Abstract
Two odorless, water-soluble exopolysaccharide (EPS) fractions, EPS-1 and EPS-2, were isolated from a newly isolated Bacillus amyloliquefaciens strain C-1 and purified by ion exchange and gel chromatography. The purified EPS-1 contained glucose/mannose/galactose/arabinose in a relative proportion of 15:4:2:1, and possessed a molecular weight of 79.6 kDa, while EPS-2 contained only glucose and mannose in a 3:1 ratio, with the molecular weights of 19.8 kDa. The antioxidant activity results showed that EPS-1 exhibited strong reducing power, superoxide radicals (O2−·), and hydroxyl free radicals (OH·) scavenging activities. For the H2O2-induced injury in HepG2 cells, EPS-1 significantly decreased the formation of reactive oxygen species, intracellular malondialdehyde levels, and restored intracellular superoxide dismutase activity. For EPS-2, there had no detectable antioxidant activities. And all these results collectively showed that as a natural antioxidant, only EPS-1 produced by C-1 had considerable potential to be used as medical compounds or functional additives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress, induced by oxygen radicals, is considered as a primary factor in various degenerative diseases, including cancer, atherosclerosis, hyperlipidemia, and diabetes [25]. Reactive oxygen species (ROS) and oxygen-derived free radicals, generated by normal metabolic processes or from exogenous factors and agents, may contribute to a variety of pathological effects, including DNA damage, carcinogenesis, and cellular degeneration [21]. Most organisms possess antioxidant defense and repair systems but they are insufficient to prevent DNA or proteins damage. Live cells protect themselves from oxidative damage through several defense mechanisms such as the enzymatic conversion of ROS into less toxic substances or through the antioxidant process [7]. Many synthetic compounds, such as butyl hydroxyl anisd (BHA), butylated hydroxytoluene (BHT), and tertiary butylhydroquinone (TBHQ), are commonly used as antioxidant in processed foods. However, synthetic antioxidants are being restricted due to their side effects such as carcinogenicity [12]. With these safety concerns, the increasing interest has heightened in finding naturally occurring antioxidants to be used in foods or medicinal materials, which had the capacity to improve food quality, terminate free radical chain reactions in biological systems, and provide additional health benefits to consumers [2].
As an important class of bio-active natural polymers, polysaccharides have been considered to be promising antioxidants and candidates of effective, nontoxic medicines and food additives in vitro and in vivo [32]. Exopolysaccharide (EPSs) is one type of metabolite in many microorganisms. They are usually biocompatible, edible, and nontoxic to humans and the environment [22]. Recently, there has been an increased interest in exploiting the EPSs for their biological activities including antitumor, immunostimulatory, cholesterol-lowering activity, and antioxidant activities [7, 31]. For example, cell-bond exopolysaccharide (cb-EPS) from Lactobacillus acidophilus can inhibit the proliferation of HT-29 colon cancer cells, and it possesses great pro-apoptotic activity in oncotherapy and adjuvant therapy [13]. In this current study, we found that fermentation solution produced by Bacillus amyloliquefaciens strain C-1 which was isolated from ready-to-eat sliced apple samples had significant antioxidant activities. To elucidate the mechanism of the active component contributed to the antioxidation, two novel water-soluble EPSs produced by C-1 were purified and characterized, and their antioxidant activities were investigated using various assays.
Materials and Methods
Strains and Cells
Bacillus amyloliquefaciens strain C-1 (16S rRNA accession no. JX028840 in GenBank) was isolated from ready-to-eat sliced apple samples by the Food Microbiology Lab of the Nutrition and Food Safety Engineering Research Center of Shaanxi Province, Xi’an, China (the China Center for Type Culture Collection, CCTCCM2012177). HepG2 cells, a human hepatoblastoma cell line, were cultured in high glucose Dulbecco’s Modified Eagle’s Medium supplemented with 100 U/ml penicillin, 100 U/ml streptomycin, and 10 % fetal bovine serum (FBS), and kept at 37 °C in a humidified atmosphere of 5 % CO2. All cells were plated in cell culture flasks at least 24 h before treatment.
