Abstract
Purpose
The fully human monoclonal antibody nivolumab binds to the programmed death-1 (PD-1) receptor, blocking interactions between PD-1 and its ligands on tumor cells and preventing T cell exhaustion in patients with cancer. The potential for corrected QT interval (QTc) prolongation was assessed in a subset of patients enrolled in a phase 2 dose-ranging study of nivolumab.
Methods
Triplicate 12-lead electrocardiograms (ECGs) obtained predose and post-dose were assessed by an independent ECG core laboratory. QTc derived from Fridericia’s formula (QTcF) was evaluated by central tendency, categorical, and concentration–response analyses.
Results
No patients had QTcF intervals or changes from baseline in QTcF (ΔQTcF) exceeding prespecified thresholds indicating borderline or prolonged QTcF (>480 ms) or ΔQTcF (>60 ms). Among 146 patients randomized to nivolumab 0.3, 2.0, or 10.0 mg/kg every 3 weeks, the maximum increases in mean (±SD) ∆QTcF at any time point were 4.9 (±13.4), 1.2 (±10.1), and 2.0 (±8.9) ms, respectively. There was no relationship between ∆QTcF and nivolumab serum concentration and no association between predicted maximum ∆QTcF and mean maximum nivolumab concentration in any dosage group.
Conclusion
Results of these intensive ECG analyses indicate that nivolumab has no clinically meaningful effect on QTc interval when administered at doses up to 10.0 mg/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One mechanism by which tumors evade immune surveillance is by exploiting inhibitory pathways that regulate immune responses. A more complete understanding of these processes has led to the development of immune checkpoint inhibitors that maintain or restore antitumor immune responses [1, 2].
Nivolumab is a recombinant, fully human monoclonal antibody that binds to the programmed death-1 (PD-1) receptor [2]. Blockade of PD-1 on activated T cells by nivolumab prevents interactions between PD-1 and PD-1 ligand 1 or 2 on tumors, thereby preventing T cell exhaustion [1]. Nivolumab has been studied in patients with melanoma [3–6], non-small cell lung cancer (NSCLC) [7], renal cell carcinoma (RCC) [8, 9], and in patients with relapsed or refractory Hodgkin lymphoma [10]. It is approved for the treatment of patients with unresectable or metastatic melanoma and disease progression following treatment with ipilimumab, patients with squamous NSCLC with progression on or after platinum-based chemotherapy, and patients with advanced RCC. Nivolumab in combination with the CTLA-4 checkpoint inhibitor, ipilimumab, is approved for the treatment of unresectable or metastatic melanoma [11].
To facilitate early identification of potential drug-related proarrhythmia, it is recommended that a thorough QT/corrected QT interval (QTc) study be conducted for all drugs in development, including anticancer and biologic agents [12–14].
Delayed cardiac repolarization (QT prolongation) resulting from blockade of rapidly activating delayed rectifier potassium current encoded by the human ether-a-go–go-related gene (hERG) in myocytes predisposes patients to torsade de pointes, ventricular fibrillation, and sudden cardiac death [15, 16]. QTc prolongation has been associated with drugs used to treat cardiovascular and non-cardiovascular conditions, including several tyrosine kinase inhibitors used to treat cancer [17, 18], and has resulted in market withdrawal of several drugs (e.g., astemizole, cisapride, and terfenadine) [19]. Drugs known to have an association with QTc prolongation are typically small molecules. No approved monoclonal antibodies or other biologics have been definitively associated with this phenomenon to date. In the case of anticancer agents, it is not possible to conduct thorough QT/QTc studies with negative (placebo) and positive controls (e.g., moxifloxacin) for ethical reasons [12]. Moreover, bulky biologic agents with high molecular weights (>140 kDa), such as nivolumab, are thought to have a low potential for blocking hERG channels because they cannot cross plasma membranes and are too large to enter and block the pores of the channel [15].
An intensive QT substudy similar to those performed with other biologic anticancer agents has been conducted in accordance with the International Conference on Harmonisation (ICH) guidelines to determine the potential for QTc prolongation with nivolumab [20, 21]. This manuscript presents the analysis of the potential effects of nivolumab on cardiac repolarization in patients with solid tumors enrolled in a phase 2 clinical trial [8].
