Abstract
A chloroaniline-degrading bacterial strain isolated from polluted sediment in the Mekong River was identified as Geobacter sp. KT5. The obtained isolate was found to utilize a wide range of trichloroanilines (TCAs), dichloroanilines (DCAs), monochloroanilines (MACs), and aniline as sources of carbon and energy. It also used Fe(III) as a terminal electron acceptor under anaerobic conditions. Among the chlorinated anilines, KT5 utilized 2,3,4-trichloroaniline (234TCA) with the highest rate (2.48 ± 0.32 µM day−1). On determining the degradation pathway for chloroanilines (CAs) in Geobacter sp. KT5, it showed that the removal of ortho and para halogen was dominant. Firstly, KT5 ortho-dechlorinated some TCAs to DCAs, and then reductively transformed them into MACs and aniline prior to complete degradation with the iron reduction stoichiometry and release of nitrogen and chlorine. The KT5 augmentation in sediment slurry enhanced the degradation of CAs and aniline; however, the anaerobic degradation rates in slurry were significantly lower compared to those in liquid media.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CAs are widely used as ingredients in the production of pesticides, pharmaceuticals, rubber, azo dyes, photographic chemicals, varnishes, cosmetics, and other products [2, 4, 26, 28]. They are also the main intermediate degradation products of acetamide and urea herbicides [15]. The widespread use of these chemicals resulted in widespread CAs accumulation in the environment. Soils and sediments are major sinks for organic, hydrophobic pollutants such as halogen aromatic components. The commercial uses of CAs and their potentials as pollutants in the aquatic and terrestrial environment were well recognized [4, 19, 29].
The aerobic microbial catabolism of CAs was well characterized by a number of reports describing the biodegradation of MCAs and DCAs [8, 11, 16, 20, 21, 27, 28]. Moreover, the aerobic degradation pathways for CAs have been elucidated. However, only some studies showed the degradation with the absence of molecular oxygen in liquid media, sediments, or aquifer slurry. The anaerobic biodegradation of CAs was conducted under nitrate-reducing conditions [3, 7, 17, 25], sulfate-reducing activities in sediment slurry [23, 24], and in a methanogenic aquifer [13]. However, CAs degradation in aquifer slurry and sediment by indigenous microorganisms was slow [14, 22,23,24]. Thus, isolating and augmenting pure cultures into contaminated sites should be conducted to enhance remediation rates. Yet, no study on chlorinated-aniline biodegradation has been done by a pure culture in an iron-reducing condition so far. Furthermore, the precise mechanism of anaerobic reductive dechlorination under iron-reducing conditions is still unclear.
This paper describes the bacterial strain KT5 isolated from sediment, which utilized chloro-substituted anilines and aniline under the iron-reducing conditions in liquid media compared to slurry sediment. Moreover, degradation metabolic routes for the isomers were investigated to propose its degradation pathways.
Materials and Methods
Chemicals and Cultivation Media
CAs (99% purity, Chem Service, USA) were dissolved in ethanol (99% purity, Fisher Scientific, USA) as 0.1 M stock solutions prior to use. Mineral salt medium (MM) components were described by Duc [9] with certain modifications, including 1,419.6 mg L−1 Na2HPO4, 1,360.9 mg L−1 KH2PO4, 98.5 mg L−1 MgCl2, 5.88 mg L−1 CaCl2·2H2O, 8.4 mg L−1 NaHCO3, 1.16 mg L−1 H3BO4, 1.15 mg L−1 ZnSO4·7H2O, 0.38 mg L−1 CuSO4·5H2O, and 0.24 mg L−1 CoCl2·6H2O. The medium was added with 500 mg L−1 ammonium chloride and 500 mg L−1 succinate as a nitrogen source and a carbon source, respectively. Media were solidified with 15 g L−1 of agar for cell cultivation.
