Abstract
A bacterial strain Acinetobacter sp. WSD with phenanthrene-degrading ability was identified based on biochemical tests and 16S rDNA gene sequence analysis. The strain was isolated from polycyclic aromatic hydrocarbons (PAHs)-contaminated groundwater from a coal-mining area of the Guozhuang karst water system in Shanxi province of northern China. Acinetobacter sp. WSD could utilize fluorine (FLO), phenanthrene (PHE) and pyrene (PYR) as its sole carbon source and was able to degrade other PAHs. Approximately 90 % of FLO, 90 % of PHE and 50 % of PYR were degraded after 6 days’ incubation. The logistic model well fitted all the experimental data. The specific degradation rates in glucose were higher than that in HCO3 −, indicating that organic carbon source promoted the growth of Acinetobacter sp. WSD and the degradation of PAHs. In presence of humic acids (HA), the total degradation rate was accelerated, although there was a delay at the beginning. In the case of high HA concentration, co-metabolism between PAHs and HA occurred. The metabolic pathway for the three compounds of PAHs was discussed using GC–MS data. Phthalic acid was found in their metabolites. Phenol, 2,5-bis(1,1-dimethylethyl), a new type of PAHs metabolites that have been reported before was found after 2 days degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are common environmental pollutants, mainly derived from anthropogenic activities, such as biomass burning, incomplete combustion of fossil fuel (coal and petroleum) and some industrial processes (Li et al. 2014; Mueller et al. 1996), among which coal processing is one of the most important sources. Coaly material enters various environmental compartments from different emission sources and on diverse pathways (Gerslova and Schwarzbauer 2014). PAHs are often highly toxic, mutagenic, and carcinogenic to living organisms (Sato and Aoki 2002). So removal or remediation of these PAHs-contaminated sites has been always a major environmental concern. PAHs release into the environment could be removed through many processes such as photo-degradation, chemical oxidation, biodegradation and adsorption. The principal process for successful removal and elimination of PAHs from the groundwater system is the microbial transformation and degradation (Harayama 1997; Wilson and Jones 1993).
In order to study the fate of PAHs in groundwater, considerable efforts have been focused on the isolation of microorganisms able to degrade PAHs. More than 20 years ago, microorganisms capable of utilizing and degrading hydrocarbons were reported in PAHs-contaminated area (Catallo and Portier 1992). The use of indigenous microflora for bioremediation is of great interest as it is often more cost-effective than commercial inoculums (Grosser et al. 1991). It is therefore of significance and interest to isolate and identify PAHs-degrading microorganisms. A wide phylogenetic diversity of bacterias such as the genera Arthrobacter (Seo et al. 2006), Bacillus cereus (Seo et al. 2006), Pseudomonas (Chávez et al. 2004), Sphingomonas (Madueño et al. 2011), Paenibacillus (Daane et al. 2002) have been reported (Seo et al. 2009).
Acinetobacter have been the subject of great interest due to their efficient capacity to degrade a wide range of alkanes and phenols (Sun et al. 2012). However, little work has been done on PAHs-degradation by Acinetobacter and its biodegradation potential, especially in karst water system.
The concentration range of total PAHs was 515.7–87,881.6 ng/g dry weight in topsoil and 1,639.1–9,036.7 ng/L in groundwater in Guozhuang karst water system of Shanxi Province in northern China (Fig. 1). Coal-washing, coal-burning and charcoal production constitute were the major ways of releasing PAHs to the Carboniferous–Permian coal-bearing strata at Guozhuang (Shao et al. 2014). The soil and groundwater may harbor diverse PAHs-degrading microbial communities and their degradation may have significant impact on the transport of PAHs in the karst aquifers. It is significant to isolate microorganisms involved in performing a complete degradation of PAHs so that potentially toxic compounds and metabolites do not accumulate in groundwater.
The present study therefore aims to investigate (1) the diversity and composition of PAHs-degrading bacterial consortia isolated from karst groundwater system; (2) the degradation ability of the isolated bacterial consortia; (3) the pathway of PAHs-biodegradation by Acinetobacter.
