Abstract
Background
Irinotecan and cisplatin are one of active regimens for patients with extensive-stage small cell lung cancer (SCLC). To determine the efficacy and toxicity of irinotecan and cisplatin with concurrent split-course thoracic radiotherapy in limited-disease (LD) SCLC, we conducted a phase II study.
Patients and methods
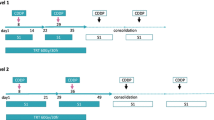
Thirty-four patients fulfilling the following eligibility criteria were enrolled: chemotherapy-naïve, good performance status (PS 0–1), age ≤75, LD-SCLC, and adequate organ function. The patients received irinotecan 40 mg/m2 i.v. on days 1, 8, and 15, and cisplatin 60 mg/m2 i.v. on day 1. Four cycles of chemotherapy were repeated every 4 weeks. Split-course thoracic radiotherapy of once-daily 2 Gy/day commenced on day 2 of each chemotherapy cycle, with 26 and 24 Gy administered in the first and second cycles, respectively.
Results
Thirty-four patients were eligible and assessable for response, toxicity, and survival. Patients’ characteristics were as follows: male/female = 29/5; PS 0/1 = 18/16; median age (range) = 67 (50–73); and stage IB/IIA/IIB/IIIA/IIIB = 2/2/3/16/11. The overall response was 100 % (CR 8, PR 26). Grade 4 leukopenia, neutropenia, grade 3–5 pneumonitis, diarrhea, and esophagitis occurred in 24, 38, 6, 3, and 0 %, respectively. There were 2 treatment-related deaths from pneumonitis. The median time to tumor progression was 14.3 months. The median overall survival time and the 2- and 5-year survival rates were 44.5 months, 66.7 and 46.1 %, respectively. No tumor progression was observed in patients with CR.
Conclusion
Irinotecan plus cisplatin with concurrent split-course thoracic radiotherapy was effective and tolerable in untreated LD-SCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2009, lung cancer was the world’s leading cause of cancer mortality [1] and 67,853 people died in Japan. Small cell lung cancer (SCLC) accounts for 11–13 % of lung cancer, and systemic chemotherapy is the mainstay of treatment. One-third of SCLC patients present with limited disease (LD), which is confined to the chest within a single radiation port. Thoracic radiotherapy (TRT) improves the local control rate by 25 %, and the combined modality of chemotherapy and TRT has been shown to improve the survival of LD-SCLC in comparison with chemotherapy alone [2, 3]. Etoposide and cisplatin (EP) have been remained the first-line standard chemotherapy regimen worldwide for SCLC over the past 30 years [4], and TRT has been used in an early concurrent schedule during four cycles of EP [5]. In addition, twice-daily treatment beginning with the first cycle of chemotherapy significantly improved survival as compared with concurrent once-daily radiotherapy [6]. Because of its inconvenience and 45 Gy on the once-daily arm corresponds to a lower biologic effective dose, once-daily TRT also remains the standard radiotherapy. On the basis of these results, EP with concurrent TRT is currently the standard care for LD-SCLC [7]; however, median survival times are only 20–27 months and the 5-year survival rate is <30 %.
Irinotecan hydrochloride is a water-soluble prodrug that is metabolized to the active metabolite SN-38, which inhibits the function of DNA topoisomerase I in cancer cells [8]. Clinical studies of irinotecan alone have shown a broad spectrum of antitumor activity against various human cancers including SCLC [9], and preclinical studies have demonstrated synergism and non-cross-resistance between the combination of cisplatin with SN-38 and irinotecan [10, 11]. A clinical trial with irinotecan plus cisplatin (IP) yielded good response rate (86 %) and median survival (13.0 months) in extensive disease (ED) SCLC [12]. A previous trial of IP with concurrent and continuous standard TRT failed in non-small-cell lung cancer (NSCLC) patients because of unacceptable toxicity and a low completion rate [13]. However, we demonstrated that split TRT method with IP was feasible in NSCLC and SCLC [14, 15].
Accordingly, we conducted a phase II trial of IP with concurrent split-course and once-daily TRT based on our phase I trial [15]. The main objectives of the trial were to evaluate the efficacy and safety of IP with TRT therapy in previously untreated patients with LD-SCLC.
Patients and methods
Study design
This was a phase II study involving 7 centers. The study protocol was approved by the institutional review board or ethics committee at each center, and patients provided written informed consent. The study was carried out in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines. This study was an independent collaborative (unsponsored) group study.