Morphological Properties and 16S rRNA Gene Sequence Analysis of Strain C-1
Strain C-1 was cultured in LB (Luria–Bertani) medium with 1 % glucose (pH 7.5). The cell morphological feature was observed by scanning electron microscopy (Hitachi, TM-1000) after growth for 60 h.
The 16S rRNA sequence was used for the identification of C-1. Primers used were: 16S-F: 5′AGAGTTTGATCCTGGCTCAG3′, 16S-R: 5′GGTACCT TGTTACGACTT3′ [11]. The PCR fragments were analyzed by gel electrophoresis, then purified and sequenced by Sangon Biotech (Shanghai, CN) Co, Ltd. The 16S rRNA sequence was used for the identification of C-1. Multiple alignments with sequences of most close similarity were analyzed using CLUSTAL W, and the phylogenetic tree was constructed by using the neighbor-joining method [9].
EPS Isolation and Purification
A 2 % (v/v) inoculum of B. amyloliquefaciens C-1 culture was sub-cultured and grown for 72 h at 30 °C. The fermented culture supernatant was concentrated, and crude EPS was precipitated by addition of 2 volumes of pre-cooled 95 % ethanol at 4 °C [30]. Crude EPS was collected and the pellet was dissolved in deionized water, and deproteinized with Sevag reagent and dialyzed. The dialyzed solution was lyophilized for further EPSs purification. The amount of crude EPSs was estimated calorimetrically by phenol–sulfuric acid method [8].
The freeze-dried sample was fractionated with an anion-exchange chromatography on the DEAE-Cellulose column (2.6 × 30 cm) (Whatman, USA), eluted with a step gradient of NaCl solution (0–1.0 M). Based on the chromatogram detected by the phenol sulfuric acid method, the EPS was resolved into two major peaks and fractions containing polysaccharides were pooled, dialyzed, and lyophilized. Further purification of EPS was performed by gel filtration using a sepharose CL-6B column (2.6 × 100 cm) (Amersham Pharmacia Biotech, Sweden) eluted with 0.9 % (w/v) NaCl. The major polysaccharide fraction was pooled, dialyzed with water, and freeze-dried.
Molecular Mass Determination of EPSs
The average molecular weight of each purified EPS was measured by gel permeation chromatography. Standard dextrans (500, 110, 70, 40, and 10 kDa, Pharmacia, USA) were passed through a sepharose CL-6B column (1.6 × 100 cm) and eluted with 0.9 % (w/v) NaCl at a flow rate of 0.67 ml per min. The elution volumes were plotted against the logarithms of their respective molecular weights. Purified EPSs were dissolved in 0.15 M NaCl and applied to the same column equilibrated.
Monosaccharide Composition of EPS
Five milligram of EPSs was hydrolyzed with 2 ml of 2 mol/l trifluoroacetic acid (TFA) at 120 °C for 2 h. The hydrolyzate was co-concentrated repeatedly with methanol to dryness, reduced with NaBH4 for 30 min at 20 °C, and acetylated with acetic anhydride and pyridine at 100 °C for 20 min. The standard sugars were prepared in the same way. The alditol acetates of EPSs were analyzed on Agilent Technologies 7890A GC equipped with flame ionization detector (FID) and a HP-5 fused silica capillary column (30 × 0.32 × 0.25 mm). The nitrogen gas was used as the carrier gas at a flow rate of 1 ml/min. The column temperature was kept at 120 °C for 2 min and then increased to 250 °C for 3 min at a rate of 8 °C per min [23]. These assays were repeated in triplicates.
