Abstract
Background
Trastuzumab when combined with fluoropyrimidine and cisplatin was proven to improve survival in patients with human epidermal growth factor receptor 2 (HER2)-positive gastric cancer (GC) in the ToGA study. The safety and efficacy of trastuzumab in combination with docetaxel and S-1 have not yet been evaluated.
Methods
This study was a multicenter, phase II study. Patients with chemotherapy-naïve HER2-positive advanced or metastatic GC were eligible. Trastuzumab was administered intravenously on day 1 of the first cycle at 8 and 6 mg/kg in subsequent cycles. Docetaxel was administered intravenously at 40 mg/m2 on day 1 of each cycle. S-1 was administered at a dosage based on body surface area for 14 days in a 3-weekly cycle. The primary endpoint was progression-free survival (PFS).
Results
A total of 23 patients were enrolled. Median PFS was 6.7 months (95% CI 4.1–10.1). The response rate (RR) was 39.1%. Median overall survival (OS) and time to treatment failure (TTF) were 17.5 and 4.4 months, respectively. Major grade 3–4 adverse events were neutropenia (39.1%), leukopenia (30.4%), and febrile neutropenia (8.7%).
Conclusion
Trastuzumab in combination with docetaxel and S-1 showed effective antitumor activity and manageable toxicities as first-line treatment for patients with HER2-positive GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the ToGA study, trastuzumab in combination with fluoropyrimidine and cisplatin improved overall survival for patients with HER2-positive unresectable advanced GC [1]. Since there have been no other successful phase III studies for HER2-positive GC, fluoropyrimidine and cisplatin have been the standard agents combined with trastuzumab [2]. Because capecitabine was mostly used as a fluoropyrimidine in the ToGA study, the combination of capecitabine and CDDP plus trastuzumab is recognized as the standard chemotherapy [1]. However, cisplatin has the potential risk of nephrotoxicity [3], and thus cisplatin-based chemotherapy always needs intensive hydration, which necessitates inpatient chemotherapy especially in Japan to avoid fluid overload. In addition, cisplatin has a dose-limitation because of the risk of irreversible ototoxicity [4], although it is rare, and platinum allergy is also sometimes an issue [5]. Thus, there are situations in which a regimen other than a cisplatin-based one is appropriate for patients with HER2-positive advanced GC. Oxaliplatin has a different toxicity profile from cisplatin [6, 7], which makes hydration unnecessary and administration to outpatients possible. Its use as a substitute for cisplatin has now been accepted. Ryu et al. demonstrated the efficacy of capecitabine and oxaliplatin in combination with trastuzumab in a phase II study, which resulted in objective RR of 67%, median PFS of 9.8 months, and OS of 21.0 months [8]. The outcomes were very attractive, but peripheral neuropathy appears to be one of the severe adverse events. Other than the doublet regimen as a backbone of trastuzumab treatment, Mitsui et al. investigated trastuzumab combined with the triplet regimen of docetaxel, cisplatin, and S-1 (DCS) in a phase II study [9]. DCS was previously reported as an active triplet combination against metastatic GC with a high RR of 87.1% [10]. Results from 16 patients in the study of DCS plus trastuzumab demonstrated a surprisingly high RR of 93.8%. In addition, non-curable factors disappeared in 56.3% of patients, all of whom underwent surgery.
As of the day when this study was planned, the JACCRO-GC03 study had just shown the efficacy and safety of docetaxel and S-1 as the first-line doublet regimen for advanced GC [11]. We had also demonstrated the efficacy of this regimen in GC with peritoneal dissemination [12]. Docetaxel and S-1 were scheduled as a three-week cycle suitable for harmonizing with triweekly administration of trastuzumab. Since docetaxel plus S-1 had not been tested as a candidate backbone regimen to be combined with trastuzumab for HER2-positive GC, a multicenter phase II prospective clinical trial was conducted to evaluate the efficacy and safety of docetaxel and S-1 with trastuzumab as first-line treatment for HER2-positive advanced GC (DASH study).
Patients and methods
Patients
Patients with histologically confirmed, untreated, HER2-positive unresectable or metastaic GC were enrolled in this phase II study at Okayama University Hospital, Okayama City, Japan, and other related hospitals. HER2 positivity was defined as immunohistochemical staining (IHC) 3 +, or IHC 2 + and fluorescent in situ hybridization (FISH)-positive. Patients were required to have measurable disease according to the Response Evaluation Criteria In Solid Tumors (RECIST) 1.1. The other eligibility criteria included, age ≥ 20 years or < 80 years; performance status (Eastern Cooperative Oncology Group: ECOG) score 0–2; adequate organ function of bone marrow, liver, and kidneys; left ventricular ejection fraction (LVEF) > 50%; and able to take food and medication orally. Patients were ineligible if they had any condition for which they could not receive S-1, docetaxel, or trastuzumab; heart failure, myocardial infarction, or coronary artery disease; other previous malignancy within 5 years; clinically evident brain metastases either currently or previously; or severe peripheral neuropathy. Pregnant women or those who could become pregnant, as well as men sexually active with women of child-bearing potential who had not had a vasectomy and were not using double-barrier methods of birth control, were also excluded.