Inclusion/exclusion criteria
Eligibility criteria for patients in this study included the following: a histologically confirmed diagnosis of LD-SCLC, no prior chemotherapy or radiotherapy, age ≤75 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤1, life expectancy greater than 12 weeks, adequate bone marrow function (leukocyte count ≥4,000/μL, platelet count ≥10 × 104/μL, and hemoglobin level ≥10 g/dL); serum bilirubin level ≤1.5 mg/dL, ALT and AST levels ≤2 times the normal upper limit, serum creatinine level ≤1.5 mg/dL and PaO2 ≥70 mmHg, and no medical problems severe enough to prevent compliance with the protocol. LD was defined as disease confined to one hemithorax, with or without ipsilateral hilar or bilateral mediastinal or supraclavicular lymph node involvement; no malignant pleural or pericardial effusion. Exclusion criteria included active infection, uncontrolled heart disease, interstitial pneumonia/active lung fibrosis on chest X-ray, and active concomitant malignancy.
Treatment
Four cycles of IP therapy were repeated at 4-week intervals. Patients received 40 mg/m2 irinotecan on days 1, 8, and 15, and 60 mg/m2 cisplatin on day 1. After completion of 4 cycles of chemotherapy, additional IP therapy was optionally permitted. TRT was administered at 6 MV or higher photons by a linear accelerator. Patients received 2 Gy per fraction once daily with a split schedule: 5 days/week from day 2 of each chemotherapy cycle, with a total of 26 and 24 Gy provided in the first and second cycles, respectively. There was a break in the split-course radiation of approximately 10 days. A radiation field included the primary tumor, the bilateral mediastinal and ipsilateral hilar lymph nodes with a margin of 1.5–2.0 cm. Radiation of the supraclavicular lymph nodes was administered only if they were involved. The inferior border extended 5 cm below the carina or to a level including ipsilateral hilar structures, whichever was lower. Prophylactic cranial irradiation (PCI) was administered to the patients achieving complete response (CR) with a total dose of 25 Gy in 10 fractions. The treatment schema is shown in Fig. 1.
Dose modification
Irinotecan was omitted on days 8 or 15 in the cycle if the leukocyte count fell below 3,000/μL, platelet count <10 × 104/μL or any diarrhea had occurred within the previous 24 h. Leukocytes ≥3,000/μL and platelets ≥10 × 104/μL were mandatory to commence the next cycle of treatment, and if levels fell below these limits, the second cycle was postponed until the counts recovered. Doses of irinotecan and cisplatin were reduced to 80 % when a leukocyte nadir count <1,000/μL or a neutrophil nadir count <500/μL for 3 or more days, if febrile neutropenia developed, if platelet nadir count <20,000/μL or if grade 3 or higher non-hematologic toxicity (excluding nausea, vomiting, and hair loss) had occurred during the previous treatment cycle.
Radiation was interrupted if grade 4 hematologic toxicity occurred during radiation and restarted after recovery to grade 3 or less. If grade 3 or greater esophagitis occurred, it was interrupted and restarted after recovery to grade 2 or less. If esophagitis did not resolve, it was discontinued. If PaO2 fell to 10 mmHg or a patient had a fever of 38 °C or higher, both radiotherapy and chemotherapy were interrupted and restarted as soon as possible after recovery.
Evaluation
The response evaluation criteria in solid tumors (RECIST) were used for the response assessment [16]. National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0, were used to grade adverse events (AEs). An extramural review was conducted to validate the eligibility of the patients, staging, and response.
Statistical analyses
The primary endpoint of this study was to estimate the objective response rate. The 2-stage accrual design described by Simon [17] was used. Assuming an overall response rate of 80 % for standard therapy, a target response rate of 95 % was established. α = 0.05, β = 0.20, and the estimated required number of patients were more than 29. Considering unfitness, dropout, and discontinuation, the sample size of this study was determined to be 35. For the survival endpoints, Kaplan–Meier methodology was used [18]. The multivariate correlation analysis was used for treatment failure within 1 year.
Results
Thirty-four patients from 7 institutions were enrolled in this trial between June 2000 and December 2009. All patients received the planned treatment and were evaluated for toxicity, response, and survival. The baseline patient characteristics are shown in Table 1.