Assay of Antioxidant Activity In Vitro
Determination of Reducing Power
The reducing power of EPS-1 and EPS-2 was determined by the modified method from [14]. The reaction mixture, which included 1 ml EPS at different concentrations (0.15, 0.30, 0.6, 1.25, 2.5, and 5 mg/ml), 2.5 ml of 0.2 M sodium phosphate buffer (pH 6.6), and 2.5 ml of K3Fe(CN)6 (1 %, v/v), was incubated at 50 °C for 20 min before the addition of 2.5 ml of 10 % trichloroacetic acid (TCA); A 2.5 ml volume of the supernatant was mixed with 0.5 ml of fresh FeCl3 and 2.5 ml of sterile H2O. After 5-min incubation, the absorbance at 700 nm was measured. Vitamin C (Vc) was used as a positive control. Higher absorbance values indicate greater reducing ability.
Assay of Superoxide Radical Scavenging Activity
Superoxide radicals were generated in the system of pyrogallol autoxidation in an alkalescent condition [20]. A 4.5-ml volume of 0.05 M Tris–HCl (pH 8.2) was incubated at 25 °C for 25 min, then 1 ml of EPS-1 and EPS-2 at different concentrations (0.15, 0.30, 0.6, 1.25, 2.5, and 5 mg/ml) were added along with 0.4 ml of 2.5 mmol pyrogallol, mixed, and reacted for 5 min. Finally, the absorbance of reaction mixture at 299 nm was measured. Vitamin C (Vc) was used as a positive control, and H2O was used as a negative control. The superoxide radical scavenging effect (%) was calculated: (A0–A1)/A0 × 100 %, where A0 is OD299 of negative control and A1 is OD299 of treated EPS.
Assay of Hydroxyl Radical Scavenging Activity
Hydroxyl radicals were generated in the H2O2–FeSO4 system and were assayed by the salicylic acid method. A 2 ml of EPS-1 and EPS-2 with different concentrations (0.15, 0.30, 0.6, 1.25, 2.5, and 5 mg/ml) were mixed with 2 ml of 6 mmol/l FeSO4 and 2 ml of 6 mmol/l H2O2. The change in absorbance caused by the color change of salicylic acid was measured at 510 nm. Vitamin C (Vc) was used as a positive control, and H2O was used as a negative control. The hydroxyl radical scavenging effect (%) was calculated: (A0–A1)/A0 × 100 %, where A0 is OD510 of negative control and A1 is OD510 of treated EPS.
Measurement of Intracellular ROS in HepG2 Cells
Intracellular ROS was estimated with a fluorescent probe, DCFH-DA [1]. The HepG2 cells were pretreated with EPS-1 and EPS-2 with final concentrations of 100 μg/ml for 12 h. Then, hydrogen peroxide (H2O2) at the final concentration of 100 μmol/l was added to each well at 0, 2, and 4 h. After incubation, cells were incubated with DCFH-DA (10 μmol/l) diluted in serum-free culture medium for 20 min at 37 °C. Imaging in live cells was performed in cover glass with an inverted confocal microscope with a 10 × objective (LSM510 NLO, Zeiss). The fluorescence was induced with excitation at 485 nm and monitored at 520 nm.
Effect of EPSs on Malondialdehyde (MDA) and Speroxide Dismutase (SOD) Activity in H2O2-Induced HepG2 Cells
After pretreatment with EPS-1 and EPS-2 for 12 h, HepG2 cells were suspended in an appropriate volume of lysis buffer (50 mmol/l Tris–HCl, pH 8.0, 50 mmol/l EDTANa2, 0.2 mol/l NaCl, 1 % Triton X-100), and the yielded cell homogenate was immediately centrifuged at 10,000g for 15 min 4 °C. The supernatant was stored at −20 °C prior to the assays. SOD activity and MDA were determined spectrophotometrically using commercially available assay kits (Nanjing Jiancheng, CN) as described previously [17].
