Abstract
Purpose
Greater scrutiny is being placed on developing a full understanding of potential cardiotoxicity of therapeutic agents, especially on the potential to prolong the QTc interval which can lead to arrhythmias such as torsade de pointes and sudden death. This trial was designed to specifically evaluate the effect, if any, of cetuximab on the QTc interval in patients with advanced solid tumors.
Methods
Cetuximab was administered as an initial dose of 400 mg/m2 on day 1 (week 1) followed by a maintenance dose of 250 mg/m2 weekly thereafter. ECG monitoring was performed at screening, baseline (week 1 preceding dosing), and during week 1 to 5 of treatment. Cetuximab concentration-to-QTc relationship was evaluated based on cetuximab serum samples obtained at the time of each ECG measurement to allow for accurate correlation between any observed QT/QTc changes and cetuximab serum concentration.
Results
At the recommended dose (400 mg/m2 on day 1 followed by 250 mg/m2 weekly), cetuximab had no clinically meaningful effect on QTc interval, PR or QRS intervals, or heart rate and there was no apparent concentration-dependent effect of cetuximab on any of these electrocardiogram parameters. Safety observations in patients treated with cetuximab in this study were consistent with the agent’s known safety profile.
Conclusion
These results suggest that cetuximab can be safely administered as a single agent without risk of effect on QTc interval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiotoxicity is a serious complication of cancer therapy with increasing importance to patient safety and long-term efficacy outcome. Cancer patients represent an aging population with greater probability of co-morbidities including cardiac disease [1]. Cardiotoxicity from anti-cancer therapy may manifest in various forms including hypertension, left ventricular dysfunction, thromboembolism, ischemia, and ventricular arrhythmias caused by a prolonged QT interval [2–4]. The QT interval, as measured by electrocardiogram (ECG), represents the total duration of ventricular activation and recovery (depolarization and repolarization) [5, 6]. Prolongation of the QT interval (or QTc interval when corrected for heart rate [7–10]) may result from a delay in cardiac repolarization which can then lead to ventricular arrhythmias. The most important and serious of these arrhythmias is torsade de pointes, which can quickly progress to ventricular fibrillation and sudden death [6]. QT prolongation may result from genetic predisposition, electrolyte imbalances, acquired cardiac disease, hypothyroidism, and hyperthermia, and may also be drug-induced [11]. Several of the classic chemotherapeutic agents including anthracyclines (doxorubicin, epirubicin), 5-fluorouracil, some platinum compounds, and taxanes (paclitaxel, docetaxel) have been implicated in QT prolongation and arrhythmias [12].
The last decade has seen the rapid expansion of molecular-targeted therapies in the treatment for cancer. Cardiotoxicity has been implicated in some small molecule multi-targeted tyrosine kinases, monoclonal antibodies targeting tyrosine kinase receptors or their ligands, antiangiogenic compounds, histone deacetylase inhibitors, and inhibitors of farnesyl protein transferase, Src/Abl kinase, and protein kinase C [1, 11]. The human epidermal growth factor receptor (HER) inhibitors, targeting the HER family of tyrosine kinase receptors, including HER2, have demonstrated potential for cardiotoxicity including decreased left ventricular ejection fraction with trastuzumab [4]. Multi-targeted tyrosine kinase inhibitors including lapatinib, imatinib, nilotinib, dasatinib, sunitinib, and sorafenib all have reported risk of cardiotoxicity including prolonged QTc [13]. For many other targeted agents, the risk of cardiotoxicity remains uncertain, often not arising until more extensive use of a drug occurs, making cardiotoxicity a leading cause for drugs to be withdrawn after approval [14].
Combination regimens of targeted therapy with radiation therapy and/or traditional chemotherapeutic agents can compound the potential for cardiac risk. Concomitant medications taken by cancer patients including antiemetics, antifungals, and various antibiotics may also prolong the QT interval. Cancer patients often experience diarrhea, nausea, and vomiting along with reduced fluid intake, creating electrolyte imbalances which can exacerbate drug-related cardiotoxicity and arrhythmias [4]. To better assess risk versus benefit, an understanding of potential drug-related effect on QTc interval must be a priority.
To facilitate earlier identification of drug-related cardiotoxicity, the International Conference on Harmonization (ICH) adopted the ICH E14 Guidelines for evaluating the potential for QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs in May 2005 [15]. The Food and Drug Administration subsequently issued a Guidance for Industry based on the ICH E14 Guidelines [16]. The guidelines recommend the conduct of a “thorough QT/QTc study” defined as a trial dedicated to evaluating a drug’s effect on cardiac repolarization. A QTc interval exceeding a value of 450 ms (men) or 460 ms (women) is considered to be associated with an increased risk of life-threatening arrhythmias; however, the exact interval length that constitutes serious prolongation remains controversial [11]. The National Cancer Institute’s criteria are used to grade QTc interval prolongation with grade 1 = QTc > 450–470 ms; grade 2 = QTc > 470–500 or 60 ms above baseline; grade 3 = QTc > 500 ms; and grade 4 QTc > 500 ms with life-threatening signs or symptoms such as torsade de pointes [17]. In cases where a “thorough QT/QTc study” in healthy volunteers is not possible because of safety concerns, alternate methods for detecting effects on the QT/QTc interval may be used (e.g., collection of ECGs at multiple time points during dosing).