Treatment administration
A total of 132 cycles of IP therapy were administered to the 34 patients: 1 cycle in 1 patient, 2 cycles in 1, 3 in 1, 4 in 29, and 5 in 2. The latter 31 patients completed the planned chemoradiotherapy regimen, and the completion rate was 91 %. Three patients could not complete the planned treatment because of pneumonia, radiation pneumonitis, and patient refusal. Thirty-five (27 %) irinotecan administrations were skipped on day 8, 63 (48 %) on day 15, including 23 (18 %) on both days. The major reasons for omission on days 8 and 15 were leukopenia 62 cases, leukopenia/thrombocytopenia 7 cases, anemia 5 cases, thrombocytopenia 5 cases, diarrhea 4 cases, leukopenia/thrombocytopenia/anemia, leukopenia/thrombocytopenia/diarrhea, stagger/arrhythmia, acute gastric mucosal lesion (AGML), fever, pulmonary toxicity, and vagal reaction 1 cases, respectively. The average delay of the treatment cycles was 5 days per cycle. Five patients delayed more than 1 month. The dose intensity of irinotecan was 19.2 mg/m2/week, which was 64.1 % of the projected dose intensity. The dose intensity of cisplatin was 12.8 mg/m2/week, which was 85.5 % of the projected dose intensity.
Toxicity
The toxicities during treatment are listed in Table 2. All 34 patients were assessable for toxicity. Thirty-one (91 %) patients experienced grade 3 or 4 hematological toxicity, and 13 (38 %) had grade 4. The principal grade 3 or 4 hematological toxicity was leukopenia and neutropenia in 31 (91 %) patients, and the principal grade 4 toxicity was neutropenia in 13 (38 %) patients. Febrile neutropenia occurred in 2 (6 %) patients. The major non-hematological toxicities were infection, gastrointestinal toxicities, and pneumonitis. One patient had grade 4 bloody diarrhea with ileus and multiple digestive ulcers during the second cycle and underwent a blood transfusion. There were two treatment-related deaths by radiation pneumonitis. A 72-year-old male patient finished 4 cycles of chemotherapy, thoracic radiotherapy, and PCI because he achieved CR. Radiation pneumonitis was first observed at 1 month and then 7 months after protocol treatment. Despite intensive care with corticosteroids, he died 3 weeks after the second pneumonitis occurred. A 66-year-old female patient finished 3 cycles of chemotherapy and thoracic radiotherapy and achieved PR. During the 4th cycle of chemotherapy, she was found on the floor of home with unconscious and taken to hospital by ambulance. She was declared dead on arrival. Chest X-ray of the patient showed an increased concentration in the extensive lung field as in acute respiratory distress syndrome, suspected as pneumonitis. Grade 3 fatigue was observed in 1 (3 %) patient.
Efficacy
All 34 patients were assessed for response. CR was observed in 8 (24 %) patients and the remaining 26 (76 %) patients had a partial response. The overall response and CR rates were 100 % (95 % CI, 100–100 %) and 24 % (95 % CI, 8–39 %), respectively. CRs at each stage were 0 % (0/2) in IB, 0 % (0/2) in IIA, 33 % (1/3) in IIB, 25 % (4/16) in IIIA, and 25 % (3/12) in IIIB, respectively.
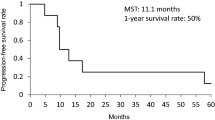
The median potential follow-up time was 81.9 (range, 22.7–132.1) months. Sixteen patients are alive at the time of this analysis, and the other 18 patients died during the follow-up period. The progression-free survival of the 34 patients is shown in Fig. 2a. Median time to tumor progression was 14.3 (95 % CI, 10.7–57.6) months, and the 1-, 2-, 3-, 4- , and 5-year progression-free survival rates were 56.9, 44.3, 40.2, 40.2, and 30.2 %, respectively. The overall survival of 34 patients is shown in Fig. 2b. Median survival time was 44.5 (95 % CI, 21.6–90.1) months, and the 1-, 2-, 3-, 4- , and 5-year survival rates were 88.2, 66.7, 54.4, 46.1, and 46.1 %, respectively.
Treatment failure pattern
Of the 34 evaluable patients in this investigation, 20 (59 %) relapsed (Table 3). The first sites of disease progression were as follows: local only in 2 (6 %) patients, local and distal in 3 (9 %), and distal only in 15 (44 %). In 12 (36 %) patients, the initial site of relapse was the brain, and all relapsed solely in the brain. Other relapse sites included pleural effusion, bone, and liver in one patient. Treatment failure within 1 year is presented in Table 4. The significant failure factors were “male”, “not CR,” and “low chemotherapy cycles”.