Enrichment, Isolation, and Identification of CAs-Degrading Bacteria
Several sediment samples (0–10 cm deep) were taken using a cylindrical collector from a site on the Mekong River (10°26′24″N, 105°35′38″E) in Dong Thap Province, South-West of Vietnam, and were transported to the laboratory within a couple of hours under the ambient temperature condition. Sediment samples were then thoroughly mixed and washed through a 1.0-mm-pore-size sieve with site water to remove stones and organic debris. After that, 2 g of the homogeneous sedimentary sample and 50 mL of MM medium were dispensed in a 100-mL serum bottle. Fe3+ (Fe(OH)3) was used as an electron acceptor at 10.0 mM. The bottles were flushed with helium gas for 20 min to create the anaerobic condition. Resazurin (0.4 mM) was used as an indicator to confirm the anaerobic media. These vials were immediately sealed with rubber septa and aluminum crimps. The bottles were incubated in a dark condition, using a shaker at 150 rpm and at room temperature (30 °C) for 6 months. Individual TCAs was added every week at 0.01 mM.
The pure isolate was obtained by serial dilution of the enriched culture and spread onto the solid MM medium supplemented with a TCA (0.05 mM). The plates were incubated at 30 °C in an anaerobic glove box with a pure nitrogen gas headspace. The strain with high effective degradation toward CAs was identified based on 16S rDNA gene sequence according to a previous report [9]. The neighbor-joining distance method and p-distance model in the MEGA 7.0 program was used to construct the phylogenetic tree.
Determination of CAs Anaerobic Utilization by Geobacter sp. KT5 in Liquid Media
The experiments were performed using 60 mL serum vials containing 20 mL of sterile MM medium with the addition of Fe3+ (10.0 mM) under a helium gas headspace. The anaerobic condition was confirmed using the indicator resazurin (0.4 mM). Individual CAs was then supplemented at 0.05 and 0.1 mM. The cells were cultivated in LB broth for 18 h to a turbidity of ~ 1.0 at 600 nm used as the inoculum at 2.0 mL L−1 into the respective fresh media. The vials without electron acceptor or without bacteria served as controls. Syringes and needles were used for substrate addition and sample collection. The incubation was conducted in the same ways as described above. Samples were collected during the incubation to examine the cell growth, residual chemical concentrations, intermediates produced, and the electron acceptor transformation. For the effects of Fe3+ concentrations on degradation, Fe3+ was amended at 0.5 mM, 1.0 mM, and 10.0 mM.
Determination of CAs Anaerobic Utilization by Geobacter sp. KT5 in Sediment Slurry
The chlorinated-aniline degradation in sediment was carried out according to a previous study [10] with some modifications. Sediment samples from the same places mentioned above were used in this experiment. The samples were air-dried within a couple of days at ambient temperature prior to determining the sediment components, while the fresh sample was used as a medium for the biodegradation experiment. The sediment components are shown in Table 1. Sterile pure water was mixed with the sediment to yield slurry containing 10% dry matter, and subsequently transferred into serum vials. Fe3+ (10.0 mM) and CAs (0.05 mM) were added into the culture. Inoculum was also supplemented as described above. The vials were then filled with helium gas, incubated at 150 rpm, and room temperature for 30 days. The chemicals in samples were extracted with acetonitrile twice. The mixture was centrifuged at 10,000 rpm for 5 min. The extract was filtered with a 0.22-µm syringe filter and concentrated. The mean recovery efficiency in the slurry phase was 95.3% of 234TCA, 93.2% of 24DCA, 94.2% of 2CA, and 92.5% of aniline.
Analytical Methods
The CAs concentrations in media were analyzed using reversed-phase high-performance liquid chromatography (HPLC) (LC-10AD, Shimadzu, Japan) with a C18 column (5 µm, 250 mm × 4.6 mm; HyperClone, Phenomenex, USA). Absorbance was measured at 240 nm. Meanwhile, a mixture of acetonitrile and ultrapure water (7:3, v/v) served as mobile phase at a flow rate of 1 mL min−1. The biodegradation intermediates were analyzed by gas chromatography–mass spectrometry [GC/MS using a GC (Agilent 6890N, CA, USA)] equipped with an inert mass selective detector (Agilent 5973, CA, USA). A DB-5 ms capillary column (0.25 mm × 30 m × 0.25 m) with a splitless mode was used with nitrogen as gas carrier at a flow rate of 1.5 mL min−1.
