Abstract
Background
We evaluated the efficacy and safety of 5-weekly S-1 and cisplatin combined with trastuzumab, a monoclonal antibody against human epidermal growth factor receptor type 2 (HER2) for HER2-positive advanced gastric cancer (AGC).
Methods
This phase II study treatment consisted of S-1 (80–120 mg per day) orally on day 1–21, cisplatin (60 mg/m2) intravenously on day 8, and trastuzumab (8 mg/kg on day 1 of the first cycle, followed by 6 mg/kg every 3 weeks) intravenously. The primary end point was 1-year survival rate. The secondary end points included overall survival, progression-free survival (PFS), response rate (RR), and safety.
Results
A total 22 patients from seven centers were enrolled. In the 20 patients evaluable for analysis, the 1-year survival rate was 70 % (95 % confidence interval (CI) 49.9–90.1 %), and median survival time, PFS, and RR were 15.3, 7.5 months and 41.2 %, respectively. Major grade 3/4 adverse events were neutropenia (30 %), anorexia (30 %), leukopenia (25 %), fatigue (20 %), and anemia (15 %).
Conclusions
Five-weekly S-1 and cisplatin combined with trastuzumab showed effective with favorable safety profile in patients with HER2-positive AGC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Combination therapies using fluoropyrimidines and cisplatin with or without epirubicin or docetaxel are commonly used as first-line chemotherapy for advanced gastric cancer (AGC) [1–4]. S-1 is an oral anticancer medicine that combines tegafur, a pro-drug of 5-FU, with two modulators, i.e., gimeracil and oteracil [5]. Phase III trial showed that S-1 was non-inferior to 5-FU [6], and that 5-weekly S-1 plus cisplatin (SP) was superior to S-1 (SPIRITS trial) [7], so SP is regarded as a standard first-line treatment of AGC in Japan [8, 9].
Human epidermal growth factor receptors (HERs) are the member of the transmembrane tyrosine kinase receptor family, and there are four members in HERs, human epidermal growth factor receptor (EGFR, also referred to as HER1), HER2, HER3, and HER4 [10]. Recently, HER2 has become an important focus of investigation in molecular target therapy for gastric cancer [11, 12].
Trastuzumab is a humanized monoclonal anti-HER2 antibody directed against the HER2 ectodomain. Although HER2 is an orphan receptor, several extracellular and intracellular antitumor mechanisms of trastuzumab have been identified [13]. Trastuzumab has been approved for the treatment of HER2-positive GC, and recent randomized ToGA trial, international phase III trial comparing chemotherapy consisting of cisplatin plus capecitabine or fluorouracil vs trastuzumab plus chemotherapy in patients with HER2-positive AGC, demonstrated a survival benefit with the addition of trastuzumab [14].
Very recently, phase II study of trastuzumab in combination with tri-weekly SP in HER2-positive GC (HERBIS-1) demonstrated promising antitumor effect and manageable toxic effects [15]. As shown in SPIRITS trails, 5-weekly SP is regarded as a standard first-line treatment of AGC in Japan [8, 9]. From these findings, we conducted this phase II study to evaluate the efficacy and safety of 5-weekly SP plus trastuzumab in HER2-positive AGC.
Patients and methods
Patients
Patients (20 ≦ age ≦ 80 years of age) were eligible for inclusion in this study if they were histologically proven unresectable or recurrent HER2-positive GC. HER2 status of GC was evaluated using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). We used a four-grade scale of HER2 IHC and FISH score previously described in ToGA study [14] and HERBIS-1 study [15]. We enrolled the patients with IHC3+, or IHC2+ and FISH positive of HER2. In this study, the patients who have unmeasurable lesions according to the response evaluation criteria in solid tumors (RECIST), version (1.1), were allowed to be enrolled. Other inclusion criteria were: patients having an Eastern Cooperative Oncology group (ECOG) performance status (PS) of 0–2, and adequate bone marrow reserve (with 12,000 cells/mm3 > leukocyte count ≧3000 cells/mm3, neutrocyte count ≧1500 cells/mm3, hemoglobin ≧9.0 g/dl, and platelet count ≧100,000 cells/mm3), hepatic function [aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ≦100 IU/l and serum bilirubin ≦2.0 mg/dl], renal function (creatinine clearance ≧60 ml/min), and cardiac function (left ventricular ejection fraction (LVEF) >50 %). Exclusion criteria included the presence of uncontrolled pleural effusion or pericardial effusion, brain tumor with active malignancy, serious allergy to medical drugs. All patients provided written informed consent before enrollment. This study was registered with UMIN-CTR, UMIN000005792.
