisms (F. mosseae + Thiobacillus) led to the maximum leaf S in this above mentioned situation (Fig. 3c).
The salinity stress (6.72 dS/m) caused about 30-fold increase in sodium (Naleaf and Naroot) in all treatments. Nonetheless, mycorrhizal treatments (single and dual inoculation) declined the concentration of Naleaf more effectively than that of Naroot. Leaf sodium contents of vegetative (50 DAS) and flowering (75 DAS) stages were similar and minimum under the non-saline conditions. The concentration of root sodium was higher than that of leaf Na, which was significantly reduced in mycorrhizal treatments (Fig. 4a and b). In all samples (roots and leaves of 50 and 75 DAS), potassium had a higher concentration under non-saline. The maximum potassium content in roots and leaves occurred in mycorrhizal inoculation followed by bacterial inoculation. As a result, the highest Kleaf (50 and 75 DAS) was belonged to mycorrhizal plants (F. mosseae) under the non-saline condition (Figs. 4-c and 4-d). The comparison of means indicated that the maximum K/Na ratio (roots and leaves of both 50 and 75 DAS) was belonged to the plants treated with F. mosseae followed by dual inoculation of ″F. mosseae + Thiobacillus sp.″. Due to the greater reduction of potassium concentration in comparison with sodium at the flowering stage, the ratio of K/Na has also decreased (Fig. 4 and f).
Means comparison of sodium leaf (a), sodium root (b), potassium leaf (c), potassium root (d), ratio of potassium and sodium from root and leaf (e and f) affected by AMF and/or bacterial inoculants under two salinity levels (non-saline and saline conditions). Different letters show significant differences at 5% probability level (Duncan's multiple range test). 0 = non-inoculated, B = Thiobacillus sp., M = Funneliformis mosseae, M + B = Funneliformis mosseae + Thiobacillus sp
Antioxidant Enzymes Activity
At both (vegetative and flowering) stages, CAT, POX, SOD and APX activity was significantly enhanced by salinity stress. Under saline conditions, the CAT activity increases was greater for dual colonized plants (F. mosseae + Thiobacillus sp.), compared to those of other treatments at vegetative stage. At the flowering stage (75 DAS), all (single or dual) inoculants exhibited identical increases for CAT activity (Fig. 5a). The rising POX and SOD activity at vegetative stage were obtained in the presence of mycorrhizal inoculum which significantly increased under stress condition. At the flowering stage, this increment of POX and SOD activity was observed for individual AMF inoculation. The same trends for non-saline condition were observed either in 50 DAS or in 75 DAS (Fig. 5b and c).
Means comparison of catalase (a), peroxidase (b), superoxide dismutase (c), ascorbate peroxidase (d), proline (e) and total soluble sugars (f) affected by AMF and/or bacterial inoculants under two salinity levels (non-saline and saline conditions). Different letters show significant differences at 5% probability level (Duncan's multiple range test). 0 = non-inoculated, B = Thiobacillus sp., M = Funneliformis mosseae, M + B = Funneliformis mosseae + Thiobacillus sp
In 50 DAS, microbial (fungi and bacteria) inoculation exhibited identical APX with untreated control plants. In 75 DAS, there was less activity of APX under both saline and non-saline conditions (Fig. 5d).
Osmolytes
Leaf proline concentration at both growing stages (vegetative and flowering) increased in mycorrhizal plants followed by dual inoculation. Inoculation of Thiobacillus revealed the similar and even lower concentrations of proline, in comparison with non-inoculated plants. The maximum leaf proline concentration (8.51 and 7.13 μmol/g FW in vegetative and flowering stages, respectively) was obtained under salinity stress with individual application of F. mosseae. The same trend (lower concentration) of leaf proline along with inoculations was observed under non-saline condition (Fig. 5e).