Materials and methods
Study design and treatment
The protocol for this randomized multicenter phase 2 study in patients with renal carcinoma was reviewed and approved by the institutional review board at each study center. Informed consent was obtained from all patients before undergoing any study procedures. The study was conducted in accordance with Good Clinical Practice guidelines as defined by the ICH.
The phase 2 study of nivolumab randomized 168 patients with progressive advanced/metastatic clear-cell renal cell carcinoma to intravenous nivolumab 0.3, 2.0, or 10.0 mg/kg every 3 weeks (ClinicalTrials.gov identifier: NCT01668784) [8]. Patients with cardiovascular disease, renal dysfunction, or electrolyte abnormalities were eligible for inclusion, and use of drugs known to prolong the QT interval was allowed. The median number of doses (cycles) received was 6.0, 7.5, and 8.0 for patients in the nivolumab 0.3-, 2.0-, and 10.0-mg/kg dose groups, respectively [8]. Data from a subset of 146 patients treated with nivolumab are included in this analysis.
Electrocardiogram assessments
Electrocardiograms (ECGs) were collected in triplicate at baseline and during treatment (Supplemental Fig. S1). ECG data were collected digitally and transmitted to an independent ECG core laboratory [BioTelemetry Research (formerly Cardiocore), Rockville, MD, USA] for assessment and interpretation by a cardiologist who was blinded to patient, time, and treatment.
ECGs were collected from all patients at baseline and then predose, at the end of the infusion, and at 3 h post-dose during doses (cycles) 1 and 7. Patients with risk factors for QT prolongation such as electrolyte abnormalities (hypokalemia, hypocalcemia, or hypomagnesemia), use of medications associated with QT prolongation, and/or a baseline QTc > 470 ms were identified and summarized by dose level.
Pharmacokinetic analyses
Blood samples were collected from all patients and analyzed for serum nivolumab concentrations using a validated electrochemiluminescent assay with a lower limit of quantification of 0.2 µg/mL. Nivolumab serum concentrations were determined and used in the concentration–response analysis.
Statistical analyses
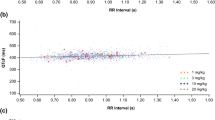
The ECG-evaluable population consisted of all treated patients who had a baseline ECG and at least one on-treatment ECG. The averages of the triplicate measurements for each time point were summarized. In order to reduce the dependence of QT on heart rate (HR), QTc derived from Fridericia’s formula (QTcF) [22] was prospectively chosen as the primary endpoint in the ECG analyses; however, scatter plots of QTc derived from Bazett’s formula (QTcB) [23], and QTcF versus HR at baseline were produced along with Pearson correlation coefficients to assess the most appropriate QTc factor.
Triplicate ECG assessments were averaged prior to analysis, and individual ECG parameters (QT, QTc, RR, HR, QRS, and PR) and corresponding changes from baseline were listed and summarized by dose, study day, and time point. Incidences of abnormal ECG results of clinical and regulatory interest [19] at screening and post-screening were tabulated and summarized using graphical displays. These included QTcF ranges (<450, 450–480, 481–500, and >500 ms) and ΔQTcF ranges (<30, 30–60, and >60 ms), change from baseline PR (ΔPR) >200 ms, change from baseline QRS (ΔQRS) ≥120 ms, and new incidences of abnormal U waves, T waves, or ECG morphology.
A concentration–response analysis of ECG parameters and change from baseline versus nivolumab concentrations was performed using two linear mixed-effects regression models fitted for change in QTcF (∆QTcF) on nivolumab concentration with random effects (for patient slopes and intercepts). As ECGs were measured on two study days (during cycles 1 and 7), two analyses were performed: (1) after pooling across study days and estimating the population slope and patient slope and intercept on the pooled data (Model 1), and (2) by including study day as a factor in the mixed-effects model and estimating the population slope and patient slope on each study day (Model 2).
Based on these two models, the predicted maximum ∆QTcF [at maximum plasma concentration (C max)] was estimated [along with two-sided 90 % confidence intervals (CIs)] for each treatment group. The mean C max for each treatment (by study day and overall) was derived by selecting the C max (on each study day and across study days) for each patient and calculating the average of these values within each treatment. The point estimate for ΔQTcF at mean C max was calculated as the ΔQTcF predicted by the estimated population regression line at the mean C max (on each study day or overall based on the pooled data) based on the fitted models previously discussed. Finally, a two-sided 90 % CI for the predicted ΔQTcF was derived.