All patients completed an informed consent form prior to study entry. This study was approved by the ethics committee of each hospital, and all procedures were in accordance with the Helsinki Declaration. The study was registered with UMIN-CTR, number UMIN000005366.
Treatment
Patients received chemotherapy in a 3-weekly cycle. Trastuzumab was administered intravenously on day 1 at a dose of 8 mg/kg in the first cycle and 6 mg/kg in the second cycle onward. S-1 was administered orally twice daily at a dose according to the body surface area (BSA) as follows: < 1.25 m2, 40 mg; ≥ 1.25 and ≤ 1.5, 50 mg; > 1.5, 60 mg, starting in the evening on day 1 for 2 weeks, followed by a drug-free interval of 1 week. Docetaxel was infused at 40 mg/m2 on day 1 of each cycle. Anti-emetic and anti-allergic medications were administered according to usual practice in each hospital. To manage toxicity, dose adjustment and treatment interruption were allowed. Toxicity was graded according to National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE) version 4.0. Treatment continued until tumor progression, unacceptable toxicity, patient refusal, or the physician’s decision to stop treatment, including conversion surgery.
Evaluations
The primary endpoint of this study was PFS. The secondary endpoints were safety, response rate (RR), overall survival (OS), and time to treatment failure (TTF). Tumor response was assessed every 2 months until disease progression according to RECIST version 1.1. PFS was defined as the time from the date of enrollment to the date of disease progression or death from any cause. In cases with conversion surgery, PFS was measured from the date of enrollment to the date of disease progression after surgery. OS was measured from the date of enrollment to the date of death from any cause, and TTF was the date of enrollment to the date of treatment discontinuation for any reason, including disease progression, treatment toxicity, and death. Safety was evaluated as the rates and grades of adverse events according to NCI-CTCAE version 4.0.
Statistical analysis
The required number of patients was calculated according to the Southwest Oncology Group One Arm Survival program [13]. According to the ToGA study, trastuzumab improved PFS by 1.2 months [1]. In the previous phase III study of S-1 and docetaxel, JACCRO-GC03, docetaxel plus S-1 showed PFS of 5.4 months (95% CI 4.5–5.9 months) [11]. Based on this evidence, assuming a null hypothesis of PFS of 4 months and an alternative hypothesis of 6 months with type-I error of 0.05 and a power of 0.8, with an accrual time of 3 years and follow-up of 2 years after the closure of recruitment, it was necessary to enroll 37 assessable patients. Thus, the sample size of this study was set at 40.
Results
Patients
From October 2011 to April 2016, 23 patients (19 males, 4 females; median age 67 years, age range 45–79 years) were enrolled at 6 institutions. The patients’ demographics and disease characteristics are shown in Table 1. The ECOG performance status for all patients was 0 or 1. Three-fourths of tumors were intestinal type adenocarcinoma. Eighty-seven percentage of patients had HER2 IHC 3 + tumors, whereas the others were IHC 2 + and FISH-positive. Seventeen patients had unresectable advanced disease with metastatic spread, and the other 6 had recurrent tumors. The most frequent metastatic site was the lymph nodes, followed by the liver, lungs, and peritoneum. Although the present study was originally planned as a study involving 40 patients, patient enrollment was delayed and the study was prematurely terminated before the projected number of patients was achieved.
Clinical outcome
Study endpoints
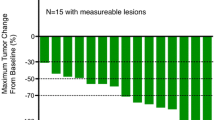
The median number of courses administered was six (average = 7.3), and the range was 2–19 courses. Objective responses consisted of a complete response in one patient (4.3%), partial response in 8 patients (34.8%), stable disease in 8 patients (34.8%), and progressive disease in 6 patients (26.1%); RR was 39.1% (95% CI 22.2–59.2), and the disease control rate was 73.9% (17/23) (Table 2). The median PFS was 205 days (6.7 months, 95% CI 4.1–10.1 months). The median OS and TTF were 532 days (17.5 months, 95% CI 11.9–31.9 months) and 134 days (4.4 months, 95% CI 4.0–6.4 months), respectively (Fig. 1).
Adverse events
Toxicities and adverse events (AE) are presented in Table 3. Major adverse events of grade 3–4 toxicities included neutropenia (39.1%), leukopenia (30.4%), febrile neutropenia (8.7%), diarrhea (4.3%), and rash (4.3%). Only one patient developed interstitial pneumonia, which eventually subsided with steroid treatment. Most patients tolerated treatment well, and two patients were administered up to 19 cycles without severe toxicity. All adverse events were well manageable, and no treatment-related deaths were observed.