Statistical Analysis
The data were analyzed by ANOVA, and P < 0.05 was selected prior to the experiments to reflect statistical significance. Unless otherwise stated, all results are expressed as the mean ± SD (n ≥ 3). All the analyses were conducted using the General Linear Model (GLM) procedure of SAS Version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results and Discussion
Characterization and Identification of Isolated Strain C-1
Observed under SEM, strain C-1 is a rod-shaped bacterium (0.6–0.8 μm in diameter and 2 μm in length, Fig. 1a). And the colonies showed sticky, translucent, white, and mucoid appearance (5 mm in diameter after 3 days on LB medium with 1 % glucose at 30 °C). The strain grew aerobically from 25 to 50 °C and its optimal temperature occurred at 30 °C. Comparison of 16S rRNA gene sequence among C-1 (1433 bp, accession no. JX028840) and other 7 bacterials from GenBank by BLAST analysis, the result showed the closest strains (99 % similarity) were B. amyloliquefaciens BFE 5335 (GU250447), B. amyloliquefaciens BFE 5359 (GU250449), and B. amyloliquefaciens M4 (JX036449). From the N-J tree (Fig. 1b), strain C-1 was identified as B. amyloliquefaciens.
Scanning electron micrograph (a) of C-1 grown on LB medium with 1 % glucose at 30 °C for 72 h. Bar, 10 μm; magnification, ×10,000; neighbor-joining tree showing the phylogenetic relationships of B. amyloliquefaciens C-1 to other Bacillus species (based on the 16S rRNA sequence) analyzed by CLUSTAL W. Sequence accession number in GenBank: B. amyloliquefaciens M4 (JX036449), B. amyloliquefaciens BFE5335 (GU250447), B. amyloliquefaciens BFE 5359 (GU250449), B. licheniformis MX5 (JX027378), B. cereus BFE5392 (GU250443), E. coli WP3 (JQ993870), S. aureus subsp. anaerobius (D83355)
Kinetics of Growth and Isolation of EPSs
As a major class of natural products, metabolites from microorganisms are one of the most reproducible, dependable, and stable source in development of food additives, bio-drugs, bio-active compounds. Among Bacillus species, strain of B. amyloliquefaciens is widely distributed in soil and considered as a useful bacterium in industry processes, as it is able to produce enzyme proteins and other useful products. The sticky characteristics of C-1 colonies indicated that B. amyloliquefaciens C-1 was able to produce metabolites consisting mainly of EPSs. The EPS produced by C-1 during the whole fermentation period is illustrated in Fig. 2a. This strain exhibited an exponential growth after incubation for 12 h and then entered stationary phase at 28 h, with cells having optical density at 600 nm of approximately 3.65. The EPS production increased gradually along with bacterial growth in LB medium with 1 % glucose (initial pH 7.5, 30 °C). The maximum OD600 of C-1 culture growth was 3.65 at 28 h, while the maximum EPS production was 121 mg/l at 60 h, which was the late stationary growth with OD600 of 2.76. It agrees with the point that EPS-producing microorganisms usually reach their optimal growth within the initial 24 h of incubation, whereas maximal EPS production occurs in later stages of growth [20]. Then the amount of EPS decreased probably due to the action of glycohydrolases in the culture that catalyzed the degradation of polysaccharides [8].
Kinetics of growth and EPS production (a) of B. amyloliquefaciens C-1 in batch cultures showing the bacterial cell counts (filled circle) and amounts of EPS produced (filled triangle). Each value represents the average of triplicate measurements; chromatography of eluted crude EPS on DEAE-cellulose column (b)
Isolation and Purification of EPSs
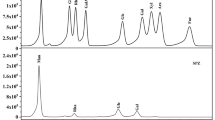
Under optimal condition of growth, crude EPS was isolated from fermentation broth of B. amyloliquefaciens C-1 by ethanol precipitation, deproteinization, dialyzation, and dryness. The extraction yield of crude EPS was approximately 12.1 % of the whole fermentation broth. The crude EPS was purified using anion-exchange chromatography with NaCl (0–1.0 M) gradient elution (Fig. 2b). This elution profile of crude EPS showed two relatively symmetrical peaks, indicating their homogeneity. It is shown that this crude EPS has two major water-soluble polysaccharides, named EPS-1 (tube49–82) and EPS-2 (tube91–109), and the extraction yields of the two components were 40.2 and 33.9 %, respectively. At the same time, absorbance at 280 nm was also determined, but no significant absorbance was shown, indicating these EPSs were free of any peptide chains.