Cell turbidity was determined at 600 nm using a spectrophotometer (DU800, Beckman Coulter, Inc, USA). Fe2+ was measured by the ferrozine assay as described in a previous report [5] using a Cary 50 Bio UV–Vis photometer (Varian, Darmstadt, Germany) at a wavelength of 508 nm. Nitrogen gas sampled from the headspace of bottles with liquid culture medium with a gas-tight syringe was measured using a gas chromatograph (Shimazu GC-14A, Japan). The gas chromatograph was equipped with a ATTM-model sieve plot GC column (30 m × 0.53 mm, Alltech, IL). Both injector and detector were maintained at 80 °C. A thermal conductivity detector was used, and helium served as gas carrier of a 6-mL min−1 flow rate. Cl− in culture media was measured using an ion chromatography (Dionex ICS-90) equipped with an ion column (IonPac AS14A 4 mm × 250 mm). The solution of mixed Na2CO3 (8.0 mM) and NaHCO3 (1.0 mM) was used as a mobile phase with 1.0 mL min−1.
Statistical Analysis
The data are shown as the mean ± standard deviation. Significant differences among means were statistically analyzed using one-way ANOVA with Duncan’s test (Statistical Package for Social Sciences (SPSS) program version 22.0).
Results
Isolation and Identification of the Bacterial Strain
After having been separated and enriched, several bacterial strains which anaerobically utilized CAs and aniline as sole organic substrates were isolated. The strain with the highest degradation rate of TCAs was named KT5. It is an anaerobic, Gram-negative bacterium and is well cultivatable at room temperature. This isolate is rod-shaped, roughly 1.2 µm in length and 0.4 µm in diameter. Its 16S rRNA sequence has 1051 bp and shows the highest degree of nucleotide identity with Geobacter isolates of the sequences available in the NCBI GenBank database. The analysis of 16S rRNA gene sequence homology using BLAST and EzBioCloud showed that the isolate is highly similar to Geobacter sp. BB (KX898554.1; 100% similarity), Geobacter sp. LAR-2 (KC211015.1; > 99% similarity), Geobacter soli GSS01 (JXBL01000001; 99.3% similarity), and Geobacter anodireducens SD-1 (CP014963; 99.3% similarity). The phylogenetic analysis placed it within the sequences in the genus Geobacter (Fig. 1). Accordingly, this strain is referred to Geobacter sp. KT5. The 16S rDNA sequence obtained was deposited in the GenBank under accession number MG984593.1. Meanwhile, the strain KT5 was deposited at the Culture Collection in Center for Biochemical Analysis (Dong Thap University, Viet Nam) under the deposition number DUCOANH2010.
Phylogenetic tree based on 16S rRNA gene fragment shows the strain KT5 position in the genus Geobacter. The tree was constructed using the MEGA version 7.0 software. The numbers at the nodes represent bootstrap values in percentages based on analyzing 1000 resampled data sets. The accession numbers corresponding to each strain are presented in parentheses
Anaerobic Degradation of Chlorinated Anilines by KT5 in Liquid Media
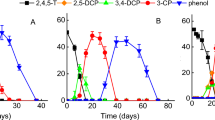
In this study, Geobacter sp. KT5 utilize a broad range of tri-, di-, monochloroanilines and aniline as sole nitrogen, organic carbon, and energy sources under anaerobic conditions (Figs. 2, 3, 4). Its cell growth and utilization of these chemicals occurred at different rates. For CAs with the same numbers in chlorine atoms, Geobacter sp. KT5 grew better in media containing the substrates with higher degradation rates. The degradation rates for 246TCA, 24DCA, and 2CA were similar. On the contrary, for TCAs, the bacteria degraded 235TCA with the lowest rate (Figs. 2, 5). It was also found that adding succinate and ammonium chloride increased the degradation rates of any TCAs by around 20% after 10 days. In addition, 2CA was transformed with a higher rate compared to other MCAs and aniline (Fig. 4). The relative descending order of degradation rates (µM day−1) by the isolate in liquid media was as follows: 234TCA (2.48 ± 0.32), 4DCA (2.19 ± 0.12), 2CA (2.07 ± 0.21), 23DCA (1.91 ± 0.21), 4CA (1.85 ± 0.16), aniline (1.66 ± 0.15), 34DCA (1.57 ± 0.22), and 3CA (1.35 ± 0.12). The effects of co-substrates (succinate and ammonium chloride) on the degradation of MCAs, DCAs, and aniline were also found out. The results showed that the degradation of any CAs was stimulated by the addition of these co-substrates (data were not shown). However, the strain KT5 did not statistically degrade other CAs. The controls in liquid media without bacteria or without the electron acceptor did not reduce any respective substrate concentrations.