Treatment
The patients received oral S-1 twice daily at a dose based on body surface area (<1.25 m2, 40 mg; 1.25 ≦ to < 1.5 m2, 50 mg; ≧1.5 m2, 60 mg) on days 1–21 of a 35-day cycle, cisplatin (60 mg/m2) intravenously on day 8 and trastuzumab (cycle 1, 8 mg/kg; cycle 2 onward, 6 mg/kg) intravenously on day 8 and every 3 weeks onward. This schedule was repeated until disease progression, development of unacceptable toxicity, or patient withdrawal of consent until maximum six cycles. Doses of S1 and cisplatin were reduced starting from the next cycle if patients had leukopenia (<1000/mm3), neutropenia (<500/mm3), febrile neutropenia, thrombocytopenia (<25,000/mm3), liver dysfunction (AST, ALT ≧200 IU/l), hyper bilirubinemia (total bilirubin ≧2 mg/dl), renal dysfunction (creatinine clearance <60 ml/min), grade 3–4 diarrhea and stomatitis. Only cisplatin dose was reduced if patients had grade 3–4 nausea, vomiting, and/or anorexia.
Evaluation
The primary end point was 1-year survival rate. The secondary end points included overall survival (OS), progression-free survival (PFS), response rate (RR), and safety. Tumors were assessed every 2 cycles until disease progression, and objective responses were evaluated according to the response evaluation criteria in solid tumors (RECIST) guidelines (version 1.1). OS was defined as the time from the date of enrollment to the date of death from any cause. PFS was defined as the time from the date of enrollment to the date of disease progression or death from any cause. Physical examination and blood examination were performed before every cycle, and left ventricular ejection fraction was assessed every 12 weeks during treatment. Adverse events were evaluated according to the National Cancer Institution Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
From the Kaplan–Meier curve of ToGA trial, 1-year survival rate is speculated to be 65 %. Assuming the 1-year survival rate of this study is 65 and 95 % confidence interval (CI) is ±15 %, at least 27 patients are required, and thus we concluded that sample size of this study is 30 patients.
Survival curves for time-to-end points were estimated by the Kaplan–Meier method and the 95 % CIs of the curves were calculated based on Greenwood’s variance. Statistical analyses were conducted by IBM SPSS Advanced Statistics Version 23.
Results
Patient characteristics
Twenty-two patients were enrolled from seven centers in Japan for this study from August 2011 to May 2014. Two patients were ineligible because of liver dysfunction and poor PS (Fig. 1). The patient demographics and disease characteristics are summarized in Table 1. The median age was 66 years (range 49–76 years). About two-thirds of patients had intestinal type adenocarcinoma. Only one patient (5 %) had recurrent cancer. The median follow-up time was 16.3 months (range 5.8–38.7). All 20 cases were IHC3+ gastric cancers.
Treatment delivery and efficacy
Of the 20 patients evaluable, the median treatment cycle was four cycles (range 1–6). Dose intensity of S-1 was 65.6 mg/body/week, cisplatin was 11.2 mg/m2/week, and trastuzumab was 2.04 mg/Kg/week. Fourteen patients discontinued protocol treatment. The reason for discontinuation was disease progression (n = 6), adverse event (n = 5), more than 21 days delay (n = 1), reduction less than minimum dose (n = 1) and complication (n = 1).
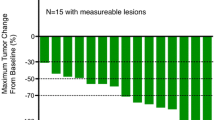
Out of total 20 patients, only 17 patients had measurable lesions, and thus, of the 17 patients evaluable, 7 (41.2 %) achieved partial response (PR) with response rate (RR) of 41.2 % (95 % CI 18.4–67.1 %) (Table 2). Seven patients (41.2 %) had stable disease (SD) with a disease control rate of 82.4 % (95 % CI 56.6–96.2 %). Two patients were finished by adverse events within two cycles and these two cases were classified to ‘Not evaluable.’