At the vegetative stage (50 DAS), concentrations of total soluble sugars (TSS) were not affected by inoculations (AMF and/or Thiobacillus) neither by salinity nor by non-salinity stresses. At the flowering stage, the highest leaf TSS was obtained from bacterial treatment plants (Thiobacillus sp. and F. mosseae + Thiobacillus sp.) under non-saline conditions. There were no significant changes of leaf TSS of inoculated plants by salinity treatments and/or microbial (fungi and bacterial) inoculants at the vegetative stage (Fig. 5f).
Discussion
The decrease in the spore density under salinity (Fig. 2a), was one of the most important factors of root colonization percentage reduction (Fig. 2b). Also, negative effect of salinity stress on the germination of spores, inhibition in hyphal growth, and proliferation in soil with a subsequent reduction in the spread of mycorrhizal colonization, suppression of arbuscules’ formation, and reduction of photosynthetic availability, indirectly cause to decrease colonization percentage under salinity stress [33, 34]. In addition, the lack of access to water and nutrients caused by ionic stress (Figs. 3 and 4), reduced the root growth and biomass (Fig. 2b), resulting in reduction of plant photosynthesis and organic carbon transfer to create mutual coexistence of microorganisms with the plants [35]. In the present study, seed and biological yields were decreased under salinity stress. Also, mycorrhiza-inoculated plants had a relatively higher yield than non-inoculated plants (Fig. 2). This was probably due to the fact that, leaf phosphorus and nitrogen losses were compensated under salinity stress by inoculations of Thiobacillus or F. mosseae as with dual inoculation (Fig. 3a and b). On the other hand, increased production of antioxidants in plants treated with mycorrhiza under salt stress (Fig. 5) can be considered as another factor in improving plant growth and yield under stress conditions.
Lower concentrations of leaf nutrients (N, P, Kleaf and Kroot, S and Naleaf and Naroot) decreased at the flowering stage (Figs. 3 and 4). Accordingly, it may be caused by the appearance of new sinks during the flowering stage and allocation of materials for producing antioxidants under salinity stress (Fig. 5), which reduced the transfer of photosynthetic materials to fungi symbiotic with roots [36]. As a result, reduced the nutrient uptake by root resulted in reduction of nutrients in the leaves and roots at this stage under both salinity conditions (Figs. 3 and 4). Furthermore, remobilization processes for growth and fertility for new sinks [37] as another factor, decrease the concentration of elements in the roots and leaves. Leaf phosphorus and nitrogen losses under salinity stress were compensated by inoculations of Thiobacillus or F. mosseae as with dual inoculation (Figs. 3a and b). Elevation of nutrients uptake by activity of mycorrhizal fungus, excretion of organic acids and Thiobacillus with oxidization of sulfur to sulfate, reducing the soil pH, soil electrical conductivity, organic carbon, and enhanced the availability of nutrients (NPK) in soil [9, 34]. So, the AMF have the ability to increase host uptake of phosphorus, nitrogen, and water absorption [9].
The increasing concentration of sulfur in leaves was observed in Thiobacillus and/or mycorrhizal plants (Fig. 3c). As the results of our experiments indicated, inoculation of AMF individual or dual with bacteria increases the antioxidant activity of the plants (Fig. 5). On the other hand, sulfur is an important component of some antioxidants like glutathione (non-enzymatic antioxidant) [38], consequently increasing this element under salinity stress and mycorrhizal treatments is unexpected due to the increase in antioxidant enzymes. Moreover, Mycorrhizal colonization enhanced the activity of Thiobacillus, production of root exudates and extensive hyphal network, which may acidify the rhizosphere thereby enhancing sulfur availability to the host plant [39].
As the results show, reduction of Na along with increase of K accumulation were observed in leaf and root (both stages) in plants with AMF (single and dual) inoculations (Fig. 4a, b, c and d). Due to the similarities in physicochemical properties of Na and K, these ions compete with each other for major binding sites in metabolic processes. Therefore, retaining K or preventing Na from accumulating in the leaves and maintaining a high cytosolic K/Na ratio is necessary for salt tolerance (Fig. 4a and b). Furthermore, Hanin et al. [11] reported that mycorrhizal colonization can prevent Na translocation to shoot tissues. This may lead to subsequent dilution of toxic effect of sodium in inoculated plants. The reason for the rise of potassium in mycorrhizal plant tissue could be due to K transporters and channels from the arbuscule or hartig net to the host plant [40]. This may result in subsequent dilution of toxic effect of sodium in inoculated plants (Fig. 4a and b).