Materials and methods
Chemicals and culture medium
A mixture of 16 PAH stock standards: naphthalene (Nap), acenaphthene (Ace), acenaphthylene (Acy), fluorene (FLO), phenanthrene (PHE), anthracene (Ant), fluoranthene (Fla), pyrene (PYR), benz[a]anthracene (BaA), chrysene (Chry), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), dibenzo[a,h]anthracene (DiA), indeno[1,2,3-cd]pyrene (InP) and benzo[ghi]perylene (BghiP) was purchased from AccuStandara (100 mg/L, USA, solvent: dichloromethane).
The mineral basal medium (MM) was composed of (L−1): Na2HPO4·2H2O 8.5 g, KH2PO4 3.0 g, NaCl 0.5 g, NH4Cl 1.0 g, MgSO4·7H2O 0.5 g, CaCl2 1.5 mg, PHE 100 mg. Soild LB plate was composed of (L−1): 10 g NaCl, 10 g peptone, 5 g yeast extract, 15 g agar, 100 mg PHE.
Isolation of aerobic phenanthrene survivors
Karst groundwater samples were collected from the Guozhuang karst water system of Shanxi Province, northern China, where PAHs-containing burnt gases and wastewater have been continually released into the aquatic environment without any treatment. In the laboratory, 1 mL groundwater sample was inoculated into 10 mL of MM. Then the solution was incubated with shaking at 150 rpm at 33 °C in the dark. About 3 days later, 1 mL culture was transferred to 10 mL of fresh MM and incubated under the same condition. This process was repeated for at least three times. Pure culture was obtained by diluting 1 μL culture to 105 times and spreading each 100 μL of culture on the LB plates. After incubation at 33 °C in the dark for 2–3 days, colonies, especially those forming clear zones on the LB plates, were selected as the candidate PHE-survivors. This process was also repeated for at least three times. All isolates were stored at −20 °C as the liquid cultures containing 20 % glycerol (v/v).
Biodegradation experiments
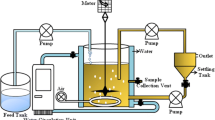
Biodegradation experiments were carried out in a 250 mL Erlenmeyer flask with 100 mL of medium containing 0.1 % (v/v) of n-hexane (used as solvent to prepare the PAHs solution, due to its low solubility in aqueous media). All individual biodegradation experiments were performed with 1 mg/L PHE, 2 mg/L FLO and 0.14 mg/L PYR as the sole carbon and energy source. Capped with cellulose stoppers, was inoculated (5 %) with previously obtained actively growing cells. Cultures were incubated in the darkness for 6 days at 150 rpm in an orbital shaker at 33 °C, initial pH was 7.0. Samples were withdrawn at different times to monitor PAHs concentration and cell density. 10 mL of solution was sampled each time and extracted twice thoroughly with 10 mL of dichloromethane (DCM) (Zhao et al. 2009). The organic phase extraction was combined and anhydrated with anhydrous sodium sulfate. 1 μL of the organic phase was analyzed by gas chromatographic mass spectrometry. The mixture biodegradation experiments have the same compositions as individual experiments.
The experimental process of the effect of environmental medium
The PAHs degradation was also performed with additional carbon source and HA. Glucose and HCO3 − with the concentration of 200 mg/L were added into the MM, respectively. The PAHs concentration was 1 mg/L PHE, 2 mg/L FLO and 0.14 mg/L PYR. The continued experiments were the same as biodegradation processes described above.
The effect of HA to PAHs degradation was performed adding 20 and 40 mg/L HA into the MM, respectively. The biodegradation experiments were the same as the method described above.
Analytical methods
Cell growth determination
Biomass concentration was measured by turbidimetry using UV spectrophotometer (UV-1750, Shimadzu, Japan) at 600 nm. Each value represents the mean of three repeats with a standard deviation less than 5 %.