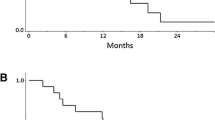
The median PFS was 7.5 (95 % CI 6.9–8.1) months, and median OS was 15.3 (95 % CI 9.9–20.7) months (Fig. 2). The OS rates at 1 year were 70.0 % (95 % CI 49.9–90.1).
Adverse event
The hematological and non-hematological toxicities of all patients are summarized in Table 3. The major adverse events were grade 3–4 leukopenia (25 %), neutropenia (30 %), anomia (15 %) and grade 3 thrombocytopenia (10 %), febrile neutropenia (5 %), nausea (15 %), anorexia (30 %), fatigue (20 %), stomatitis (5 %), and diarrhea (15 %). There was no treatment-related death.
We planned to enroll 30 patients at first, but contrary to our expectation, it took longer time to enroll HER2-positive GC patients, so the study was closed at 20 patients’ enrollment.
Discussion
Here, we reported the results of our multicenter phase II study of 5-weekly SP plus trastuzumab in patients with HER2-positive AGC. One-year survival rate (primary endpoint of this study) was 70 %, and this was similar to those of previous clinical studies ToGA trial (65 %) [14] and HERBIS-1 (67.9 %) [15]. OS (15.3 M) and PFS (7.5 M) were also similar to those of ToGA trial (16.0, 6.7 M) [14] and HERBIS-1 (16.0, 7.8 M) [15]. The toxicity profile of our regimen was tolerable, and the incidence of grade 3–4 adverse events were similar to those of the SP regimen in the SPIRITS [7] study and HERBIS-1 [15].
RR (41.2 %) of our trial was a little bit lower than those of ToGA trial (47 %) [14] and HERBIS-1 (68 %) [15]. In this 5-weekly clinical trials, dose intensity of cisplatin is 12 mg/m2/week, and this is lower than that of ToGA trial (26.7 mg/m2/week) [14] and HERBIS-1 (20 mg/m2/week) [15]. Actually, relative dose intensity of cisplatin was 93.3 % (11.2 mg/m2/week) in this trial, and this might be one of the reasons why RR of this study was lower than those of ToGA and HERBIS-1.
We did not limit subjects to patients with measurable lesions assessable according to RECIST guidelines, so we included eight cases of peritoneum metastasis. For these eight cases, the median cycle of this regimen was 4 (range 1–6), which is nearly equal to that of total 20 cases in this study. From these things, this regimen might be useful for AGC patients with peritoneum metastasis.
The limitation of our study is the lower number of analyzed subjects (20 cases). Originally, we planned to enroll 30 patients, so the reliability of the result isn’t enough.
We enrolled one patient with HER2-positive mixed adenoneuroendocrine carcinoma (MANEC). Histo-pathologically, MANEC contains adenocarcinoma component more than 30 % of the tumor by WHO classification [16, 17], which was thus included in the analysis of this trail.
Recently, SOS study showed that tri-weekly S-1 plus cisplatin (SP) was superior to 5-weekly SP in PFS, although there was no difference in OS [18], so both tri-weekly SP and 5-weekly SP can be the recommended first-line chemotherapy for patient with advanced GC. The PFS (7.8 months) of tri-weekly SP+ trastuzmab (HERBIS-1) was almost equal to the PFS (7.5 months) of our study. OS and 1-year survival rates were also almost equal between two studies, so 5-weekly as well as tri-weekly SP+ trastuzmab are useful regimens for HER2 positive AGC.
Very recently phase III study comparing tri-weekly oxaliplatin plus S-1 (SOX) with tri-weekly SP for AGC revealed that SOX is as effective as SP, and SOX is one of the alternative options to SP [19]. From these things, we are conducting SOX+ trastuzmab for the patients of HER2-positive AGC (UMIN000017182).