Although root colonization decreases under salinity stress, the positive effects of coexistence (fungi and bacteria) in comparison with control plants are evident. The oxidative stress created in the plant following salinity stress causes oxygen radicals including singlet oxygen (1O2), hydrogen peroxide (H2O2), and/or hydroxyl radicals (OH•). As indicated in Fig. 4, antioxidant enzymes activity (CAT, POX, SOD and APX) increased under salinity stress to counteract and eliminate its destructive effects. The efficiency of SOD is related to catalysis of the dismutation of superoxide anion (O2−) to H2O2 and O2. Then, the action of enzymes (POD, APX, GPX and CAT) turns hydrogen peroxide to water and oxygen protecting organisms against free radicals [34, 41]. POX and CAT reduce the levels of lipid peroxidation by eliminating the excess ROS. Mycorrhizal symbiosis raised up the ability to protect the plants by enhancing antioxidant enzymes activities under salinity stress and non-saline condition (Figs. 3 and 5). Higher APX and POD activities in AMF inoculation are correlated with plant growth improvement under salt stress [41]. Higher activity of antioxidant enzymes in mycorrhizal plants was often associated with increased biomass along with higher P or N concentration (Figs. 2b and 3). Also, the availability of Fe, Cu, Zn, and Mn in AMF-inoculated plants might be the reason of increased activity some metalloenzymes such as SOD, CAT, and APX [42].
The results of the present study also revealed that, the antioxidants’ activity dropped at the flowering stage (Fig. 5). The composition of antioxidants is largely depended on maturation stage, growing conditions, and part of the plant analyzed [43]. Because senescence affects the expression of antioxidant gene products, SOD and CAT activities are reduced in leaves upon aging and with maturity [34, 44]. The results demonstrated that co-inoculation with bacteria and AMF or single-inoculation was more efficient on antioxidant enzymes (Fig. 5).
Enhanced leaf proline in stressed plants (Supplementary Table 3) might be due to activate stress genes [45]. To reduce salt stress-induced damages, proline may retain osmotic pressure balance between intracellular and extracellular space to improve the moisture retention capacity [46]. The reason for the elevation of proline in mycorrhizal plants (Fig. 5e) in salt stress is accumulation of the maximum content of proline in root tissues of mycorrhizal plants adjusting osmotic capacity to absorb water under stress conditions [42].
As an osmotic adjustment, reduction of the total soluble carbohydrates when L. iberica plants that were exposed to salinity (Fig. 5f), could be caused by osmotic stress-induced decline in photosynthesis and the subsequent shortage of photo assimilates [47]. Sensitivity of L. iberica to salinity stress varied with the growth stage, where the amount of soluble sugar in the vegetative stage was not affected (Fig. 5f). Total soluble sugar in plants inoculated by Thiobacillus under salinity stress plants (Supplementary Table 3) may be due to the positive effect of sulfur fertilizer on photosynthesis along with the increase in the total content of pigments [48]. According to Lewis et al. [49], a demand of plants for carbon is relatively less, and sinks activity declines at the onset of flowering. Furthermore, the flowering may decrease the net photosynthesis and mesophyll conductance [50].