The cell growth (a) and MCAs utilization (b) by Geobacter sp. KT5 in MM liquid medium as sources of nitrogen and organic carbon. Individual 2CA (square), 3CA (triangle), 4CA (circle), and aniline (diamond) were added into the culture medium at 0.05 mM. Error bars show the standard error of at least three replicates
The cell growth (a) and TCAs utilization (b) by Geobacter sp. KT5 in the liquid medium with the presence of ammonium chloride and succinate. Individual 234TCA (square), 246TCA (circle), and 235TCA (triangle) were supplemented at 0.05 mM. Error bars show the standard error of at least three replicates
In addition, the cell growth on CAs and the degradation of CAs at 0.1 mM were carried out. However, the degradation of TCAs was completely inhibited, while the degradation rates of other MCAs and DCAs were 20–30% lower than those at 0.05 mM after 20 days (data were not shown).
The Transformation of Electron Acceptor During Degradation of CAs
During the anaerobic degradation process, Fe3+ was simultaneously converted to Fe2+ (Table 2), while Fe3+ transformation was not found in the controls. These results indicated that chloro-substituted anilines and aniline were utilized in relation to Fe3+ reduction. It is supposed that CAs are completely degraded to CO2, the degradation of TCAs, DCAs, MCAs, and aniline could be stated as stoichiometric Eqs. 1, 2, 3, and 4, respectively.
The degradation rates of CAs at 1.0 and 10.0 mM Fe3+ were not statistically different in most treatments, but were significantly higher than those at 0.5 mM Fe3+ (P < 0.05). Although Fe3+ supplemented at 0.5 and 1.0 mM was smaller than theoretical calculation based on the equations, not all Fe3+ concentrations were transformed. From the known amounts of substrates utilized and Fe2+ produced as well as the stoichiometric equations given above, the amount of the electron acceptor transformed was calculated (Table 2). Fe2+ production was from 63.9 to 91.9% of those expected for degradation. In most treatments, the ratios of theoretical amount to measured amount of the electron acceptor were 234TCA < 24DCA ≈ 2CA < aniline. For nitrogen produced, these ratios were from only 42.4–61.8%. The calculation from the Table 2 presented that these ratios of a substrate were not statistically different among trials with different concentrations of Fe3+.
Anaerobic Biodegradation Intermediates, Enzyme Activities, and the Biodegradation Pathways for CAs
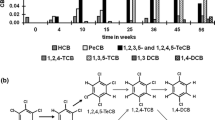
When 234TCA was transformed, some metabolites were produced in liquid media, in which their transient accumulation with peak retention times (RT) was revealed in the HPLC profile (Fig. 6). The first intermediate product with the RT in HPLC of 4.98 min and m/z 161 in GC/MS analyses was identified to be 34DCA. However, 23DCA and 24DCA were not detected as intermediates. Moreover, 2CA was not accumulated during the degradation of 23DCA and 24DCA. Both 3CA and 4CA were detected in the degradation process of 234TCA and 34DCA (Table 3). Aniline (RT 3.4 min and m/z 93) and 4-aminobenzoic acid (RT 2.8 min and m/z 138) were found during any CAs transformation. These results indicated that KT5 anaerobically transformed some TCAs to DCAs, MCAs, and aniline prior to the complete mineralization. The dechlorination process as well as the production of metabolites is also presented in Table 3. Accordingly, the plausible complete mineralization pathway of 234TCA is hypothetically proposed in Fig. 7. The intermediates of CAs anaerobic degradation in slurry were determined, and the metabolites were similar to the results in liquid media.
During some CAs transformations, the released-chlorine amount was lower than expected, based on the equations and the amount of CAs degraded. The ratio of chlorine released to chlorine theoretically produced (calculated based on the amounts of substrates transformed and chlorine atoms remained in metabolites, shown in Table 3) was nearly 100% (data were not shown). This result indicated that the anaerobic dechlorination in CAs degradation was the main route. Meanwhile, chlorine released in the controls without bacteria or without the electron acceptor was negligible.
