Abstract
Background
Amrubicin and cisplatin is one of the active regimens used to treat patients with extensive-disease (ED)-small cell lung cancer (SCLC), whereas combined therapy involving chemotherapy and concurrent thoracic radiotherapy is the standard treatment for limited-disease (LD)-SCLC.
Purpose
This study aimed to determine the maximum tolerated dose (MTD) and dose-limiting toxicities (DLT) of amrubicin and cisplatin with concurrent thoracic radiotherapy (TRT) for LD-SCLC.
Patients and methods
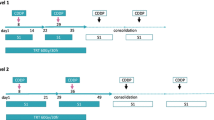
Patients that fulfilled the following eligibility criteria were enrolled: being aged ≤ 75 years and chemotherapy-naïve and having a performance status (PS) of 0–1, LD-SCLC, and adequate organ function. The patients received escalating doses of amrubicin on days 1, 2, and 3, and a fixed 60-mg/m2 dose of cisplatin on day 1. Four cycles of chemotherapy were administered, with each cycle lasting 4 weeks. TRT involving 2 Gy/day, once daily, commenced on day 2 of the first cycle of chemotherapy. The initial dose of amrubicin was 20 mg/m2 (level 1), and the dose was escalated to 25 mg/m2 (level 2) and then 30 mg/m2 (level 3).
Results
Eight patients from three institutions were enrolled at three dose levels. The patients’ characteristics were as follows: male/female: 3/5; median age (range): 68.5 (60–73); PS 0/1: 4/4; stage IIIA/IIIB disease: 3/5. Both level 3 patients experienced DLT (grade 4 neutropenia and/or leukopenia lasting > 4 days). Level 3 was defined as the MTD, and level 2 was recommended as the dose for this regimen. Seven patients exhibited partial responses, and 1 displayed progressive disease (response rate: 88%). The median progression-free survival and overall survival periods were 11.1 and 39.5 months, respectively. No treatment-related deaths occurred.
Conclusions
When this regimen was combined with TRT for LD-SCLC, the MTD was 30 mg/m2 for amrubicin and 60 mg/m2 for cisplatin. In addition, neutropenia and leukopenia were DLT, and doses of 25 mg/m2 for amrubicin and 60 mg/m2 for cisplatin are recommended for this regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer deaths worldwide. It is also the leading cause of cancer deaths in Japan, with an estimated 77,500 deaths from lung cancer occurring in 2018 (20.4% of all cancer deaths) [1]. Small cell lung cancer (SCLC) accounts for one-tenth of such deaths, and one-third of SCLC patients present with limited disease (LD), i.e., disease that is confined to a single radiation port within the chest. Thoracic radiotherapy (TRT) improves the local control rate of LC-SCLC by 25%, and combining chemotherapy with TRT has been shown to improve the survival of LD-SCLC patients compared with chemotherapy alone [2, 3]. Etoposide and cisplatin (EP) has been the standard first-line chemotherapy regimen worldwide for SCLC for the past 30 years [4], and TRT has been used in an early concurrent twice-daily schedule during four cycles of EP [5]. Although our previous study of irinotecan plus cisplatin (IP) with concurrent TRT demonstrated promising survival data, high incident rate of pneumonitis was problem [6].
Amrubicin is a chemically synthesized anthracycline compound, which exerts an antitumor effect by being converted to an active metabolite (amrubicinol). Amrubicinol inhibits cell proliferation 5–54 times more strongly than amrubicin [7]. Amrubicin inhibits cell proliferation by inhibiting topoisomerase II, which leads to increased DNA cleavage due to the stabilization of the cleavable complex [8]. Amrubicin monotherapy had a marked effect on patients with untreated extensive-disease (ED)-SCLC, in which it produced a 75.8% response rate and a median survival time of 11.7 months [9]. Furthermore, it achieved a response rate of 87.8% and a median survival time of 13.6 months when it was used in combination with cisplatin [10]. Since ED-SCLC patients treated with IP exhibited a response rate of 84.4% and a median survival time of 12.8 months, respectively, and those treated with EP therapy displayed a response rate of 67.5% and a median survival time of 9.4 months [11], the combination of amrubicin plus cisplatin (AP) is considered to be a potentially useful treatment for LD-SCLC.