PAHs analysis
The PAHs concentration was determined by means of gas chromatography (GC 6850, Agilent) equipped with a DB-5 MS capillary column (30 m × 250 μm film thickness × 0.25 mm, Agilent), operating with helium carrier gas, coupled to an Agilent MD 5975 mass spectrometer (MS). The GC injector was operated in splitless mode, 1 μL aliquot was injected using an autosampler. GC oven was programmed to hold 80 °C for 1 min, then elevated by 10 °C/min to 200 °C, which was held for 2 min. The temperature was finally brought up to 290 °C by 4 °C/min, keeping for 10 min. The degradation products of FLO, PHE and PYR were identified by comparison with the NISTS05 database of spectra.
Analysis of 16S rDNA sequences
The isolated strains were identified by 16S rDNA gene sequence after amplification of the gene by PCR using the set of primers 27F (Escherichia coli position 8–27, 5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (Escherichia coli position 1510–1492, 5′-ACG GTT ACC TTG TTA CGA CTT-3′) (Ikenaga et al. 2002). The PCR conditions (30 cycles of 5 min at 94 °C, 30 s at 94 °C, 30 s at 55 °C, 1 min at 72 °C, 10 min at 72 °C) were performed in a PCR thermal cycler (PTC-200 DNA Engine, MJ Research) with Taq DNA polymerase (SuperTherm). DNA sequence of the cloned 16S rDNA fragments was compared using BLASTN at http://www.ncbi.nlm.nih.gov/BLAST/ maintained by National Center of Biotechnology Information (NCBI).
Adsorption test
PAHs adsorption on biomass was determined as follows: 10 mL of culture medium was centrifuged at 8,000 rpm for 5 min. After removing the supernatant, biomass samples were washed and resuspended in n-hexane (1 mL) three times. Then, the PAHs contents were determined by GC–MS using the method described above.
Results and discussion
Isolation of PHE-degrading bacteria
PAHs-contaminated groundwater samples were used for isolating the PHE-degrading bacteria. A PHE-degrading mixed culture was obtained by screening the culture supernatant several times for its growth on the MM with PHE as the sole carbon source. WSD was selected as one of the excellent isolates. Microscopic observation and Gram-staining test indicated that strain WSD was bacteria with rod form and classified as Gram-negative bacteria. The 16S rDNA gene sequence of strain WSD was submitted in the GenBank database. Alignment of the strain indicated that WSD was most closely related to Acinetobacter (99.9 % homology), which was a coco-bacilli, oxidase-negative and catalase-positive bacteria (Narciso-da-Rocha et al. 2013). Therefore, strain WSD was named as Acinetobacter sp. WSD.
Growth and biodegradation assays in flasks
Acinetobacter sp. WSD was tested for its ability to grow on different PAHs-contaminants acting as single carbon source such as low-molecular weight FLO (initial concentration 2 mg/L), and high-molecular weight PHE (1 mg/L), PYR (0.14 mg/L), as well as a mixture of them. When the biomass reached the maximum after culture in the LB for 2–3 days, the bacteria was cleaned using MM three times in order to remove carbon source, and then the culture broth was used as inocula; 500 μL of inocula was transferred into 10 mL MM containing different PAHs. The course of biodegradation of PAHs in the Acinetobacter sp. WSD culture (Fig. 2) shows that the initial PAHs degradation activity was observed once the microorganisms were inoculated. The degradation reached equilibrium after 6 days; more than 90 % of FLO, 90 % of PHE and 50 % of PYR were degraded. Approximately, 12 h lag phase of biodegradation and subsequent high biodegradation rate was observed for each compound, which indicated that an acclimatization process, such as an induction or a de-repression of enzymes or an adaptation to the toxic chemical, occurred and allowed the bacteria to cope with the toxicity of PAHs before further degradation (Hongsawat and Vangnai 2011). Meanwhile, PAHs degradation was associated with the concomitant increase in OD600nm, indicating that strain WSD could utilize FLO, PHE and PYR as sole carbon source for growth. Moreover, the solution was treated with an extracting agent n-hexane, measuring the possible residue of PAHs concentration in the final solution in order to determine PAHs adsorption. The results showed that a maximum of 7.5 % of FLO, 8.5 % of PHE, 5 % of PYR were adsorbed on the biomass. By contrast, Moscoso et al. (2012) found that a maximum of 17 % of PAHs was adsorbed on the biomass in their study, thus proposed that the contaminants had undergone biodegradation.