Anaerobic Degradation of Chlorinated Anilines by KT5 in a Sediment Slurry Culture
Some CAs compounds with higher transformation rates and aniline were selected to determine anaerobic degradation in sediment slurry. Data given in Table 4 presented that the degradation rates in soil slurry inoculated with KT5 were significantly higher than those in non-inoculated media with or without sterilization. Since the dissipation rates in this medium were significantly lower compared to those in liquid medium, the degradation rates were examined after 1 month. Even though the declines of any compound in non-sterile and sterile slurry inoculated with Geobacter sp. KT5 were not statistically different (Table 4), significant concentrations of substrates were lost in the sterile controls without bacteria. Thus, the order of calculated chemical concentrations lost in non-sterile slurry was 234TCA ≈ aniline > 2CA ≈ 24DCA, and the degradation by KT5 was 234TCA > 2CA ≈ 24DCA > aniline.
Discussion
The degradation rates of chlorinated anilines compounds by Geobacter sp. KT5 were likely to depend on the halogen positions rather than on the number of halogen in the molecule. One previous report stated that the ability to degrade chlorinated compounds depends on structure, number of chlorine substituents, and position of chlorine in the molecules [1]. Although KT5 utilized some TCAs with the rates similar to those of DCAs and MCAs, they grew at lower levels in media with TCAs. These phenomena were probably caused by a large amount of TCAs transformed to intermediates and not completely degraded. Moreover, TCAs toxicity might have inhibited the cell growth. On the other hand, bacteria degraded aniline with the rates similar to those in some CAs, but they grew at a higher level, probably because a large amount of aniline was completely degraded to CO2.
In this study, the dechlorination rates were found to adopt the order ortho > para > meta. Similarly, the halogen was easier to be removed from MCAs at the ortho, followed by para and meta positions in anaerobic estuarine sediment with sulfate as an electron acceptor described in previous reports [23, 24]. A number of studies on dechlorination of chlorinated aromatic compounds under anaerobic conditions have been reported. For examples, the degradation pathways for CAs [12, 14, 23, 24] of microbial consortia and other substrates such as 2,4-dichlorophenoxyacetic acid by Thauera sp. DKT [10] based on their metabolites were described. The dechlorination of tetra-, tri-, di-substituted anilines to MCAs by anaerobic microbial metabolism was determined, and MCAs were consistent in the pond sediment and the transformation was not found [14, 22]. The free energy released during the reductive dehalogenation of a polyhalogenated aromatic compound is more negative compared to a monosubstituted halogenated substrate [6], which might preferentially favor aryl halide release from a polyhalogenated aromatic compound. However, this phenomenon was not apparent in KT5. The 234TCA transformation in anaerobic aquifer slurry produced 34DCA and 24DCA with higher concentrations for 34DCA [13], which was different from KT5 in that the isolate transformed 234TCA to only 34DCA.
The first pure culture, Paracoccus sp., had a capacity to degrade some MCAs and DCAs under anaerobic conditions with the simultaneous reduction of nitrate to nitrite [3]. In another report, Paracoccus isolated from soil converted 4CA into triazene with nitrate as an electron acceptor [17]. Rhodococcus sp. strain 2 transformed 34DCA into some intermediates (3,4-dichloroacetanilide, 3,4-dichloro-N-(3,4-dichlorophenyl) benzamide, and 1,2-dichlorobenzene) under nitrate-reducing conditions [25]. However, CAs degradation by these bacterial strains was carried out in media containing co-substrates. Geobacter sp. KT5, on the other hand, transformed CAs via dechlorination, and could utilize CAs as the sole organic growth substrates in anaerobic media.
During 234TCA and 34DCA transformation in liquid media, 3CA accumulated was 3 times as much as 4CA (Table 3) probably because 3CA was more difficult to degrade than 4CA by the bacterial strain. However, 2CA was not detected in the degradation of 234TCA, 23DCA, and 24DCA, illustrating that the bacteria first removed chlorine at ortho position. Meanwhile, 3CA was the most persistent in medium, which was in line with those results in other reports [13, 14, 22,23,24]. The amino group tends to release electrons to aromatic resonance structures making the ring more reactive to electrophilic attack, particularly at the para and the ortho positions [18], which probably contributes to the preferential release of halides attached at these sites.