Conclusion
Overall, salinity stress led the osmotic stress in plants to enhance the activity of antioxidant enzymes, proline content, and Naleaf plus Naroot uptake. On the other hand, it reduced the absorption of NPK by the plants. These are very important and effective factors in reducing plant yield under salinity stress. Although salt stress reduced the percentage of colonization and spore density, AMF inoculation improved the antioxidant enzymes activity, osmolytes, and nutrients concentration of Lallemantia iberica plants under salinity condition. Therefore, using inoculants, especially co-inoculation of AMF and Thiobacillus bacteria, was more successful in most of the measured cases (about 59% of all measured traits) in comparison with applying the individual mycorrhiza. Thiobacillus increased the sulfur uptake and TSS content. Despite higher N, P, S, Kleaf, Kroot, Naleaf, Naroot, CAT, SOD, APX, proline and TSS were diminished with the development of a new sink at the flowering stage. These data can provide additional support for future research on medicinal plants resistance to saline condition. Of course, further research is needed to investigate the role of bio-fertilizers on plant support to improve growth at saline conditions.
Abbreviations
- AMF:
-
Arbuscular mycorrhizal fungi
- POX:
-
Peroxidase
- APX:
-
Ascorbate peroxidase
- ROS:
-
Reactive oxygen species
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- DARI:
-
Dryland Agricultural Research Institute
- TSS:
-
Total soluble sugars
- DAS:
-
Days after sowing
- WUE:
-
Water use efficiency
- GR:
-
Glutathione reductase
References
Fathi M, Emam-Djomeh Z, Sadeghi-Varkani A (2018) Extraction, characterization and rheological study of the purified polysaccharide from Lallemantia ibrica seeds. Int J Biol Macromol 120:1265–1274. https://doi.org/10.1016/j.ijbiomac.2018.08.159
Amanzadeh Y, Khosravi Dehagni N, Gohari AR, Monsef-Esfehani HR, Sadat Ebrahimi SE (2011) Antioxidant activity of essential oil of Lallemantia iberica in flowering stage and post-flowering stage. Res J Biol Sci 6:114–117. https://doi.org/10.3923/rjbsci.2011.114.117
Awad A, Barta SL, Fink CS, Bradford PG (2008) β-sitosterol enhances tamoxifen effectiveness on breast cancer cells by affecting ceramide metabolism. Mol Nutr Food Res 52:419–426. https://doi.org/10.1002/mnfr.200700222
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.09
Samadi S, Khaiyamiand M, Hasanzadeh A (2007) A Comparison of important physical and chemical characteristics of six Lallemantia iberica (Bieb.) varieties. Pak J Nutr 6:387–390
Yadegari M (2014) Study of ratio and speed germination of twelve medicinal plants under several treatments of salinity. Adv Environ Biol 8:425–430
Foyer CH (2018) Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot 154:134–142. https://doi.org/10.1016/j.envexpbot.2018.05.003
Rahimzadeh S, Pirzad A (2017) Arbuscular mycorrhizal fungi and Pseudomonas in reduce drought stress damage in flax (Linum usitatissimum L.): a field study. Mycorrhiza 27:537–552. https://doi.org/10.1007/s00572-017-0775-y
Mohamed AA, Eweda WEE, Heggo AM, Hassan EA (2014) Effect of dual inoculation with arbuscular mycorrhizal fungi and sulphur-oxidising bacteria on onion (Allium cepa L.) and maize (Zea mays L.) grown in sandy soil under greenhouse conditions. Ann Agric Sci 59:109–118. https://doi.org/10.1016/j.aoas.2014.06.015
Pirzad A, Mohammadzadeh S (2018) Water use efficiency of three mycorrhizal Lamiaceae species (Lavandula officinalis, Rosmarinus officinalis and Thymus vulgaris). Agric Water Manag 204:1–10. https://doi.org/10.1016/j.agwat.2018.03.020
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1664–1462. https://doi.org/10.3389/fpls.2016.01787