The logistic Eq. (1) was used to model the obtained data, in which D is the PAHs degradation degree at a specific moment of the time t, D 0 and D max are the initial and maximum degradation percentage, respectively, and μ D is the maximum specific degradation rate (Rosales et al. 2012).
The parameters were obtained by fitting the logistic model. From Table 1, the maximum specific growth rates varied significantly from FLO to PYR, and all of them increased in the second batch after 12 h lag phase. The μ D values were much higher than those reported for bacterial culture of B. pumilus, Dyella ginsengisol and Mycobacterium barrasi (Chang et al. 2008): PHE: 1.04 vs. 0.768 days−1, PYR: 0.95 vs. 0.36 days−1. Moreover, D max values reflected almost complete degradation of FLO and PHE by the strain Acinetobacter sp. WSD, which indicated the biodegradation of the bacteria for low-molecular weight PAHs was relatively strong. By contrast, the D max of PYR was almost 50 %, indicating that high-molecular weight PAHs was hard to be degraded by Acinetobacter.
Using single PAHs is an unrealistic simulation, since they are usually present as multi-component mixtures in natural environment (Chávez et al. 2004). Hence, when a mixture of the three selected PAHs was used as carbon and energy source, different behaviors could be expected. As a matter of fact, higher maximum specific degradation rates and microbial growth occurred under co-metabolic conditions (Fig. 2b and Table 1). A fact that is advantageous in terms of bioprocess economy. However, the maximum degradation percentage basically remained at the same level except PYR (increasing from 50 to 55 % in the mixture). In addition, the reasons for the slight differences detected can also be explained in the light of pH values variation, even though pH was adjusted at the beginning of the experiment (Moscoso et al. 2012). Peng et al. (2008) pointed out that biodegradation of single PAHs in PAHs mixture is strongly influenced by their different bioavailabilities.
The degradation of all PAHs was conducted as the same as individual PAHs biodegradation experiments. Sixteen kinds of PAHs could be degraded at varying degrees by Acinetobacter sp. WSD (Fig. 3). Low molecular weight PAHs such as Nap and Ant were completely degraded after 6 days. For high molecular weight PAHs, the degradation percentage could reach 65 %, indicating that the D max of high molecular weight PAHs were higher under co-metabolic condition. So the existence of multiple PAHs could promote the biodegradation of single PAHs. However, the R 2 value between concentration of total PAHs (∑PAHs) and FLO degradation, ∑PAHs and PHE degradation, ∑PAHs and PYR degradation were 0.34, 0.27, and 0.12, respectively. Biodegradation percentages of individual PAHs were not related to levels of PAHs contamination in aqueous solution. Potin et al. (2004) indicated that the initial of individual PAHs influenced the level of PAHs degradation significantly.
Effect of carbon source on degradation of PAHs
Glucose and HCO3 − were used to investigate the effect of additional carbon source on PAHs degradation, the HCO3 − was the main component of the karst groundwater. The maximum specific degradation rates increased in the addition of external carbon source, although the max degradation percentage of PAHs remained at the same level (Fig. 4). The OD600nm at 2 days was 0.12 in the absence of glucose and 0.21 in its presence, which indicated that supplement of glucose and HCO3 − have caused increase in the growth of bacteria through the supplement of additional carbon source. It was suggested that addition of carbon source as nutrient may enhance the rate of pollutant degradation by stimulating the growth of microorganisms responsible (Haritash and Kaushik 2009).
Significant difference was observed in PAHs degradation percentage in cultures without carbon source addition and with 200 mg/L of glucose or HCO3 −. The results showed that Acinetobacter sp. WSD could use PAHs, glucose and HCO3 − as its carbon source at the same time, and that additional carbon source in the form of glucose or HCO3 − may have increased the metabolism of PAHs in our experiments. This was different from findings of Wong et al. (2002) who reported that supplement of glucose at 450 mg/L had a negative influence on degradation activity of PAHs. It was likely due to the assimilation of glucose prior to PHE, which inhibited the production of relevant enzymes for PAHs degradation. Hence, additional carbon source became a promoting substrate at low supplement rates. At higher concentration, it became a competitive substrate in PAHs degradation. Our findings had important implications for understanding the hydrogeochemical behavior of PAHs in karst water systems, because dissolution or precipitation of carbonate minerals can elevate or attenuate HCO3 − concentration of the groundwater, which may affect the PAHs degradation in the aquifers.