Diagnostic plots of population-averaged and patient-specific residuals were graphically displayed over the corresponding nivolumab concentrations.
Safety was assessed at baseline and at each subsequent clinical visit. Adverse events (AEs) were graded by severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Results
Overall, 146 patients with renal cell carcinoma treated with nivolumab contributed data to the ECG analysis. The average age was 61 years (range 37–81 years), and the majority of patients were white (94 %) and male (71 %). Forty of 146 patients (27 %) were taking a medication associated with QTc prolongation and/or had an electrolyte imbalance (defined as potassium, calcium, or magnesium levels below the lower limit of normal) at any point over the course of treatment (Supplemental Table S1). Among patients randomized to treatment with nivolumab 0.3, 2.0, and 10.0 mg/kg, 22.4, 17.6, and 30.4 %, respectively, were receiving a concomitant medication associated with QTc prolongation. No patients had a QTcF > 470 ms at baseline, and the distribution of patients with acquired risk factors for QT prolongation (electrolyte disturbance or medication) was balanced across the dosage groups; thus, no patients were omitted from the analysis on this basis.
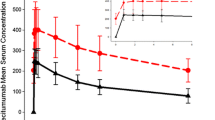
QTcF was confirmed as the more appropriate QTc factor based on an analysis of scatter plots of baseline QTcF and QTcB intervals versus baseline HR, with Pearson correlation coefficients indicating less dependency on HR for QTcF (QTcB Pearson coefficient = 0.41; QTcF Pearson coefficient = −0.17). Within the dosage range evaluated, nivolumab did not affect the QTc interval. There was no evidence of a dose-related effect of nivolumab on the QTc interval or ∆QTcF during cycle 1 or 7 (Table 1; Fig. 1). No uncorrected QT interval exceeded 483 ms at any time during the study, and the longest observed QT interval (483 ms) occurred 2.5 h after the baseline reading in a patient in the lowest dosage group (0.3 mg/kg).
No patients had QTcF intervals or ΔQTcF values that exceeded the prespecified thresholds considered to indicate borderline or prolonged QTcF (>480 ms) or ΔQTcF (>60 ms) (Fig. 1). Indeed, no patients had a QTcF interval >470 ms or a ΔQTcF > 45 ms at any time point during this study. In addition, there was no evidence of dose-related changes in the PR or QRS intervals or in HR during cycle 1 or 7 (Table 2).
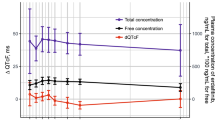
No significant relationship was found between ∆QTcF and nivolumab concentrations according to the linear mixed-effects regression models (Table 3; Fig. 2). When data were pooled across days, the slope of the concentration versus the ∆QTcF curve was 0.0; when time was included as a variable, the slope was positive for the first dose (0.01) and negative for the seventh dose (−0.03) (Table 3; Fig. 2). These small changes were not statistically significant because the 95 % CIs for the slope estimates include 0.
There was also no association between the predicted maximum ∆QTcF and the mean maximum nivolumab concentration in each dosage group, either when pooled across study days or when assessed by study day (Fig. 3). These predicted effects are in agreement with the mixed-effects regression models. The largest predicted maximum ∆QTcF [−10.3 ms (90 % CI −23.8, 3.3)] was associated with a nivolumab concentration of 353.36 µg/mL (cycle 7, day 1); however, for both models and for all three treatment groups, the upper boundary of the 90 % CI for predicted ∆QTcF was <5 ms, which is not a clinically meaningful increase (Table 3; Fig. 3b).