During the degradation processes, Fe3+ was reduced to Fe2+, and N2 was produced in media. When Fe3+ was supplemented at 0.5 mM, the degradation rates were low due to a shortage of electron acceptor. The amounts of Fe2+ and N2 were smaller than expected for the degradation of any compound, even though Fe3+ was added at 10.0 mM (higher than requirement). These mostly resulted from the fact that CAs and aniline were transformed into intermediates and not completely degraded to CO2. The small amount of Fe3+ transformed during degradation of 234TCA indicated that this substrate was converted to a number of metabolites. The amounts of N2 produced were smaller than those of Fe3+ transformed probably because bacteria used nitrogen for cell synthesis and/or nitrogen was produced under other forms.
The mineralization rates of any CAs and aniline in slurry sediment were slower than the rates in the liquid media. The cell activities in the sediment can be influenced by its physico-chemical properties, such as available nutrients, pH, and other environmental factors. The loss of some chemicals in the sterile controls without bacteria was significant, which did not occur in liquid media. Previous reports showed that the loss of CAs was presumably due to abiotic processes such as sorption to aquifer solids [13, 14, 23, 24].
In the sterile slurry supplemented with Geobacter sp. KT5, aniline was lower disintegrated compared to 234TCA and 2CA; however, the degradation rate for aniline was similar to that of 234TCA and higher than 2CA in non-sterile slurry. The decomposition of CAs and aniline in the non-sterilized sample in most trials was higher than in sterilized sediment. These phenomena were probably due to the activities of native microorganisms. Also, non-sterile sediment inoculated with strain KT5 resulted in higher degradation compared to the sterile sediment in some treatments, indicating that the bacterial strain could well adapt to the complicated sediment media and well cooperate with indigenous microorganisms.
Conclusion
To our knowledge, Geobacter sp. KT5 is the first to demonstrate its anaerobic utilization of some CAs for growth under iron-reducing conditions. The findings presented in this study indicate that Geobacter sp. KT5 transformed TCAs to DCAs, MCAs, and aniline prior to completely degrading them.
Change history
22 March 2019
The original version of this article unfortunately contained a mistake. The authors would like to correct the heading “Anaerobic Biodegradation Intermediates, Enzyme Activities, and the Biodegradation Pathways for CAs” in the Results section. The correct heading should read as “Anaerobic Biodegradation Intermediates and the Biodegradation Pathways for CAs”.
References
Bhatt P, Kumar MS, Mudliar S, Chakrabarti T (2007) Biodegradation of chlorinated compounds—a review. Crit Rev Environ Sci Technol 37:165–198
Boehncke A, Kielhorn J, Konnecker G, Pohlenz-Michel C, Mangelsdorf I (2003) 4-Chloroaniline. Concise International Chemical Assessment Document 48. World Health Organization, Geneva
Bollag JM, Russel S (1976) Aerobic versus anaerobic metabolism of halogenated anilines by a Paracoccus sp. Microb Ecol 3:65–73
Boon N, Goris J, De Vos P, Verstraete W, Top EM (2001) Genetic diversity among 3-chloroaniline- and aniline-degrading strains of the Comamonadaceae. Appl Environ Microbiol 67:1107–1115
Braunschweig J, Bosch J, Heister K, Kuebeck C, Meckenstock RU (2012) Reevaluation of colorimetric iron determination methods commonly used in geomicrobiology. J Mirobiol Methods 89:41–48
Brown JF Jr, Feng H, Bedard DL, Brennan MJ, Carnahan JC, May RJ (1987) Environmental dechlorination of PCBs. Environ Toxicol Chem 6:579–593
Bunce NJ, Merrick RL, Corke CT (1983) Reductive transformations of nitrate with 3,4-dichloroaniline and related compounds by Escherichia coli. J Agric Food Chem 31(5):1071–1075
Duc HD (2016) Biodegradation of 3-chloroaniline by suspended cells and biofilm of Acinetobacter baumannii GFJ1. Appl Biol Chem 59(5):703–709