The maximum specific degradation rates in glucose were higher than in HCO3 −, which implied that Acinetobacter sp. WSD more inclined to use organic carbon than inorganic carbon, though the PAHs degradation efficiencies did not show significant difference among them. This was the same with Warskow and Juni (1972) reported that some Acinetobacter grew better in glucose than in inorganic carbon.
11.5 % of FLO, 15.5 % of PHE, 15 % of PYR were adsorbed on the biomass in the presence of glucose, which were higher than in the presence of HCO3 −, indicating that the addition of glucose may have promoted PAHs adsorbed on biomass as reported by Yang et al. (2011). With the addition of glucose, the concentration of organic carbon increased, which could promote the adsorption of PAHs. Also the addition of glucose promoted the growth of the microorganisms, which led to the increase of the amount of adsorption.
Effect of humic acids on degradation of PAHs
The effect of humic acids (HA) on the degradation of PAHs was evaluated. After 6 days, most of FLO and PHE were removed that there was no significant differences among the amounts of degradation at the two different concentrations of HA (Fig. 5a, b). However, the degradation rates of FLO and PHE were stimulated by increasing HA concentrations. HA can promote the growth of microorganisms through providing nutrients (Filip and Kubát 2001). And HA shortened the onset of biodegradation by a carrier effect of HA on PAHs towards degrading bacterial cells, which supplemented the diffusive uptake from the freely dissolved phase. From the standpoint of bioavailability, interaction between microorganisms, HA (low concentration) and PAHs has been reported to increase PAHs bioconcentration (Perminova et al. 2001). It is noteworthy that HA greatly enhanced the ability of Acinetobacter sp. WSD to degrade PYR. The percentage of PYR degradation increased from 50 to 70 % in the presence of HA (Fig. 5c). This enhancement could be explained that the addition HA contains hydroxyl groups, which could be converted to the corresponding polarity matrix under the action of microbes, then affect enzyme activity and promoting the PAHs degradation (Itoh et al. 2000). A previous studies demonstrated that addition of HA enhanced further the rate of PAHs transformation and the amount of biodegradation (Bogan and Sullivan 2003; Mayer et al. 2007; Smith et al. 2009).
HA was obviously consumed in the biodegradation process (Fig. 5d). And higher HA concentration (40 mg/L instead of 20 mg/L) was inhibitory for PAHs degradation rates. The inhibition of PAHs degradation may be attributed to the associated mineral salts that remained after freeze-drying of the HA. The solution could not be dialyzed since HA have molecular weights as low as 500 Da (Chin et al. 1994). HA as the dissolved organic matter was an important part sequestrating the organic pollutants to decrease the availability of PAHs significantly (Nam et al. 1998). And with the increase of the amount of added HA, competitve degradation might occur between HA and PAHs. Therefore, in the real environment, HA will influence the microbial PAHs degradation through the ways including nutrition supports, carrier for PAHs to cells and the effects on the availability through sequestrations. The effect of promoting degradation of PAHs was dominant because of the low concentration of HA in Guozhuang karst water system.
Identification of PAHs degradation pathway
The aforementioned results showed the great potential of Acinetobacter sp. WSD for PAHs degradation. It is therefore worthwhile to identify the metabolic PAHs degradation pathway. Oxygen is a most common electron acceptor in bacterial respiration, also participating in the activation of the substrate via oxygenation reactions (Cao et al. 2009). For the case of Guozhuang karst water system, before their transport into karst aquifer, the PAHs-containing wastewater or gas emissions were first discharged or deposited in the surface water containing free oxygen. Leaking river water then transported PAHs into the karst aquifer (Shao et al. 2014). Thus the initial step may include the oxidation of the benzene ring by mono or dioxygenase enzymes, converting the aromatic compounds to hydroxy aromatic intermediates which are further dehydrogenated to form carbonyl compounds. Besides, this process may contain ortho- or meta-cleavage pathway depending on the intradiol or extradiol ring-cleaving (Moscoso et al. 2012).