Each polysaccharide was further purified through sepharose CL-6B column and eluted with 0.9 % NaCl (Fig. 3a, b). It showed that EPS-1 and EPS-2 only had a single peak profile, indicating a purified polysaccharide. Extraction yields of EPS-1 and EPS-2 reached up to 57.1 and 62.5 %, respectively. The fraction was collected separately, dialyzed, and lyophilized for further characterization.
Molecular Weight (MW) and Monosaccharide Compositions of EPS-1 and EPS-2
To characterize the structures of the EPS-1 and EPS-2, the apparent average molecular weight of EPS-1 and EPS-2 were determined by gel permeation chromatography as shown in Fig. 4. The calibration curve for MW determination was made using a series of β-glucan standards: lg MW = −0.0203x + 6.7953 (r 2 = 0.9913), where x is elution volume. Based on the calibration curve, the MW of EPS-1 and EPS-2 were 79.6 and 19.8 kDa, respectively. The monosaccharide compositions of EPS-1 and EPS-2 were analyzed by gas chromatography, and the result was shown in Table 1. It revealed that the monosaccharide contents were different for the two polysaccharides. In general, glucose was the most abundant monosaccharide in both EPS-1 and EPS-2. EPS-1 was comprised of d-glucose, d-mannose, d-galactose, and d-arabinose in the molar ratio of 15:4:2:1, respectively. Several reports explained that galactose and arabinose are associated with the antioxidant activities of polysaccharides [5, 28]. However, the EPS-2 was comprised of d-glucose and d-mannose in a 3:1 ratio. It may be an important factor of EPS-2 that EPS-2 would have different antioxidant activity compared with EPS-1 in our further assays. EPS from Streptococcus macedonicus Sc136 was composed of d-glucose, d-galactose, and N-acetyl-d-glucosamine [27]. Another EPS produced from V. harveyi strain VB23 was composed primarily of d-galactose and d-glucose [4]. All the results indicated that the monosaccharide compositions were quite different from different bacterial cultures.
Antioxidant Activity of EPSs In Vitro
For measuring antioxidant property of EPS-1 and EPS-2 from strain C-1, different methods including reductive power assay, superoxide radicals, and hydroxyl radical scavenging assays have been used corresponding to different levels of antioxidant action. The reducing power of a compound may serve as a significant indicator of its potential antioxidant activity [14]. As shown in Fig. 5a, EPS-2 showed no obvious reductive ability when compared with Vc. While the EPS-1 showed significant reducing power in dose-dependent manner, suggesting a high potential in hydrogen-donating ability which could react with free radicals to convert them into more stable products and thereby terminate radical chain reactions [10]. However, none of the EPS-1 samples had a reductive activity approaching that of the same concentration of Vc. At 5 mg/ml EPS-1, its reducing power (0.82) was less than half of that of Vc (2.1).
Superoxide radicals are harmful free radicals for cellular components, and the presence of superoxide anions can magnify cellular damages, as they produce other types of free radicals and oxidizing agents [3]. Therefore, the superoxide radical scavenging ability is of great importance to its potential antioxidant activity. The scavenging activities of superoxide radical by EPSs from strain C-1 are shown to be dose-dependent in Fig. 5b. The superoxide radical scavenging effect of C-1 EPS-1 ranged from 2.4 % at 0.15 mg/ml to 30.8 % at 5 mg/ml, which was similar to that of Cordyceps militaris SU5-08, Bacillus edudis [16]. And for EPS-2, little superoxide radicals scavenging activity was found. And the scavenging effect ranged from 1.9 % at 0.15 mg/ml to 8.5 % at 5 mg/ml, respectively. It has been reported that the mechanism of superoxide anion scavenging may be associated with the dissociation energy of O–H bonds [24].