Discussion
This multicenter phase II study of IP with concurrent split-course radiotherapy showed a promising median survival time and 5-year survival rate of 44.5 months and 46.1 %, respectively. The high response rate of 100 % that no recurrence was observed in patients with a CR and long follow-up contributed to this good survival result. Meanwhile, two treatment-related deaths by pneumonitis were observed.
A phase III study in Japan showed IP to be more effective than EP in extensive disease (ED) SCLC [19]. Median survival and 1-year survival rates in IP and EP arms were 12.8 versus 9.4 months and 58.4 versus 37.7 %, respectively. Although three confirmatory trials comparing IP with EP for ED-SCLC in the United States and Europe did not show the superiority of IP [20–22], it is suggested that IP is an equally effective regimen with a different toxicity profile. A meta-analysis of six trials involving 1,476 patients demonstrated that IP could achieve a greater overall response and prolong OS compared with EP for previously untreated SCLC [23]; therefore, we adopted IP as the chemotherapy regimen for LD-SCLC. Although the Fox Chase Cancer Center Group reported the tolerability of IP with standard thoracic radiotherapy [24], a previous Japanese dose-finding trial of IP with concurrent 60 Gy radiotherapy was not completed because of unacceptable toxicity for NSCLC [13]. Then, we incorporated split-course radiotherapy because of safety considerations for normal tissue.
The median survival times of phase II trials of EP-based concurrent chemoradiotherapy plus IP chemotherapy and previous IP-based concurrent chemoradiotherapy were 20.2–26.1 months [25–28]. In these trials, Sohn et al. previously reported IP regimen with concurrent TRT and seemed similar to this trial in sample size, good median survival time, neutropenia, pneumonitis, and an unfortunate treatment-related death rate of two cases; however, a large difference still exists between the median survival time of 26.1 and the 44.5 months. First possible reason is related to radiotherapy timing. TRT was begun on day 1 of the first chemotherapy cycle in this study compare with second cycle in their study. Because early radiotherapy has a survival advantage when platinum-based chemotherapy is used concurrently [29], this study had high local control activity with local failure occurring in only 15 % (5/34), compared with 36 % (12/33) in the Sohn study. Second possible reason is split-course radiotherapy. Although split-course thoracic radiotherapy did not provide a survival advantage evidence in patients with LD-SCLC [30], it has been recently reconsidered by a favorable toxicity profile especially in acute esophagitis [31]. Third possible reason is that our study was followed up for a long time. The goal of treatment in LD-SCLC is to achieve a cure. The CR patients in this study have never suffered a recurrence, have survived for a long period, and might be cured. In addition, the patients might be able to receive new agent treatment. A long follow-up study is therefore favorable in this situation. Finally, the 95 % confidence interval of median survival time was broad as 21.6–90.1 months in present study.
Hyperfractionated radiation has been preferred for LD-SCLC based on a positive result in one large intergroup phase III study [6]; however, their once-daily arm of 1.8 Gy fractions to 45 Gy was deemed to be underpowered. The two treatment arms were not equitoxic as reflected by a rate of severe esophagitis of 27 % on twice-daily arm compared with 11 % on the once-daily arm [32]. Duke University Group retrospectively analyzed 65 patients treated with continuous once-daily 1.8–2 Gy fractions to approximately 60 Gy (range, 58–66) for LD-SCLC [33]. All their patients received chemotherapy, and it was concluded that chemotherapy plus approximately 60 Gy of once-daily RT for LD-SCLC was generally well tolerated. Mayo Clinic Group reported long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily in LD-SCLC [34]. Patients in the once-daily arm received 1.8 Gy fractions to 50.4 Gy, and no survival difference was found. Thus, we consider that once-daily radiotherapy remains the treatment option for LD-SCLC and used in the present study. Two phase III studies that compare standard-dose RT of 45 Gy in twice-daily fractions for 3 weeks with once-daily schedules with a higher total dose are on going (CALGB-RTOG and EORTC).