Mean maximum predicted ∆QTcF with time-matched nivolumab serum concentration. a Model 1, pooled across days. Estimated population slope = 0.0 (95 % CI −0.02, 0.02), b Model 2, study day included as a factor. Estimated population slope: cycle 1, day 1 = 0.01 (95 % CI −0.01, 0.02); cycle 7, day 1 = −0.03 (95 % CI −0.08, 0.02). CI confidence interval, QTcF corrected QT interval derived from Fridericia’s formula
None of the observed AEs were potentially suggestive of repolarization abnormalities, e.g., seizures or convulsions (n = 3), syncope (n = 3), presyncope (n = 2), QTc prolongation (n = 1), and tachycardia (n = 7), or were associated with abnormal ECG findings potentially related to proarrhythmia. The patient with a QTc prolongation had a baseline QTcB of 434 ms prior to the first dose of nivolumab. Three hours after receiving nivolumab 0.3 mg/kg, the mean QTcB was 454 ms (mean of three replicate measurements). The largest ∆QTcF was 8 ms. The event lasted less than 24 h and did not result in study drug discontinuation. The patient did not experience any other AEs during the study.
Discussion
The results of this analysis demonstrate that, over the dose range studied, nivolumab did not have a clinically meaningful effect on the QTc interval in patients with solid tumors. These data are considered robust given that ECGs were performed in triplicate at baseline and during treatment and that a larger number of patients (n = 146) participated in the evaluation compared with studies involving other monoclonal antibodies [e.g., cetuximab and pertuzumab (n = 37)] [20, 21].
The data obtained from this analysis are also applicable to the approved dosing regimen of nivolumab 3.0 mg/kg every 2 weeks. A total of 146 patients received nivolumab 0.3–10.0 mg/kg for a median of 6.5–8 doses (cycles). When administered at a dose of 3.0 mg/kg every 2 weeks, nivolumab had a mean elimination half-life of 26.7 days [11]; thus, the observations collected during the seventh dose in the present study would have occurred under steady state conditions. The steady state C max of approximately 132 mg/mL observed in patients treated with nivolumab 3.0 mg/kg every 2 weeks, the approved dose and schedule for the treatment of melanoma, NSCLC, and RCC, is within the range of steady state mean C max values observed in the present study (10.48, 70.34, and 353.36 µg/mL after administration of nivolumab 0.3, 2.0, and 10.0 mg/kg, respectively).
No patient had a QTcF > 480 ms or a ∆QTcF > 60 ms at any time point before or during treatment with nivolumab in the present analysis. The upper boundary of the 90 % CI for predicted ∆QTcF was <5 ms for all three dosage groups in both linear regression models. It is important to note that patients with preexisting cardiovascular disease, renal dysfunction, or electrolyte abnormalities were not excluded from the study and that use of drugs known to prolong the QT interval was not prohibited during the study. Among the overall population of 168 patients, cardiovascular abnormalities were present in 75–80 % of patients across the three dosage groups, making this the most common concomitant medical condition in the study population. As a result, many patients were predisposed to QTc prolongation: 27 % of patients had electrolyte abnormalities, and 18–30 % of patients in the three dose groups were taking medications associated with QTc prolongation. The absence of safety signals and the absence of a relationship between nivolumab serum concentrations and ECG parameters in patients prone to electrolyte imbalances and in whom use of QT-prolonging medications was allowed provides confidence when extrapolating the results to other cancer patient populations.
This analysis has several strengths. It was conducted in a large number of patients and did not exclude patients with cardiovascular diseases, which was the most common concomitant medical condition in the patients enrolled in the study. The nivolumab dose range went up to 10.0 mg/kg, which allowed for an evaluation of dose response beyond the approved 3.0 mg/kg dose. Predefined criteria were used to categorize QTc and ∆QTc, and robust methods were used for data collection and interpretation. Limitations of the study include the absence of a placebo group in two studies and the absence of a positive control. However, these limitations are inherent to studies of anticancer therapies for ethical reasons [12].
No evidence of QTc interval prolongation was apparent when nivolumab was administered at doses three times that recommended for monotherapy (10.0 mg/kg vs. the approved dose of 3.0 mg/kg) in this intensive study. This finding is consistent with expectations for biologic agents of large molecular size that are not likely to interact with the hERG channel and interfere with transmembrane ion transport.
In addition, nivolumab in combination with ipilimumab has recently been approved for the treatment of patients with BRAF V600 wild-type, unresectable or metastatic melanoma [11]. It was previously shown that ipilimumab does not have QT prolongation potential [24]. Per the ICH guidance [25], combinations of two or more drugs are unlikely to need a thorough QT/QTc study or intensive late-stage monitoring when the component drugs administered alone have been shown to lack effects on QT/QTc in separate studies. Thus, the combination of nivolumab and ipilimumab is unlikely to have an effect on the QTc interval.