Hydroxyl free radicals and their derivative radicals are highly potent oxidants, which can react with most biomacromolecules in living cells and induce severe biological damage and lipid peroxidation. As shown in Fig. 5b, the EPS-1 had an obvious hydroxyl radical scavenging activity from 6 % increased to 60.4 % in a dose-dependent manner, which was higher than that of 50.8 % for Boletus edudis, 49.4 % for Pholiota adipose, 26.2 % for Antrodia camphorate at 5 mg/ml, respectively [16]. It has been proposed that the hydrogen or electron abstraction mechanism might be the best explanation of why polysaccharides can inhibit the formation of hydroxyl radicals [6, 26]. While for EPS-2, those scavenging activity was less than 10 % of EPS-1 activity at the same concentration.
Multiple mechanisms account for the antioxidant activity of different compounds, including prevention of chain initiation, binding to transition metal ion catalysts, decomposition of peroxides, and prevention of scavenging ability [17]. Among the biological characteristics and functions studied above, the results revealed that although there were 2 EPS fractions purified from C-1 culture, the functional EPS was only EPS-1, which exhibited good antioxidant activity in vitro for superoxide radicals, hydroxyl radical scavenging activity, and reductive power (Fig. 5). It might be attributed to the functional groups in the EPS fraction, which can donate electrons to reduce the radicals to a more stable form or react with the free radicals to terminate the radical chain reaction [15]. These characteristics are similar to those of EPS fractions from Bifidobacterium animalis [29] and Paenibacillus polymyxa [18]. For EPS-2, the function was still unknown. Maybe it contributes to the anti-tumor activity, immunostimulatory, anti-microbial activity which was shown in crude C-1 EPS [19].
Effects of EPSs on Intracellular ROS, MDA and SOD in H2O2-Treated HepG2 Cells
According to the results above, the effects of antioxidant activities of EPS-1 exhibit strong free radical scavenging effects, whereas EPS-2 nearly had no antioxidant activities. In order to investigate in-depth the antioxidant activities of the EPS-1 and EPS-2, protective effects of EPS-1 and EPS-2 on HepG2 cells against H2O2 injury were evaluated. Figure 6a showed that pretreated cells with EPS-1 for 2 and 4 h significantly decreased the fluorescence of ROS induced by H2O2. Particularly, the decreased fluorescence with increased time indicated that the inhibition of ROS by EPS-1 is time-dependent. Compared with the EPS-1, EPS-2 nearly did not inhibit the formation of intracellular ROS (P > 0.01). MDA is often used as a marker of the lipid peroxidation being consequently an indicator of oxidative damage in cell membranes. As shown in Fig. 6b, when treated with 500 μg/ml EPS-1, the intracellular MDA levels were significantly decreased (P < 0.05) compared with those cells treated by H2O2, indicating that EPS-1 could significantly improve the antioxidant status by resisting the lipid peroxidation in H2O2-treated HepG2 cells. However, no significant differences in the ability of EPS-2 (500 μg/ml) to inhibit MDA (P > 0.01) were observed. Finally, the effects of EPS-1 and EPS-2 on the H2O2-induced changes of SOD activity in HepG2 cells were also investigated. As shown in Fig. 6c, H2O2 treatment decreased intracellular SOD activity compared with control cells (P < 0.05); however, pretreatments of EPS-1 for 12 h could restore SOD activity of H2O2-treated HUVECs. In contrast, the EPS-2 showed the similar decresed level of SOD activities as the H2O2 treated control. These findings further indicated that only EPS-1 could significantly improve the antioxidant status in H2O2-treated HepG2 cells, which is consistent with the antioxidant activities such as reducing ability and scavenging superoxide radical ability. Many EPSs were reported to be potential antioxidants secreted by bacteria and fungus. For example, EPS from mycelial culture of Cordyceps sinensis fungus Cs-HK1 displayed moderate antioxidant activities with a trolox equivalent antioxidant capacity and a ferric reducing ability of plasma [15]. EPS from L. plantarum C88 inhibited the formation of MDA and raised the activities of SOD and total antioxidant capacities (T-AOC) in Caco-2 cells [33]. But it is currently unclear how polysaccharides affect the intracellular antioxidant system. EPS-1 responded to H2O2-induced oxidative stress by increasing enzymatic (such as SOD) and non-enzymatic (such as MDA) intracellular antioxidant defenses, and it probably could be the mechanism of the protective effect of EPS-1 produced by B. amyloliquefaciens C-1 on H2O2-induced oxidative damage in HepG2 cells. It may protect bacterial cells from desiccation, bacteriophage attack, and phagocytosis. The great differences existed in antioxidant activities of EPS-1 and EPS-2 possibly depended on their monosaccharide composition, type of linkages and chain conformation. Therefore, the beneficial health effects of EPS-1 produced by B. amyloliquefaciens C-1 might be associated with its prominent antioxidant activities.