Pokorna D, Zabranska J (2015) Sulfur-oxidizing bacteria in environmental technology. Biotechnol Adv 33:1246–1259. https://doi.org/10.1016/j.biotechadv.2015.02.007
Wang YF, Wang SP, Cui XY, Chen ZZ, Schnug E, Haneklau S (2003) Effects of sulphur supply on the morphology of shoots and roots of alfalfa (Medicago sativa L.). Grass Forage Sci 58:160–167. https://doi.org/10.1046/j.1365-2494.2003.00366.x
Garcia Júnior O (1992) O enxofre e suas transformações microbianas. In: Tsai SM, Neves MCP (eds) Cardoso EJB. Microbio-logia do solo, Sociedade Brasileira de Ciência do Solo Campinas, pp 1–15
Mostafavin SR, Pirdashti H, Ramaznpour MR, Andarkhoran AA, Shahsavari A (2008) Effect of mycorrhizal, Thiobacillus and sulfur nutrition on the chemical composition of Soybean (Glycine max L.) Merr Seed. Pak J Biol Sci 11:826–835. https://doi.org/10.3923/pjbs.2008.826.835
Issam IB, Antoin HS (2007) Methods of analysis for soils of arid and semi-arid regions. Food and Agriculture organization of the United Nations, Rome
Øvreås L, Torsvik V (1998) Microbial diversity and community structure in two different agricultural soil communities. Microb Ecol 36:303–315. https://doi.org/10.1007/s002489900117
Soil Survey Staff (2014) Keys to soil taxonomy, 12th edn. USDA, NRCS, Washington
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161. https://doi.org/10.1016/s0007-1536(70)80110-3
McGonigle TP, Miller MH, Evans DG, Fairchild DL, Swan GA (1990) A new method which gives an objective measure of colonisation of roots by vesicular arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244. https://doi.org/10.1016/S0007-1536(63)80079-0
McGill WB, Figueiredo CT (1993) Total nitrogen. In: Carter MR (ed) Soil sampling and methods of analysis, society of soil science. Lewis, Boca Raton, pp 201–211
Waling I, Vark WV, Houba G, Van der Lee JJ (1989) Soil and plant analysis, a series of syllabi. Part 7. Plant Anal Proced. Wageningen Agriculture University, Wageningen
Chapman HD, Pratt PF (1962) Methods of analysis for soils, plants and waters. Soil Sci 93:68. https://doi.org/10.1097/00010694-196201000-00015
Tabatabai MA, Bremner JM (1970) A simple turbidimetric method of determining total sulfur in plant material. Agron J 62:805–806. https://doi.org/10.2134/agronj1970.00021962006200060038x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
MacAdam JW, Nelson CJ, Sharp RE (1992) Peroxidase activity in the leaf elongation zone of tall fescue: I. spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol 99:872–878. https://doi.org/10.1104/pp.99.3.872
Dhindsa RH, Plumb-Dhindsa R, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101. https://doi.org/10.1093/jxb/32.1.93
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/bf00018060
Irigoyen JJ, Einerich DW, Sanchez-Diaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol Plant 84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x
Bothe H (2012) Arbuscular mycorrhiza and salt tolerance of plants. Symbiosis 58:7–16. https://doi.org/10.1007/s13199-012-0196-9
Hajiboland R, Aliasgharzadeh A, Laiegh SF, Poschenrieder C (2010) Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 331:313–327. https://doi.org/10.1007/s11104-009-0255-z
ŘezáčováV SR, Sochorová L, Konvalinková T, ProcházkováV ŠV, Hršelová H, Beskid O, Hujslová M, Gryndlerová H, Gryndler M, Püschel D, Jansa J (2018) Mycorrhizal symbiosis induces plant carbon re-allocation differently in C3 and C4 Panicum grasses. Plant Soil 425:441–456. https://doi.org/10.1007/s11104-018-3606-9
Johnson CR, Graham JH, Leonard RT, Menge JA (1982) Effect of flower bud development in chrysanthemum on vesicular-arbuscular mycorrhiza formation. New Phytol 90:671–675. https://doi.org/10.1111/j.1469-8137.1982.tb03277.x