Discussion
S-1 and docetaxel in combination with trastuzumab were evaluated in patients with measurable Her2-positive advanced GC. This combination chemotherapy regimen demonstrated a good antitumor effect and tolerability. With the actual sample size of 23 patients, median PFS was 6.7 months, with 4.1 months as the lower limit of the 95% CI, which was longer than the initially anticipated PFS of 6 months and longer than the preplanned null hypothesis threshold of 4.0 months. As compared with the JACCRO-GC03 study with a PFS of 5.3 months, the present study showed that the addition of trastuzumab to S-1 and docetaxel improved PFS. In addition, one of the responders in the present study achieved resectable disease, and treatment was successfully converted to curative surgery. These outcomes suggested that this new trastuzumab-containing chemotherapy also has a potential antitumor effect.
The only available phase III evidence involved a combination of fluoropyrimidine and cisplatin with trastuzumab as the standard chemotherapy, but cisplatin comes with potential nephrotoxicity and ototoxicity. The necessity for hydration and a hospital stay for some days every 3 weeks sometimes compromises patients’ quality of life. Even in cases in which a remarkable response was obtained, the cisplatin-based treatment needed to be discontinued after 6 or 7 cycles because of ototoxicity owing to its cumulative dose. Since trastuzumab in combination with docetaxel and S-1 does not have critical nephrotoxicity or ototoxicity, unlike cisplatin, inpatient treatment and a limitation of cycle number is unnecessary. In fact, all patients could be treated as outpatients in the present study. Although high incidences of leukopenia and neutropenia were noted, they were manageable with ordinary management protocols. Alopecia was relatively prominent due to docetaxel, which might be one of the reasons why there were few female participants in the study.
Combination chemotherapy regimens other than capecitabine-based or cisplatin-based ones have been pursued by other investigators as well [8, 9, 14,15,16,17,18]. The most investigated alternative agents are S-1 and oxaliplatin. S-1 has been preferentially used in Japan, and oxaliplatin has recently been shown to be as effective as cisplatin in GC. These observed responses appear to be promising. However, even oxaliplatin has potential drawbacks, such as severe neurotoxicity, after multiple cycles of administration [7]. In addition, conclusion about which fluoropyrimidine is the equivalent to allow similar results seen in the ToGA study remains to be clearly demonstrated. Thus, there would still be room for more appropriate candidates for combination backbone chemotherapy with trastuzumab.
An explicit limitation of the study is the small sample size, a single-arm, and non-randomized study. However, owing to the low incidence of HER2-positive GC itself [2], designing a large, prospective, phase III study to demonstrate the definitive combination would be unrealistic. Nevertheless, multiple options would help clinicians to wisely choose the chemotherapy appropriate for individual patients based on different comorbidities and living situations.
In conclusion, docetaxel and S-1 in combination with trastuzumab might be an option for a patient who does not fit cisplatin- or oxaliplatin-based anti-HER2 chemotherapy. The result provides background for further validation of first-line backbone chemotherapy in combination with trastuzumab.
References
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK, To GATI (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697. https://doi.org/10.1016/S0140-6736(10)61121-X
Pazo Cid RA, Anton A (2013) Advanced HER2-positive gastric cancer: current and future targeted therapies. Crit Rev Oncol Hematol 85(3):350–362. https://doi.org/10.1016/j.critrevonc.2012.08.008
Cornelison TL, Reed E (1993) Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol 50(2):147–158. https://doi.org/10.1006/gyno.1993.1184
Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, Kanz L (1998) Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer 77(8):1355–1362
Leguy-Seguin V, Jolimoy G, Coudert B, Pernot C, Dalac S, Vabres P, Collet E (2007) Diagnostic and predictive value of skin testing in platinum salt hypersensitivity. J Allergy Clin Immunol 119(3):726–730. https://doi.org/10.1016/j.jaci.2006.11.640
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United K (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358 (1):36–46. https://doi.org/10.1056/NEJMoa073149