In conclusion, although this was the small number phase II study, our results suggest that 5-weekly SP plus trastuzumab is effective with favorable safety profile in patients with HER2-positive AGC. This study firstly disclosed that Japanese gold-standard 5-weekly SP combined with trastuzumab is effective and tolerable for the HER2-positive AGC.
References
Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B, Bodenstein H, Schmoll HJ, Bleiberg H, Nordlinger B, Couvreur ML, Baron B, Wils JA (2000) Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 18(14):2648–2657
Waters JS, Norman A, Cunningham D, Scarffe JH, Webb A, Harper P, Joffe JK, Mackean M, Mansi J, Leahy M, Hill A, Oates J, Rao S, Nicolson M, Hickish T (1999) Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer 80(1–2):269–272. doi:10.1038/sj.bjc.6690350
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24(31):4991–4997. doi:10.1200/JCO.2006.06.8429
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, Forster G, McCloud PI (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20(4):666–673. doi:10.1093/annonc/mdn717
Shirasaka T (2009) Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 39(1):2–15. doi:10.1093/jjco/hyn127
Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, Nasu J, Ohtsu A, Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 10(11):1063–1069. doi:10.1016/S1470-2045(09)70259-1
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221. doi:10.1016/S1470-2045(08)70035-4
Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28(9):1547–1553. doi:10.1200/JCO.2009.25.4706
Ajani JA, Buyse M, Lichinitser M, Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha R, Carrato A, Ferry D, Moiseyenko V (2013) Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer 49(17):3616–3624. doi:10.1016/j.ejca.2013.07.003
Kataoka H (2009) EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. J Dermatol Sci 56(3):148–153. doi:10.1016/j.jdermsci.2009.10.002
Gravalos C, Jimeno A (2008) HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 19(9):1523–1529. doi:10.1093/annonc/mdn169
Shimoyama S (2014) Unraveling trastuzumab and lapatinib inefficiency in gastric cancer: future steps (review). Mol Clin Oncol 2(2):175–181. doi:10.3892/mco.2013.218
Hudis CA (2007) Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med 357(1):39–51. doi:10.1056/NEJMra043186
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK, To GATI (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, Imamura H, Gamoh M, Sakai D, Shimokawa T, Komatsu Y, Doki Y, Tsujinaka T, Furukawa H (2014) Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer 110(5):1163–1168. doi:10.1038/bjc.2014.18
Boo YJ, Park SS, Kim JH, Mok YJ, Kim SJ, Kim CS (2007) Gastric neuroendocrine carcinoma: clinicopathologic review and immunohistochemical study of E-cadherin and Ki-67 as prognostic markers. J Surg Oncol 95(2):110–117. doi:10.1002/jso.20616
Namikawa T, Oki T, Kitagawa H, Okabayashi T, Kobayashi M, Hanazaki K (2013) Neuroendocrine carcinoma of the stomach: clinicopathological and immunohistochemical evaluation. Med Mol Morphol 46(1):34–40. doi:10.1007/s00795-012-0006-8
Ryu MH, Baba E, Lee KH, Park YI, Boku N, Hyodo I, Nam BH, Esaki T, Yoo C, Ryoo BY, Song EK, Cho SH, Kang WK, Yang SH, Zang DY, Shin DB, Park SR, Shinozaki K, Takano T, Kang YK, Investigators SOSs (2015) Comparison of two different S-1 plus cisplatin dosing schedules as first-line chemotherapy for metastatic and/or recurrent gastric cancer: a multicenter, randomized phase III trial (SOS). Ann Oncol 26(10):2097–2101. doi:10.1093/annonc/mdv316
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Yasui H, Hironaka S, Shimada K, Miwa H, Hamada C, Hyodo I (2015) Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol 26(1):141–148. doi:10.1093/annonc/mdu472
Acknowledgments
We wish to thank Professor Suzuki and Dr. Nishiyama for statistical analyses advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataoka, H., Mori, Y., Shimura, T. et al. A phase II prospective study of the trastuzumab combined with 5-weekly S-1 and CDDP therapy for HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol 77, 957–962 (2016). https://doi.org/10.1007/s00280-016-3013-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3013-y