Fluorenone and salicylic acid were identified in FLO degradation by Acinetobacter sp. WSD (Fig. 6), indicating that the initial route of FLO degradation was mono-oxygenation. This result matched well with those obtained by Grifoll et al. (1994) who found fluorenone as the main intermediate and protocatechuate as the end product, the toxicity of protocatechuate was relatively weak.
Usually, the existence of mono-oxygenases and dioxygenases favors the oxidative degradation, through two possible degradation pathways: salicylate or protocatechuate. The results of GC–MS analysis demonstrated that Acinetobacter sp. WSD completely degraded PHE via salicylate as has been previously documented (Zhang et al. 2011). In this case, as shown in Fig. 6, the precursor of salicylate and the absence of protocatechuate, suggested degradation route via salicylate instead of protocatechuate.
PYR, a four-ring PAH has been often used as a model high molecule weight PAHs for biodegradation, although its cleavage has not been well understood. With its metabolites such as phenanthrene-4,5-dicarboxylate and salicylate were identified by our GC–MS analysis, it can be proposed that after the initial dioxygenation steps, PYR degradation pathway should be the same as PHE. Kim et al. (2007) found 3,4-dihydroxyphenanthrene and salicylate as metabolic intermediates when studied PYR degradation by Mycobacterium. As a result, the schematic pathway for the degradation of PYR by the Acinetobacter sp. WSD was proposed.
PAHs degradation products usually include alkanes, carbon dioxide and water. It is important to note that after 2 days biodegradation phenol, 2,5-bis(1,1-dimethylethyl) was formed, which has not been reported as a degradation product of PAHs in previous studies. And to the end of the degradation process, its concentration did not decrease, indicating that this compound could not be degraded or utilized by Acinetobacter sp. WSD. Wang et al. (2011) also found phenol, 2,5-bis(1,1-dimethylethyl) in the effluent of coal gasification wastewater after aerobic biodegradability. And in our investigation of the soil and groundwater samples in the karst system in 2013, we also detected phenol, 2,5-bis(1,1-dimethylethyl). However, little has been known about the degradation pathway resulting in its formation, including its toxicity and physicochemical property.
Conclusions
In view of the results obtained in the present study, Acinetobacter sp. WSD isolated from PAHs-contaminated karst groundwater was found to be efficient in the degradation of PAHs. The degradation percentage of low ring PAHs was higher than that of high ring PAHs. Significant increase in PAHs biodegradation was shown by the addition of glucose and bicarbonate, indicating that dissolution or precipitation of carbonate minerals in karst system may affect the degradation. The presence of HA can lead to a direct increase in PYR degradation. Of particular interest was a possible role in the degradation of heavier PAHs with their lower solubilities and higher sorption, on which even lower HA concentrations could have impact. The results of metabolic pathway study help will improve our ability to predict the fate of PAHs compounds in karst groundwater system.