The main toxicities of this regimen were pneumonitis and neutropenia. Ohe et al. [35] retrospectively analyzed the risk factors for treatment-related death in chemotherapy and TRT for lung cancer. Pulmonary fibrosis identified on a plain chest X-ray film, the combination of irinotecan plus cisplatin, advanced age, and elevated lactate dehydrogenase were associated with treatment-related death from TRT. Pneumonitis is an important problem in chemoradiotherapy using an IP regimen. Although esophagitis (≥G3) was observed in 2–29 % in other trials [25–28], it did not occur in this trial; therefore, it seems that split-course concurrent radiotherapy could reduce the risk of esophagitis without loss of activity compared with continuous use. Diarrhea (≥G3) was reduced from 19 % in our NSCLC trial [14] to 3 % in this trial, which can be explained by the reduced dose of irinotecan, 50–60 to 40 mg/m2.
In conclusion, our study demonstrated the activity of irinotecan and cisplatin with concurrent split-course radiotherapy for patients with LD-SCLC.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B, Onoshi T, Osterlind K, Tattersall MHN, Wagner H (1992) A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327:1618–1624
Warde P, Payne D (1992) Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 10:890–895
Mascaux C, Paesmans M, Berghmans T, Branle F, Lafitte JJ, Lemaitre F, Meert AP, Vermylen P, Sculier JP, European Lung Cancer Working Party (ELCWP) (2000) A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 30:23–36
Curran WJ (2001) Combined-modality therapy for limited-stage small cell lung cancer. Semin Oncol 28:S14–S22
Turrisi AT III, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, Wagner H, Aisner S, Johnson DH (1999) Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265–271
Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T, Fukuda H, Saijo N (2002) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 20:3054–3060
Hsiang YH, Liu LF (1988) Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 48:1722–1726
Masuda N, Kudoh S, Fukuoka M (1996) Irinotecan (CPT-11): pharmacology and clinical applications. Crit Rev Oncol/Hematol 24:3–26
Fukuda M, Nishio K, Kanzawa F, Ogasawara H, Ishida T, Arioka H, Bojanowski K, Oka M, Saijo N (1996) Synergism between cisplatin and topoisomerase I inhibitors, NB-506 and SN-38, in a human small cell lung cancer cells. Cancer Res 56:789–793
Kanzawa F, Sugimoto Y, Minato K, Kasahara K, Bungo M, Nakagawa K, Fujiwara Y, Liu LF, Saijo N (1990) Establishment of a camptothecin analogue (CPT-11)-resistant cell line of human non-small cell lung cancer: characterization and mechanism of resistance. Cancer Res 50:5919–5924
Kudoh S, Fujiwara Y, Takada Y, Yamamoto H, Kinoshita A, Ariyoshi Y, Furuse K, Fukuoka M (1998) Phase II study of irinotecan combined with cisplatin in patients with previously untreated small-cell lung cancer. West Japan Lung Cancer Group. J Clin Oncol 16:1068–1074
Yokoyama A, Kurita Y, Saijo N, Tamura T, Noda K, Shimokata K, Matsuda T (1998) Dose-finding study of irinotecan and cisplatin plus concurrent radiotherapy for unresectable stage III non-small-cell lung cancer. Br J Cancer 78:257–262
Fukuda M, Soda H, Fukuda M, Kinoshita A, Nakamura Y, Nagashima S, Takatani H, Tsukamoto K, Kohno S, Oka M (2007) Irinotecan and cisplatin with concurrent split-course radiotherapy in locally advanced nonsmall-cell lung cancer: a multiinstitutional phase 2 study. Cancer 110:606–613
Oka M, Fukuda M, Kuba M, Ichiki M, Rikimaru T, Soda H, Tsurutani J, Nakamura Y, Kawabata S, Nakatomi K, Narasaki F, Nagashima S, Takatani H, Fukuda M, Kinoshita A, Kohno S (2002) Phase I study of irinotecan and cisplatin with concurrent split-course radiotherapy in limited-disease small cell lung cancer. Eur J Cancer 38:1998–2004
Therasse P, Arbuch SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Simon R (1989) Optional two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85–91
Hanna N, Bunn PA Jr, Langer C, Einhorn L, Guthrie T Jr, Beck T, Ansari R, Ellis P, Byrne M, Morrison M, Hariharan S, Wang B, Sandler A (2006) Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 24:2038–2043