Therefore, we planned a dose escalation phase I trial of AP combined with TRT for previously untreated LD-SCLC. As no regimen involving twice-daily irradiation combined with third-generation platinum doublet chemotherapy has been established, we decided to employ once-daily concurrent radiotherapy.
Patients and methods
The study protocol was reviewed and approved by the Nagasaki Thoracic Oncology Group (NTOG), and the ethics committee of each institution. Written informed consent was obtained from all study participants. This study was an independent collaborative (unsponsored) group study. It is registered at the University Hospital Medical Information Network (UMIN) in Japan (Registration Number: UMIN000005816).
Study design and patients
The patient eligibility criteria for this study were as follows: (1) a histologically and/or cytologically confirmed diagnosis of SCLC; (2) not having received any prior treatment for SCLC; (3) having LD, which was defined as disease limited in scope to one hemithorax according to positron emission tomography, with or without regional metastases, including ipsilateral hilar, bilateral mediastinal, or bilateral supraclavicular lymph node metastases; (4) not having any synchronous active malignancies; (5) being aged ≤ 75 years; (6) having an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1; (7) exhibiting adequate organ function (a leukocyte count of ≥ 4000/µl, a hemoglobin level of ≥ 10.0 g/dl, a platelet count ≥ 10.0 × 104/µl, a serum total bilirubin of ≤ 1.5 mg/dl, hepatic transaminase levels of ≤ 2× the upper normal limit, and a serum creatinine level of ≤ the upper normal limit); (8) having consulted with radiotherapy physicians and having been judged to have appropriate lung function levels that can be maintained even after radiotherapy prior to registration; and (9) providing written informed consent. The exclusion criteria were as follows: (1) being classified with stage IA disease that was eligible for lobectomy; (2) exhibiting pericardial effusion with subjective symptoms; (3) displaying pleural effusion or malignant pleural effusion that was confirmed on a chest X-ray; (4) requiring emergency radiotherapy due to symptoms of superior vena cava syndrome; (5) having severe heart disease; i.e., (i) a history of uncontrolled angina, (ii) having suffered a myocardial infarction in the previous 3 months, or (iii) suffering heart failure; (6) having uncontrolled hypertension or diabetes mellitus; (7) having a severe infection; (8) being pregnant; or (9) experiencing complications that it was considered would interfere with the implementation of therapy.
Treatment and study design
The patients received escalating doses of amrubicin on days 1, 2, and 3, and a fixed dose of 60 mg/m2 of cisplatin on day 1. Four cycles of chemotherapy were administered, with each cycle lasting 4 weeks. TRT, involving 2 Gy/day, once daily, was started on day 2 (26 Gy/13 fractions [fr]) and day 30 (24 Gy/12 fr). The initial dose of amrubicin was 20 mg/m2 (level 1), and the dose was escalated to 25 mg/m2 (level 2) and then 30 mg/m2 (level 3). This study aimed to determine the maximum tolerated dose (MTD) and dose-limiting toxicities (DLT) of AP with concurrent TRT for LD-SCLC. DLT were defined as any of the following that occurred during the first 28 days of treatment: grade 3/4 non-hematological toxicities; grade 4 thrombocytopenia; grade 4 neutropenia or leukopenia that lasted for > 4 days; febrile neutropenia; or the second cycle not starting by day 35. For the dose escalation, 3 patients were enrolled at each level, and the dose was escalated to the next level if none of the patients experienced DLT. If ≥ 2 patients experienced DLT, the dose level was defined as the MTD. If 1 of the 3 patients experienced DLT, an additional 3 patients were treated at the same level. If none of the additional patients experienced DLT, the dose was escalated to the next level. If ≥ 1 of the additional patients experienced DLT, the dose level was defined as the MTD. The recommended dose for this regimen was defined as the level below the MTD.
Response and toxicity evaluations
Screening was conducted within the 28 days before the first dose of the study drug was administered. During the treatment period, a physical examination, a complete blood count, blood chemistry tests, and chest radiography were performed once a week. Toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (version 4.0). Radiographic tumor assessments were conducted at the screening and every 4 weeks after the initiation of the study treatment, as per the RECIST guidelines, version 1.0.