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Yasui H, Hironaka S, Shimada K, Miwa H, Hamada C, Hyodo I (2015) Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol 26(1):141–148. https://doi.org/10.1093/annonc/mdu472
Ryu MH, Yoo C, Kim JG, Ryoo BY, Park YS, Park SR, Han HS, Chung IJ, Song EK, Lee KH, Kang SY, Kang YK (2015) Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer 51(4):482–488. https://doi.org/10.1016/j.ejca.2014.12.015
Mitsui Y, Sato Y, Miyamoto H, Fujino Y, Takaoka T, Miyoshi J, Kagawa M, Ohnuma H, Hirakawa M, Kubo T, Osuga T, Sagawa T, Sato Y, Takahashi Y, Katsuki S, Okuda T, Takimoto R, Kobune M, Nobuoka T, Hirata K, Kato J, Takayama T (2015) Trastuzumab in combination with docetaxel/cisplatin/S-1 (DCS) for patients with HER2-positive metastatic gastric cancer: feasibility and preliminary efficacy. Cancer Chemother Pharmacol 76(2):375–382. https://doi.org/10.1007/s00280-015-2807-7
Sato Y, Takayama T, Sagawa T, Takahashi Y, Ohnuma H, Okubo S, Shintani N, Tanaka S, Kida M, Sato Y, Ohta H, Miyanishi K, Sato T, Takimoto R, Kobune M, Yamaguchi K, Hirata K, Niitsu Y, Kato J (2010) Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother Pharmacol 66(4):721–728. https://doi.org/10.1007/s00280-009-1215-2
Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY, Hosaka H, Tsuji A, Takagane A, Inokuchi M, Tanabe K, Okuno T, Ogura M, Yoshida K, Takeuchi M, Nakajima T, Jaccro, Group KS (2014) Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 140 (2):319–328. https://doi.org/10.1007/s00432-013-1563-5
Shigeyasu K, Kagawa S, Uno F, Nishizaki M, Kishimoto H, Gochi A, Kimura T, Takahata T, Nonaka Y, Ninomiya M, Fujiwara T (2013) Multicenter phase II study of S-1 and docetaxel combination chemotherapy for advanced or recurrent gastric cancer patients with peritoneal dissemination. Cancer Chemother Pharmacol 71(4):937–943. https://doi.org/10.1007/s00280-013-2086-0
Lawless JF (2003) Statistical models and methods for lifetime data. 2nd edn. Wiley, Hoboken
Chua C, Tan IB, Yamada Y, Rha SY, Yong WP, Ong WS, Tham CK, Ng M, Tai DW, Iwasa S, Lim HY, Choo SP (2015) Phase II study of trastuzumab in combination with S-1 and cisplatin in the first-line treatment of human epidermal growth factor receptor HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol 76(2):397–408. https://doi.org/10.1007/s00280-015-2811-y
Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, Wang J, Xu N, Cheng Y, Bai Y, Liu W, Wang L, Shen L (2016) Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer 16:68. https://doi.org/10.1186/s12885-016-2092-9
Kataoka H, Mori Y, Shimura T, Nishie H, Natsume M, Mochizuki H, Hirata Y, Sobue S, Mizushima T, Sano H, Mizuno Y, Nakamura M, Hirano A, Tsuchida K, Adachi K, Seno K, Kitagawa M, Kawai T, Joh T (2016) A phase II prospective study of the trastuzumab combined with 5-weekly S-1 and CDDP therapy for HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol 77(5):957–962. https://doi.org/10.1007/s00280-016-3013-y
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, Imamura H, Gamoh M, Sakai D, Shimokawa T, Komatsu Y, Doki Y, Tsujinaka T, Furukawa H (2014) Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer 110(5):1163–1168. https://doi.org/10.1038/bjc.2014.18
Miura Y, Sukawa Y, Hironaka S, Mori M, Nishikawa K, Tokunaga S, Okuda H, Sakamoto T, Taku K, Nishikawa K, Moriwaki T, Negoro Y, Kimura Y, Uchino K, Shinozaki K, Shinozaki H, Musha N, Yoshiyama H, Tsuda T, Miyata Y, Sugimoto N, Shirakawa T, Ito M, Yonesaka K, Yoshimura K, Boku N, Nosho K, Takano T, Hyodo I (2017) Five-weekly S-1 plus cisplatin therapy combined with trastuzumab therapy in HER2-positive gastric cancer: a phase II trial and biomarker study (WJOG7212G). Gastric Cancer. https://doi.org/10.1007/s10120-017-0725-6
Acknowledgements
The authors would like to thank Dr. Nonaka, Department of Surgery, Tsuyama Central Hospital, and Dr. Takahata, Department of Surgery, Okayama Saiseikai General Hospital, for collaboration in the initial planning of this protocol. The authors are grateful to the clinical research coordinators in Chugoku Central Hospital and Fukuyama Medical Center for data collection. This study had no funding supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The all authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of all participating institutions (clinical trial registration no. UMIN-CTR, number UMIN000005366).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kagawa, S., Muraoka, A., Kambara, T. et al. A multi-institution phase II study of docetaxel and S-1 in combination with trastuzumab for HER2-positive advanced gastric cancer (DASH study). Cancer Chemother Pharmacol 81, 387–392 (2018). https://doi.org/10.1007/s00280-017-3505-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3505-4