Antioxidant activities assays in HepG2 cells which was treated with EPS-1 and EPS-2 of B. amyloliquefaciens C-1. ROS production determined by confocal microscope using the dichlorofluorescein (DCF) (magnification, ×100) (a), SOD activity analysis (b), and MDA analysis (c) in HepG2 cells with H2O2-stimulation. Results are presented as means ± SDs
Conclusion
In summary, EPS-1 and EPS-2 were isolated with high purity and identified as typical polysaccharides from B. amyloliquefaciens C-1. EPS-1 was composed of glucose, mannose, galactose, and arabinose in a ratio of 15:4:2:1 with molecular weight of 79.6 kDa, while EPS-2 was composed of glucose and mannose in a ratio of 3:1 with molecular weight of 19.8 kDa. EPS-1 exhibited strong antioxidant activity by quenching hydroxyl and superoxide anion radicals in vitro and in HepG2 cells against H2O2-induced injury. However, no significant antioxidant activities of EPS-2 were observed. Therefore, it is suggested that EPS-1, but not EPS-2 might provide a source of natural antioxidants with potential value for functional foods or therapeutics. Further works should be done for detailed structural characterization of EPSs and the corresponding relationships between their structures and functionalities.
References
Aharoni-Simon Reifen R, Tirosh O (2006) ROS-production-mediated activation of AP-1 but not NFkappaB inhibits glutamate-induced HT4 neuronal cell death. Antioxid Redox Sig 8:1339–1349
Ananthi S, Raghavendran HRB, Sunil AG et al (2010) In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem Toxicol 48:187–192
Athukorala Y, Kim KN, Jeon YJ (2006) Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol 44:1065–1074
Bramhachari PV, Dubey SK (2006) Isolation and characterization of exopolysaccharide produced by Vibrio harveyi strain VB23. Lett Appl Microbiol 43:571–577
Capek P, Machova E, Turjan J (2009) Scavenging and antioxidant activities of immunomodulating polysaccharides isolated from Salvia officinalis L. Int J Food Microbiol 44:75–80
Chen H, Wang Z, Qu Z et al (2009) Physicochemical characterization and antioxidant activity of a polysaccharide isolated from oolong tea. Eur Food Res Technol 229:629–635
Chen H, Yan X, Zhu P et al (2006) Antioxidant activity and hepatoprotective potential of agaro-oligosaccharides in vitro and in vivo. Nutr J 5:31–41
Degeest B, Mozzi F, De Vuyst L (2002) Effect of medium composition and temperature and pH changes on exopolysaccharide yields and stability during Streptococcus thermophilus LY03 fermentations. Int J Food Microbiol 79:161–174
Han B, Liu HZ, Hu XM et al (2006) Preliminary characterization of a thermostable DNA polymerase I from a mesophilic Bacillus sphaericus strain C3-41. Arch Microbiol 186:203–209
He J, Ru Q, Dong D et al (2012) Chemical characteristics and antioxidant properties of crude water soluble polysaccharides from four common edible mushrooms. Molecules 17:4373–4387
Kaci Y, Heyraud A, Barakat M et al (2005) Isolation and identification of an EPS-producing Rhizobium strain from arid soil (algeria): characterization of its EPS and the effect of inoculation on wheat rhizosphere soil structure. Res Microbiol 156:522–531
Kanmani P, Satish KR, Yuvaraj N et al (2011) Production and purification of a novel exopolysaccharide from lactic acid bacterium Streptococcus phocae P180 and its functional characteristics activity in vitro. Bioresour Technol 102:4827–4833
Kim Y, Oh S, Yun HS et al (2010) Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumor cells. Lett Appl Microbiol 51:123–130
Kumar CG, Joo HS, Choi JW et al (2004) Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzyme Microb Technol 34:673–681