Results
Patient enrollment
Eight patients from 3 institutions were enrolled in this trial between April 2011 and January 2014. All the patients who received the planned treatment underwent evaluations of toxicities, the response to treatment, and survival. The patients’ baseline characteristics are shown in Table 1. The patients ranged in age from 60 to 73 years (median age 68.5 years), and 5 of the patients (63%) were female.
Treatment administration
A total of 34 cycles of this treatment were administered (median: 4 cycles per patient): 4 cycles were administered to 7 patients (88%), and 6 cycles were administered to 1 patient (13%). Three patients were treated at dose levels 1 and 2, and 2 patients were treated at dose level 3.
Dose escalation
At levels 1 and 2, none of the 3 patients experienced DLT. The first patient treated at level 3 experienced grade 4 neutropenia lasting ≥ 4 days, grade 4 leukopenia lasting ≥ 4 days, and grade 4 thrombocytopenia. The second patient treated at level 3 experienced grade 4 neutropenia lasting ≥ 4 days. Both of these patients experienced DLT; therefore, level 3 was considered to be too toxic and was determined to be the MTD. Thus, level 2 (25 mg/m2 amrubicin and 60 mg/m2 cisplatin) was regarded as the recommended dose level for this regimen.
Toxicities
The worst grades of hematological and non-hematological toxicities experienced by each patient are listed in Tables 2 and 3. Leukopenia and neutropenia were the principal toxicities. Grade 3–4 leukopenia and neutropenia were noted in 7 (88%) and 6 (75%) patients, respectively, which led to grade 3 febrile neutropenia in 1 (13%) patient. No grade ≥ 3 non-hematological toxicities were observed. All cases of pneumonitis were considered to be radiation induced, rather than drug induced. There were no cases of ≥ grade 3 pneumonitis or treatment-related deaths.
Efficacy
The treatment response was assessable in all eight patients. Objective tumor responses were observed in seven patients, giving an overall response rate of 88% (95% confidence interval, 47–100%). At levels 1 and 2, all three patients exhibited partial responses. At level 3, one of two patients displayed partial responses, and the other patient had progressive disease. In May 2019, two patients were still alive, and the other six patients had died. The median progression-free survival (PFS) period was 11.1 months (Fig. 1) and the median overall survival (OS) period was 39.5 months (Fig. 2).
Discussion
The present study is the first prospective study to evaluate the tolerability and MTD of AP with concurrent thoracic radiotherapy for patients with LD-SCLC. Level 3 (30 mg/m2 amrubicin and 60 mg/m2 cisplatin) was determined to be the MTD, and level 2 (25 mg/m2 amrubicin and 60 mg/m2 cisplatin) was regarded as the recommended dose for this regimen.
AP was compared with IP for ED-SCLC in a phase III trial and was demonstrated to be inferior to IP (median OS period: 15.0 months [AP] vs. 17.7 months [IP]). Therefore, IP continues to be the standard first-line therapy for ED-SCLC in Japan [12]. On the other hand, AP was shown to not be inferior to EP, i.e., it prolonged the OS period by 1.5 months, and became a treatment option for ED-SCLC in China [13]. Since IP failed as a treatment for LD-SCLC [5] and seems to be incompatible with radiotherapy, AP is a candidate chemotherapy regimen for LD-SCLC. Actually, the administration of one cycle of EP in combination with concurrent thoracic radiotherapy, followed by 3 cycles of AP achieved a promising 5-year OS rate of 57.8% in a feasibility study [14].
Regarding the toxicities encountered in the current study, leukopenia and neutropenia were both seen in all patients, and grade 3–4 leukopenia and neutropenia occurred in 7 (88%) and 6 (75%) patients, respectively. At levels 1 and 2, grade 4 leukopenia and neutropenia were seen in 1 (17%) and 3 (50%) patients, respectively, but all of them recovered within 3 days, and none of them developed febrile neutropenia. At level 3, both patients experienced severe myelosuppression and DLT, and this level was considered to be the MTD. As the most common severe toxicity associated with amrubicin was myelosuppression [15], attention must be paid to leukopenia and neutropenia in patients treated with this drug. As for non-hematological toxicities, 7 patients experienced radiation-induced pneumonitis, but no patients developed drug-induced pneumonitis. It is important to note that no treatment-related deaths or cases of severe pneumonitis were observed in the present study, and the AP chemotherapy regimen seemed to exhibit good compatibility with radiotherapy among LD-SCLC patients. There were also no treatment-related deaths in the abovementioned feasibility study of chemoradiotherapy followed by AP [14].