In conclusion, the results of this intensive ECG analysis in patients with solid tumors indicate that, when administered at doses up to 10.0 mg/kg, nivolumab does not have a clinically meaningful effect on the QTc interval.
References
Shih K, Arkenau HT, Infante JR (2014) Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 74:1993–2013
Deeks ED (2014) Nivolumab: a review of its use in patients with malignant melanoma. Drugs 74:1233–1239
Weber JS, D’Angelo SP, Minor D et al (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375–384
Long GV, Atkinson V, Ascierto PA et al (2015) Nivolumab improved survival vs dacarbazine in patients with untreated advanced melanoma. J Transl Med 13:O6
Topalian SL, Sznol M, McDermott DF et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030
Robert C, Long GV, Brady B et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330
Rizvi NA, Mazieres J, Planchard D et al (2015) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16:257–265
Motzer RJ, Rini BI, McDermott DF et al (2015) Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 33:1430–1437
McDermott DF, Drake CG, Sznol M et al (2015) Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 33:2013–2020
Ansell SM, Lesokhin AM, Borrello I et al (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372:311–319
Opdivo® [prescribing information] Bristol-Myers Squibb, Princeton. Revised Nov 2015
Rodriguez I, Erdman A, Padhi D et al (2010) Electrocardiographic assessment for therapeutic proteins—scientific discussion. Am Heart J 160:627–634
Rock EP, Finkle J, Fingert HJ et al (2009) Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J 157:827–836
Giorgi MA, Bolanos R, Gonzalez CD, Di Girolamo G (2010) QT interval prolongation: preclinical and clinical testing arrhythmogenesis in drugs and regulatory implications. Curr Drug Saf 5:54–57
Vargas HM, Bass AS, Breidenbach A et al (2008) Scientific review and recommendations on preclinical cardiovascular safety evaluation of biologics. J Pharmacol Toxicol Methods 58:72–76
Roden DM (2008) Cellular basis of drug-induced torsades de pointes. Br J Pharmacol 154:1502–1507
Ghatalia P, Je Y, Kaymakcalan MD, Sonpavde G, Choueiri TK (2015) QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br J Cancer 112:296–305
Shah RR, Morganroth J, Shah DR (2013) Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf 36:295–316
Shah RR (2007) Cardiac repolarisation and drug regulation: assessing cardiac safety 10 years after the CPMP guidance. Drug Saf 30:1093–1110
Deeken JF, Shimkus B, Liem A et al (2013) Evaluation of the relationship between cetuximab therapy and corrected QT interval changes in patients with advanced malignancies from solid tumors. Cancer Chemother Pharmacol 71:1473–1483
Garg A, Li J, Clark E et al (2013) Exposure-response analysis of pertuzumab in HER2-positive metastatic breast cancer: absence of effect on QTc prolongation and other ECG parameters. Cancer Chemother Pharmacol 72:1133–1141
Fridericia LS (1920) The duration of systole in an electrocardiogram in normal humans and patients with heart disease. Acta Med Scand 53:469–486
Bazett HC (1920) An analysis of the time-relations of electrocardiograms. Heart 7:353–370
Agrawal S, Williams D, Waxman I, Lambert A, Roy A, Darbenzio R (2015) Absence of QT prolongation effect by nivolumab or ipilimumab in patients with solid tumors. Clin Pharmacol Ther 97:PII–117
International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH) Steering Committee. ICH harmonised tripartite guideline: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, E14. Geneva, Switzerland, ICH, 2005. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. Accessed 20 Oct 2015
Acknowledgments
Editorial support was provided by Blair Jarvis of inScience Communications, Springer Healthcare (Philadelphia, PA, USA), and funded by Bristol-Myers Squibb (Princeton, NJ, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of and own stock in Bristol-Myers Squibb.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agrawal, S., Waxman, I., Lambert, A. et al. Evaluation of the potential for QTc prolongation in patients with solid tumors receiving nivolumab. Cancer Chemother Pharmacol 77, 635–641 (2016). https://doi.org/10.1007/s00280-016-2980-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-2980-3