References
Bogan BW, Sullivan WR (2003) Physicochemical soil parameters affecting sequestration and mycobacterial biodegradation of polycyclic aromatic hydrocarbons in soil. Chemosphere 52(10):1717–1726
Cao B, Nagarajan K, Loh K-C (2009) Biodegradation of aromatic compounds: current status and opportunities for biomolecular approaches. Appl Microbiol Biotechnol 85(2):207–228
Catallo W, Portier R (1992) Use of indigenous and adapted microbial assemblages in the removal of organic chemicals from soils and sediments. Water Sci Technol 25(3):229–237
Chang BV, Chang I, Yuan SY (2008) Biodegradation of phenanthrene and pyrene from mangrove sediment in subtropical Taiwan. J Environ Sci Health A 43(3):233–238
Chávez FP, Lünsdorf H, Jerez CA (2004) Growth of polychlorinated-biphenyl-degrading bacteria in the presence of biphenyl and chlorobiphenyls generates oxidative stress and massive accumulation of inorganic polyphosphate. Appl Environ Microbiol 70(5):3064–3072
Chin Y-P, Aiken G, O’Loughlin E (1994) Molecular weight, polydispersity and spectroscopic properties of aquatic humic substances. Environ Sci Technol 28(11):1853–1858
Daane L, Harjono I, Barns S, Launen L, Palleron N, Häggblom M (2002) PAH-degradation by Paenibacillus sp. and description of Paenibacillus naphthalenovorans sp. nov., a naphthalene-degrading bacterium from the rhizosphere of salt marsh plants. Int J Syst Evol Microbiol 52(1):131–139
Filip Z, Kubát J (2001) Microbial utilization and transformation of humic substances extracted from soils of long-term field experiments. Eur J Soil Biol 37(3):167–174
Gerslova E, Schwarzbauer J (2014) Hydrocarbon-based indicators for characterizing potential sources of coal-derived pollution in the vicinity of the Ostrava City. Environ Earth Sci 71(7):3211–3222
Grifoll M, Selifonov S, Chapman PJ (1994) Evidence for a novel pathway in the degradation of fluorene by Pseudomonas sp. strain F274. Appl Environ Microbiol 60(7):2438–2449
Grosser R, Warshawsky D, Vestal JR (1991) Indigenous and enhanced mineralization of pyrene, benzo[a]pyrene, and carbazole in soils. Appl Environ Microbiol 57(12):3462–3469
Harayama S (1997) Polycyclic aromatic hydrocarbon bioremediation design. Curr Opin Biotechnol 8(3):268–273
Haritash A, Kaushik C (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169(1):1–15
Hongsawat P, Vangnai AS (2011) Biodegradation pathways of chloroanilines by Acinetobacter baylyi strain GFJ2. J Hazard Mater 186(2):1300–1307
Ikenaga M, Muraoka Y, Toyota K, Kimura M (2002) Community structure of the microbiota associated with nodal roots of rice plants along with the growth stages: estimation by PCR-RFLP analysis. Biol Fertil Soils 36(6):397–404
Itoh K, Fujita M, Kumano K, Suyama K, Yamamoto H (2000) Phenolic acids affect transformations of chlorophenols by a Coriolus versicolor laccase. Soil Biol Biochem 32(1):85–91
Kim S-J, Kweon O, Jones RC, Freeman JP, Edmondson RD, Cerniglia CE (2007) Complete and integrated pyrene degradation pathway in Mycobacterium vanbaalenii PYR-1 based on systems biology. J Bacteriol 189(2):464–472
Li H, Chen J, Jiang L (2014) Elevated critical micelle concentration in soil–water system and its implication on PAH removal and surfactant selecting. Environ Earth Sci 71(9):3991–3998
Madueño L, Coppotelli B, Alvarez H, Morelli I (2011) Isolation and characterization of indigenous soil bacteria for bioaugmentation of PAH contaminated soil of semiarid Patagonia, Argentina. Int Biodeterior Biodegrad 65(2):345–351
Mayer P, Fernqvist MM, Christensen PS, Karlson U, Trapp S (2007) Enhanced diffusion of polycyclic aromatic hydrocarhons in artificial and natural aqueous solutions. Environ Sci Technol 41(17):6148–6155
Moscoso F, Teijiz I, Deive F, Sanromán M (2012) Efficient PAHs biodegradation by a bacterial consortium at flask and bioreactor scale. Bioresour Technol 119(9):270–276
Mueller JG, Cerniglia C, Pritchard PH (1996) Bioremediation of environments contaminated by polycyclic aromatic hydrocarbons. Biotechnol Res Ser 6:125–194