Lara PN Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR, Chansky K, Gandara DR (2009) Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomics results from SWOG S0124. J Clin Oncol 27:2530–2535
Zatloukal P, Cardenal F, Szczesna A, Gorbunova V, Moiseyenko V, Zhang X, Cisar L, Soria JC, Domine M, Thomas M (2010) A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol 21:1810–1816
Jiang J, Liang X, Zhou X, Huang L, Huang R, Chu Z, Zhan Q (2010) A meta-analysis of randomized controlled trials comparing irinotecan/platinum with etoposide/platinum in patients with previously untreated extensive-stage small cell lung cancer. J Thorac Oncol 5:867–873
Langer CJ, Somer R, Litwin S, Feigenberg S, Movsas B, Malale C, Sherman E, Millenson M, Nicoloau N, Huang C, Treat J (2007) Phase I study of radical thoracic radiation, weekly irinotecan, and cisplatin in locally advanced non-small cell lung carcinoma. J Thorac Oncol 2:203–209
Han JY, Cho KH, Lee DH, Kim HY, Kim EA, Lee SY, Lee JS (2005) Phase II study of irinotecan plus cisplatin induction followed by concurrent twice-daily thoracic irradiation with etoposide plus cisplatin chemotherapy for limited-disease small-cell lung cancer. J Clin Oncol 23:3488–3494
Kubota K, Nishiwaki Y, Sugiura T, Noda K, Mori K, Kawahara M, Negoro S, Watanabe K, Imamura F, Tamura T, Saijo N (2005) Pilot study of concurrent etoposide and cisplatin plus accelerated hyperfractionated thoracic radiotherapy followed by irinotecan and cisplatin for limited-stage small cell lung cancer: Japan Clinical Oncology Group 9903. Clin Cancer Res 11:5534–5538
Saito H, Takada Y, Ichinose Y, Eguchi K, Kudoh S, Matsui K, Nakagawa K, Takada M, Negoro S, Tamura K, Ando M, Tada T, Fukuoka M, West Japan Thoracic Oncology Group 9902 (2006) Phase II study of etoposide and cisplatin with concurrent twice-daily thoracic radiotherapy followed by irinotecan and cisplatin in patients with limited-disease small-cell lung cancer: West Japan Thoracic Oncology Group 9902. J Clin Oncol 24:5247–5252
Sohn JH, Moon YW, Lee CG, Kim GE, Chung KY, Chang J, Kim SK, Kim YS, Choi BW, Choi HJ, Kim JH (2007) Phase II trial of irinotecan and cisplatin with early concurrent radiotherapy in limited-disease small-cell lung cancer. Cancer 109:1845–1850
Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J, Kester A, Rutten K, Lambin P (2007) Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Cancer Treat Rev 33:461–473
Blackstock AW, Bogart JA, Matthews C, Lovato JF, McCoy T, Livengood K, Ho C, White D, Atkins JN, Miller AA (2005) Split-course versus continuous thoracic radiation therapy for limited-stage small-cell lung cancer: final report of a randomized phase III trial. Clin Lung Cancer 6:287–292
Gielda BT, Marsh JC, Zusag TW, Faber LP, Liptay M, Basu S, Warren WH, Fidler MJ, Batus M, Abrams RA, Bonomi P (2011) Split-course chemoradiotherapy for locally advanced non-small cell lung cancer. A single-institution experience of 144 patients. J Thorac Oncol 6:1079–1086
Sorensen M, Pijls-Johannesma M, Felip E (2010) Small-cell lung cancer: ESMO clinical guidelines for diagnosis, treatment and follow-up. Ann Oncol 21:v120–v125
Miller KL, Marks LB, Sibley GS, Clough RW, Garst JL, Crawford J, Shafman TD (2003) Routine use of approximately 60 Gy once-daily thoracic irradiation for patients with limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 56:355–359
Schild SE, Bonner JA, Shanahan TG, Brooks BJ, Marks RS, Geyer SM, Hillman SL, Farr GH Jr, Tazelaar HD, Krook JE, Geoffroy FJ, Salim M, Arusell RM, Mailliard JA, Schaefer PL, Jett JR (2004) Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 59:943–951
Ohe Y, Yamamoto S, Suzuki K, Hojo F, Kakinuma R, Matsumoto T, Ohmatsu H, Nishiwaki Y (2001) Risk factors of treatment-related death in chemotherapy and thoracic radiotherapy for lung cancer. Eur J Cancer 37:54–63
Conflict of interest
The authors report no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuda, M., Nakamura, Y., Kinoshita, A. et al. Phase II study of irinotecan and cisplatin with concurrent split-course radiotherapy in limited-disease small cell lung cancer. Cancer Chemother Pharmacol 70, 645–651 (2012). https://doi.org/10.1007/s00280-012-1952-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1952-5