Leung PH, Zhao S, Ho KP et al (2009) Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem 114:1251–1256
Lin RS, Liu HH, Wu SQ et al (2012) Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int J Biol Macromol 51:153–157
Liu C, Chang J, Zhang L et al (2012) Purification and antioxidant activity of a polysaccharide from bulbs of Fritillaria ussuriensis Maxim. Int J Biol Macromol 50:1075–1080
Liu J, Luo JG, Ye H et al (2012) Preparation, antioxidant and antitumor activities in vitro of different derivatives of levan from endophytic bacterium Paenibacillus polymyxa EJS-3. Food Chem Toxicol 50:767–772
Luo WJ, Wan Y, Zhang RJ et al (2014) Evaluation the antitumor effects of exopolysaccharide produced by newly isolated Bacillus amyloliquenfaciens strain C-1. J Pure Appl Microbiol 8:469–474
Raza W, Makeen K, Wang Y et al (2011) Optimization, purification, characterization and antioxidant activity of an extracellular polysaccharide produced by Paenibacillus polymyxa SQR-21. Bioresour Technol 102:6095–6103
Seifried HE, Anderson DE, Fisher EI et al (2007) A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Bichem 18:567–579
Shih IL (2010) Microbial exo-polysaccharides for biomedical applications. Mini Rev Med Chem 10:1345–1355
Sun Y, Wang S, Li T et al (2008) Purification, structure and immunobiological activity of a new water-soluble polysaccharide from the mycelium of Polyporus albicans (Imaz.) Teng. Bioresour Technol 99:900–904
Tsiapali E, Whaley S, Kalbfleisch J et al (2001) Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic Bio Med 30:393–402
Valko M, Leibfritz D, Moncol J et al (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Valko M, Rhodes CJ, Moncol J et al (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Vincent SJ, Faber EJ, Neeser JR et al (2001) Structure and properties of the exopolysaccharide produced by Streptococcus macedonicus Sc136. Glycobiology 11:131–139
Wang M, Zhu P, Jiang C et al (2012) Preliminary characterization, antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis. Food Chem Toxicol 50:2964–2970
Xu RH, Shang N, Li PL (2011) In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 17:226–231
Ye SH, Liu F, Wang JH et al (2012) Antioxidant activities of an exopolysaccharide isolated and purified from marine Pseudomons PF-6. Carbohyd Polym 87:764–770
Yoo SH, Yoon EJ, Cha J et al (2004) Antitumor activity of levan polysaccharides from selected microorganisms. Int J Biol Macromol 34:37–41
Zhang L, Liu C, Li D et al (2013) Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int J Biol Macromol 54:270–275
Zhang Z, Teruya K, Eto H et al (2013) Induction of apoptosis by low-molecular-weight fucoidan through calcium- and caspase-dependent mitochondrial pathways in MDA-MB-231 breast cancer cells. Biosci Biotech Bioch 77:235–242
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (31200031), the Fundamental Research Funds for the Central Universities (xjj2012125), Open Project Program of the State Key Laboratory of Food Science and Technology, Nanchang University (SKLF-KF-201201), and China Postdoctoral Science Foundation funded Project (2013M532055).
Conflict of interest
The authors declare that they have no competing interests to this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Deng, J., Yuan, Y. et al. Two Novel Exopolysaccharides from Bacillus amyloliquefaciens C-1: Antioxidation and Effect on Oxidative Stress. Curr Microbiol 70, 298–306 (2015). https://doi.org/10.1007/s00284-014-0717-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0717-2