Nam K, Chung N, Alexander M (1998) Relationship between organic matter content of soil and the sequestration of phenanthrene. Environ Sci Technol 32(23):3785–3788
Narciso-da-Rocha C, Vaz-Moreira I, Svensson-Stadler L, Moore ER, Manaia CM (2013) Diversity and antibiotic resistance of Acinetobacter spp. in water from the source to the tap. Appl Microbiol Biotechnol 97(1):329–340
Peng RH, Xiong AS, Xue Y, Fu XY, Gao F, Zhao W, Tian YS, Yao QH (2008) Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol Rev 32(6):927–955
Perminova IV, Grechishcheva NY, Kovalevskii DV, Kudryavtsev AV, Petrosyan VS, Matorin DN (2001) Quantification and prediction of the detoxifying properties of humic substances related to their chemical binding to polycyclic aromatic hydrocarbons. Environ Sci Technol 35(19):3841–3848
Potin O, Rafin C, Veignie E (2004) Bioremediation of an aged polycyclic aromatic hydrocarbons (PAHs)-contaminated soil by filamentous fungi isolated from the soil. Int Biodeterior Biodegrad 54(1):45–52
Rosales E, Pérez-Paz A, Vázquez X, Pazos M, Sanromán M (2012) Isolation of novel benzo[a]anthracene-degrading microorganisms and continuous bioremediation in an expanded-bed bioreactor. Bioprocess Biosyst Eng 35(5):851–855
Sato H, Aoki Y (2002) Mutagenesis by environmental pollutants and bio-monitoring of environmental mutagens. Curr Drug Metab 3(3):311–319
Seo J-S, Keum Y-S, Hu Y, Lee S-E, Li QX (2006) Phenanthrene degradation in Arthrobacter sp. P1-1: initial 1,2-, 3,4- and 9,10-dioxygenation, and meta- and ortho-cleavages of naphthalene-1,2-diol after its formation from naphthalene-1,2-dicarboxylic acid and hydroxyl naphthoic acids. Chemosphere 65(11):2388–2394
Seo J-S, Keum Y-S, Li QX (2009) Bacterial degradation of aromatic compounds. Int J Environ Res Public Health 6(1):278–309
Shao YX, Wang YX, Xu XQ, Wu X, Jiang Z, He SS, Qian K (2014) Occurrence and source apportionment of PAHs in highly vulnerable karst system. Sci Total Environ 490:153–160
Smith KEC, Thullner M, Wick LY, Harms H (2009) Sorption to humic acids enhances polycyclic aromatic hydrocarbon biodegradation. Environ Sci Technol 43(19):7205–7211
Sun J-Q, Xu L, Tang Y-Q, Chen F-M, Wu X-L (2012) Simultaneous degradation of phenol and n-hexadecane by Acinetobacter strains. Bioresour Technol 123(11):664–668
Wang W, Han H, Yuan M, Li H, Fang F, Wang K (2011) Treatment of coal gasification wastewater by a two-continuous UASB system with step-feed for COD and phenols removal. Bioresour Technol 102(9):5454–5460
Warskow AL, Juni E (1972) Nutritional requirements of Acinetobacter strains isolated from soil, water, and sewage. J Bacteriol 112(2):1014–1016
Wilson SC, Jones KC (1993) Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): a review. Environ Pollut 81(3):229–249
Wong J, Lai K, Wan C, Ma K, Fang M (2002) Isolation and optimization of PAH-degradative bacteria from contaminated soil for PAHs bioremediation. Water Air Soil Pollut 139(1–4):1–13
Yang X-P, Wang S-M, Zhang D-W, Zhou L-X (2011) Isolation and nitrogen removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Bacillus subtilis A1. Bioresour Technol 102(2):854–862
Zhang S, Wang Q, Xie S (2011) Microbial community changes in contaminated soils in response to phenanthrene amendment. Int J Environ Sci Technol 8(2):321–330
Zhao H-P, Wu Q-S, Wang L, Zhao X-T, Gao H-W (2009) Degradation of phenanthrene by bacterial strain isolated from soil in oil refinery fields in Shanghai China. J Hazard Mater 164(2):863–869
Acknowledgments
The research work was financially supported by National Natural Science Foundation of China (No. 40902071, and No. 41120124003), the Ministry of Science and Technology of China (2012AA062602), and the Ministry of Education of China (111 project and Priority Development Projects of SRFDP (20120145130001)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shao, Y., Wang, Y., Wu, X. et al. Biodegradation of PAHs by Acinetobacter isolated from karst groundwater in a coal-mining area. Environ Earth Sci 73, 7479–7488 (2015). https://doi.org/10.1007/s12665-014-3920-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3920-3