Duc HD (2017) Degradation of chlorotoluenes by Comamonas testosterone KT5. Appl Biol Chem 60(4):457–465
Ha DD (2018) Anaerobic degradation of 2,4-dichlorophenoxyacetic acid by Thauera sp. DKT Biodegradation 29:499–510
Hongsawat P, Vangnai AS (2011) Biodegradation pathways of chloroanilines by Acinetobacter baylyi strain GFJ2. J Hazard Mater 186:1300–1307
Ismail ZZ, Pavlostathis SG (2010) Influence of sulfate reduction on the microbial dechlorination of pentachloroaniline in a mixed anaerobic culture. Biodegradation 21:43–57
Kuhn EP, Suflita JM (1989) Sequential reductive dehalogenation of chloroanilines by microorganisms from a methanogenic aquifer. Environ Sci Technol 23:848–852
Kuhn EP, Townsend GT, Suflita JM (1990) Effect of sulfate and organic carbon supplements on reductive dehalogenation of chloroanilines in anaerobic aquifer slurries. Appl Environ Microbiol 56:2630–2637
Lacorte S, Perrot MC, Fraisse D, Barcelo D (1999) Determination of chlorobenzidines, in industrial effluent by solid-phase extraction and liquid chromatography with electrochemical and mass spectrometric detection. J Chromatogr A 833:181–194
Loidl M, Ditzelmüller C, Ditzelmüller G, Ferschl A, Streichsbier F (1990) Degradation of aniline and monochlorinated anilines by soil-born Pseudomonas acidovorans strains. Arch Microbiol 155:56–61
Minard RD, Russel S, Bollag JM (1977) Chemical transformation of 4-chloroaniline to a triazene in a bacterial culture medium. J Agric Food Chem 25:841–844
Morrison RT, Boyd RN (1981) Organic chemistry, 3rd edn. Allyn & Bacon, Boston
Piet GJ, Smeenk JGMM (1985) Behavior of organic pollutants in pretreated Rhine water during dune infiltration. In: Ward CH, Giger W, McCarty PL (eds) Ground water quality. Wiley, New York
Reber H, Helm V, Karanth NGK (1979) Comparative studies on the metabolism of aniline and chloroanilines by Pseudomonas multivorans strain An 1. Eur J Appl Microbiol Biotechnol 7:181–189
Schukat B, Janke D, Krebs D, Fritsche W (1983) Cometabolic degradation of 2- and 3-chloroaniline because of glucose metabolism by Rhodococcus sp. An 117. Curr Microbiol 9:81–86
Struijs J, Rogers JE (1989) Reductive dehalogenation of dichloroanilines by anaerobic microorganisms in fresh and dichlorophenol-acclimated pond sediment. Appl Environ Microbiol 55:2527–2531
Susarla S, Yoneza Y, Masunage S (1998) Reductive transformations of halogenated aromatics in anaerobic estuarine sediment: kinetics, products and pathways. Water Res 32(3):639–648
Susarla S, Yonezawa Y, Masunaga S (1997) Reductive dehalogenation of chloroanilines in anaerobic estuarine sediment. Environ Technol 18(1):75–83
Travkin V, Baskunov BP, Golovlev EL, Boersma MG, Boeren S, Vervoort J, van Berkel WJ, Rietjens IM, Golovleva LA (2002) Reductive deamination as a new step in the anaerobic microbial degradation of halogenated anilines. FEMS Microbiol Lett 209:307–312
Yao XF, Khan F, Pandey R, Pandey J, Mourant RG, Guo RKJJH, Russell RJ, Oakeshott JG, Pandey G (2011) Degradation of dichloroaniline isomers by a newly isolated strain Bacillus megaterium IMT21. Microbiology 157:721–726
Zeyer J, Wasserfallen A, Timmis KN (1985) Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol 50:447–453
Zhang LL, He D, Chen JM, Liu Y (2010) Biodegradation of 2-chloroaniline, 3-chloroaniline, and 4-chloroaniline by a novel strain Delftia tsuruhatensis H1. J Hazard Mater 179:875–882
Zoeteman BCJ, Harmsen K, Linders JBHJ, Morra CFH, Slooff W (1980) Persistent organic pollutants in river water and ground water of the Netherlands. Chemosphere 9:231–249
Acknowledgements
The authors are very thankful to the Center for Chemical Analysis, Dong Thap University, for all their supports and encouragements during their working on this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ha Danh, D., Nguyen Thi, O. Anaerobic Degradation of Chloroanilines by Geobacter sp. KT5. Curr Microbiol 76, 248–257 (2019). https://doi.org/10.1007/s00284-018-1617-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-018-1617-7