Regarding the radiotherapy protocol employed in the present study, we selected once-daily radiotherapy because no standard concurrent twice-daily regimen involving the administration of third-generation platinum doublet chemotherapy at 45 Gy/30 fr has been established. Turrisi et al. demonstrated the superiority of twice-daily radiotherapy over once-daily radiotherapy as a treatment for LD-SCLC. However, a total dose of 45 Gy was adopted in the once-daily arm, and grade 3 esophagitis was significantly more common in the twice-daily arm [16]. Therefore, a phase 3 trial, the CONVERT study, was designed to demonstrate the superiority of 45 Gy twice-daily radiotherapy over once-daily radiotherapy involving a higher dose of 66 Gy in the setting of concurrent chemotherapy [17]. Although no differences in survival outcomes were detected between the two groups, the median OS period of the twice-daily group was longer than that of the once-daily group (30 vs. 25 months), and twice-daily radiotherapy continues to be considered the standard protocol. As a good response rate (88%) was observed in the present study, but none of the patients achieved complete remission, the use of twice-daily radiotherapy might improve the outcomes of such treatment for LD-SCLC.
As for the recommended dose of amrubicin that should be used in combination with a fixed dose of 60 mg/m2 cisplatin, 40 mg/m2 was adopted in a phase I–II study [10]. However, because of the high incidence of severe hematological toxicities, the dose of amrubicin was reduced to 35 mg/m2 in a subsequent phase III study [12]. In another phase III study, 40 mg/m2 amrubicin and 60 mg/m2 cisplatin were adopted, but 3 (2%) patients suffered treatment-related deaths, mainly due to myelosuppression [13]. Thus, 35 mg/m2 amrubicin and 60 mg/m2 cisplatin seems to be optimal regimen for treating SCLC with chemotherapy alone. As chemotherapy involving a third-generation cytotoxic agent plus cisplatin combined with concurrent thoracic radiotherapy requires about a 20% reduction in the dose of the third-generation cytotoxic agent compared with that used for chemotherapy alone [18], and a fixed dose of 60 mg/m2 cisplatin, it seems reasonable that the recommended dose of amrubicin was determined to 25 mg/m2 in the present study.
In conclusion, the use of the AP regimen in combination with TRT to treat LD-SCLC was examined. As a result, the MTD was determined to be 30 mg/m2 for amrubicin and 60 mg/m2 for cisplatin, neutropenia and leukopenia were identified as DLT, and doses of 25 mg/m2 for amrubicin and 60 mg/m2 for cisplatin were recommended.