Malagoli P (2005) Dynamics of nitrogen uptake and mobilization in field-grown winter oilseed rape (Brassica napus) from stem extension to harvest: I. Global N flows between vegetative and reproductive tissues in relation to leaf fall and their residual N. Ann Bot 95:853–861. https://doi.org/10.1093/aob/mci091
Rais L, Masood A (2013) Sulfur and nitrogen co-ordinately improve photosynthetic efficiency, growth and proline accumulation in two cultivars of mustard under salt stress. J Plant Biochem Physiol 1:1–6. https://doi.org/10.4172/jpbp.1000101
Miransari M (2013) Arbuscular mycorrhizal fungi and uptake of nutrients. Symbiotic Endophytes. Springer, Berlin, pp 253–270
Dominguez-Nuñez JA, Benito B, Berrocal-Lobo M, Albanesi A (2016) Mycorrhizal fungi: role in the solubilization of potassium. In: Meena V, Maurya B, Verma J, Meena R (eds) Potassium Solubilizing Microorganisms for Sustainable Agriculture. Springer, New Delhi, pp 77–98
Ahmad H, Hayat S, Ali M, Liu T, Cheng Z (2018) The combination of arbuscular mycorrhizal fungi inoculation (Glomus versiforme) and 28-homobrassinolide spraying intervals improves growth by enhancing photosynthesis, nutrient absorption, and antioxidant system in cucumber (Cucumis sativus L.) under salinity. Ecol Evol 8:5724–5740. https://doi.org/10.1002/ece3.4112
Evelin H, Kapoor R (2013) Arbuscular mycorrhizal symbiosis modulates antioxidant response in salt-stressed Trigonella foenum-graecum plants. Mycorrhiza 24:197–208. https://doi.org/10.1007/s00572-013-0529-4
Rodriguez-Amaya DB (2003) Food carotenoids: analysis, composition and alterations during storage and processing of foods. Forum Nutr 56:35–37
Wang SV, Jiao H (2001) Changes in oxygen-scavenging systems and membrane lipid peroxidation during maturation and ripening in black berry. J Agric Food Chem 49:1612–1619. https://doi.org/10.1021/jf0013757
Bharti N, Barnawal D (2019) Amelioration of salinity stress by PGPR: ACC deaminase and ROS scavenging enzymes activity (Chap. V)). In: Kishore Singh A, Kumar A, Kumar Singh P (eds) PGPR Amelioration in Sustainable Agriculture. Charlotte Cockle Publisher, Chennai, pp 85–106
Lei P, Xu Z, Liang J, Luo X, Zhang Y, Feng X, Xu H (2016) Poly (γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul 78:233–241. https://doi.org/10.1007/s10725-015-0088-0
Kohler J, Hernández JA, Caravaca F, Roldán A (2009) Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ Exp Bot 65:245–252. https://doi.org/10.1016/j.envexpbot.2008.09.008
Bakry AB, Mervat SS, El-karamany MF (2015) Effect of humic acid and sulfur on growth, some biochemical constituents, yield and yield attributes of flax grown under newly reclaimed sandy soils. ARPN J Agric Biol Sci 10:247–259
Lewis JD, Wang XZ, Griffin KL, Tissue DT (2002) Effects of age and ontogeny on photosynthetic responses of a determinate annual plant to elevated CO2 concentrations. Plant Cell Environ 25:359–368. https://doi.org/10.1046/j.0016-8025.2001.00815.x
Urban L, Jegouzo L, Damour G, Vandame M, Francois C (2008) Interpreting the decrease in leaf photosynthesis during flowering in mango. Tree Physiol 28:1025–1036. https://doi.org/10.1093/treephys/28.7.1025
Acknowledgements
We thank Dr. Shahram Manafi, (Department of Soil Science, Urmia University) for his helps to identify soil used in the experiment.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Shabnam Heydari is a PhD student in Urmia University, and Professor Alireza Pirzad (Corresponding author) is supervisor of her thesis.
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest in this manuscript with person and /or institute.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heydari, S., Pirzad, A. Mycorrhizal Fungi and Thiobacillus Co-inoculation Improve the Physiological Indices of Lallemantia iberica Under Salinity Stress. Curr Microbiol 77, 2523–2534 (2020). https://doi.org/10.1007/s00284-020-02034-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02034-y