References
National Cancer Center Japan, Center for Cancer Control and Information Services (2018). Available from http://ganjoho.jp/reg_stat/statistics/stat/short_pred.html. Cited 25 March 2018
Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B, Onoshi T, Osterlind K, Tattersall MHN, Wagner H (1992) A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327:1618–1624
Warde P, Payne D (1992) Dose thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 10:890–895
Hanna N, Bunn PA Jr, Langer C, Einhorn L, Guthrie T Jr, Beck T, Ansari R, Ellis P, Byrne M, Morrison M, Hariharan S, Wang B, Sandler A (2006) Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 24:2038–2043
Kubota K, Hida T, Ishikura S, Mizusawa J, Nishio M, Kawahara M, Yokoyama A, Imamura F, Takeda K, Negoro S, Harada M, Okamoto H, Yamamoto N, Shinkai T, Sakai H, Matsui K, Nakagawa K, Shibata T, Saijo N, Tamura T, Japan Clinical Oncology Group (2014) Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): a randomized phase 3 study. Lancet Oncol 15:106–113
Fukuda M, Nakamura Y, Kinoshita A, Soejima Y, Yamaguchi H, Ikeda T, Izumikawa K, Takatani H, Fukuda M, Soda H, Hayashi N, Tsukamoto K, Oka M, Kohno S (2012) Phase II study of irinotecan and cisplatin with concurrent split-course radiotherapy in limited-disease small cell lung cancer. Cancer Chemother Pharmacol 70:645–651
Yamaoka T, Hanada M, Ichii S, Morisada S, Noguchi T, Yanagi Y (1998) Cytotoxicity of amrubicin, a novel 9-aminoanthracycline, and its active metabolite amrubicinol on human tumor cells. Jpn J Cancer Res 89:1067–1073
Hanada M, Mizuno S, Fukushima A, Saito Y, Noguchi T, Yamaoka T (1998) A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II-DNA complex. Jpn J Cancer Res 89:1229–1238
Yana T, Negoro S, Takada M, Yokota S, Takada Y, Sugiura T, Yamamoto H, Sawa T, Kawahara M, Katakami N, Ariyoshi Y, Fukuoka M (2007) Phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan Thoracic Oncology Group (WJTOG) study. Invest New Drugs 25:253–258
Ohe Y, Negoro S, Matsui K, Nakagawa K, Sugiura T, Takada Y, Nishiwaki Y, Yokota S, Kawahara M, Saijo N, Fukuoka M, Ariyoshi Y (2005) Phase I-II study of amrubicin and cisplatin in previously untreated patients with extensive-stage small-cell lung cancer. Ann Oncol 16:430–436
Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N, Japan Clinical Oncology Group (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85–91
Satouchi M, Kotani Y, Shibata T, Ando M, Nakagawa K, Yamamoto N, Ichinose Y, Ohe Y, Nishio M, Hida T, Takeda K, Kimura T, Minato K, Yokoyama A, Atagi S, Fukuda H, Tamura T, Saijo N (2014) Phase III study comparing amrubicin plus cisplatin with irinotecan plus cisplatin in the treatment of extensive-disease small-cell lung cancer: JCOG0509. J Clin Oncol 32:1262–1268
Sun Y, Cheng Y, Hao X, Wang J, Hu C, Han B, Liu X, Zhang L, Wan H, Xia Z, Liu Y, Li W, Hou M, Zhang H, Xiu Q, Zhu Y, Feng J, Qin S, Luo X (2016) Randomized phase III trial of amrubicin/cisplatin versus etoposide/cisplatin as first-line treatment for extensive small-cell lung cancer. BMC Cancer 16:265–272
Sekine I, Sumi M, Satouchi M, Tsujino K, Nishio M, Kozuka T, Niho S, Yamamoto N, Harada H, Ishikura S, Tamura T (2016) Feasibility study of chemoradiotherapy followed by amrubicin and cisplatin for limited-disease small cell lung cancer. Cancer Sci 107:315–319
Ogawara D, Fukuda M, Nakamura Y, Kohno S (2010) Efficacy and safety of amrubicin hydrochloride for treatment of relapsed small cell lung cancer. Cancer Manag Res 2:191–195
Turrisi AT 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, Wagner H, Aisner S, Johnson DH (1999) Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265–271
Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, Bezjak A, Cardenal F, Fournel P, Harden S, Le Pechoux C, McMenemin R, Mohammed N, O’Brien M, Pantarotto J, Surmont V, Van Meerbeeck JP, Woll PJ, Lorigan P, Blackhall F, CONVERT Study Team (2017) Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomized, superiority trial. Lancet Oncol 18:1116–1125
Fukuda M, Soda H, Fukuda M, Kinoshita A, Nakamura Y, Nagashima S, Takatani H, Tsukamoto K, Kohno S, Oka M (2007) Irinotecan and cisplatin with concurrent split-course radiotherapy in locally advanced non-small cell lung cancer: a multiinstitutional phase 2 study. Cancer 110:606–613
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shimada, M., Yamaguchi, H., Fukuda, M. et al. Dose escalation study of amrubicin and cisplatin with concurrent thoracic radiotherapy for limited-disease small cell lung cancer. Cancer Chemother Pharmacol 84, 1059–1064 (2019). https://doi.org/10.1007/s00280-019-03940-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03940-0