Cetuximab (Erbitux®) is a recombinant, human/mouse chimeric monoclonal antibody that inhibits human EGFR activity by binding specifically to the extracellular domain of the receptor on both normal and tumor cells [18]. It competitively inhibits the binding of epidermal growth factor and other ligands, such as transforming growth factor-alpha, and blocks phosphorylation and activation of receptor-associated kinases [18]. The result is inhibition of cell growth, induction of apoptosis, inhibition of cell motility and invasion, and decreased production of pro-angiogenic factors such as vascular endothelial growth factor. Cetuximab has been approved for the treatment for metastatic colorectal cancer [19–22] and squamous cell carcinoma of the head and neck [23–25].
The current study was designed to determine the effect, if any, of cetuximab on QT/QTc interval and followed the 2005 ICH E14 Guidelines for non-thorough QT (NTQT) studies for cancer patients [15, 26]. ECG measurements were obtained at screening, baseline (week 1 preceding dosing), and during week 1 to 5 of treatment in male and female patients receiving the recommended clinical dosing regimen of cetuximab (400 mg/m2 on day 1 followed by 250 mg/m2 weekly starting on day 8). Due to tolerability and ethical considerations, the study could not be conducted in healthy volunteers. Garnett et al. [27] suggested the assessment of concentration-to-QT relationship as a valid alternative in cases such as this where cancer therapy is involved. Therefore, serum samples were collected, time-matched to each ECG measurement, to allow for accurate correlation between QT/QTc changes and cetuximab serum concentration.
Methods
This study was conducted at 20 centers in the United States, and appropriate approval was obtained from the Institutional Review Board (IRB) prior to initiation of the study at each site. Each patient freely gave written informed consent prior to study participation. This study was registered on ClinicalTrials.gov as NCT00698841.
Study design and treatment
This was a multicenter, single-arm study of cetuximab monotherapy conducted in patients with advanced solid tumor malignancies. The study was designed to determine the effect, if any, of cetuximab on QT/QTc interval and followed the 2005 ICH E14 Guidelines for non-thorough QT (NTQT) studies for cancer patients [15, 26].
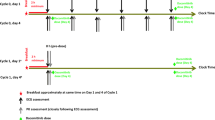
The study design, summarized in Fig. 1, consisted of a screening period for the determination of study eligibility, a baseline ECG period (week 1), an on-treatment ECG period (weeks 1–8), and an on-treatment post-ECG period (following completion of day 29 ECGs). Patients who discontinued treatment on or prior to the week 8 infusion of cetuximab were followed for toxicity during the post-treatment period.
Cetuximab was administered as intravenous infusions at an initial dose of 400 mg/m2 administered over 120 min on day 1 (week 1) followed by a maintenance dose of 250 mg/m2 administered over 60 min weekly thereafter. The administration of the recommended premedication (diphenhydramine HCl 50 mg) was required for the first 5 doses of cetuximab. Diphenhydramine HCl 50 mg was also required to be administered during the baseline monitoring to account for any change in QT interval related to its administration.
ECG monitoring was performed at screening, baseline (week 1 preceding dosing), and during week 1 to 5 of treatment. Serum concentration samples were obtained at time-matched points during the administration in week 1 to 5. Following the completion of the ECG monitoring in week 5, the addition of other therapy (e.g., chemotherapy and/or radiation therapy) was allowed as per investigator discretion.
Patients who were stable or responding to cetuximab at week 9 were eligible to continue treatment off-study with cetuximab and/or other therapy.
Eligibility criteria
Patients were eligible if they were more than 18 years of age, had histologically or cytologically documented advanced or metastatic malignant disease of solid tumor origin that was considered measurable and/or evaluable, and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Prior cancer therapies were allowed provided that a patient had adequately recovered from prior therapy and a minimum of 14 days (21 days for an investigational agent or EGFR receptor antagonists) had elapsed between the last treatment and enrollment in the study. Patients had to have adequately recovered from any recent surgery or radiation therapy, with 21-day elapsing since these interventions.
Exclusion criteria included the following: a QTc interval (corrected QT interval) >470 ms, resting heart rate <50 or >100 bpm, sustained supine systolic blood pressure (SBP) of >150 or <90 mmHg or a diastolic blood pressure (DBP) of <45 or >95 mmHg, or any other clinically relevant abnormality identified on a screening electrocardiogram (ECG) that prevented accurate measurement of the QT interval. Patients with a history of any of the following were ineligible: myocardial infarction ≤6 months prior to study entry, severe congestive heart failure, uncontrolled angina, uncontrolled arrhythmias, congenital long QT syndrome, history of risk factors for ventricular tachycardia or torsade de pointes, fainting, or unexplained loss of consciousness or convulsions, significant peripheral artery disease (PAD), arterial thrombotic events requiring surgical or medical intervention within 6 months prior to study initiation (e.g., transient ischemic attack (TIA), cerebrovascular accident (CVA), and active angina pectoris or angina pectoris requiring surgical or medical intervention). Patients with an implantable pacemaker or automatic implantable cardioverter defibrillator (AICD) were also ineligible.
Medications known to prolong QT/QTc interval were prohibited during the 7-day period prior to the screening ECG and during the ECG collection period. However, medications in this category taken by a patient on a regular schedule and dose and initiated >21 days prior to the screening ECG were allowed to continue. These patients were included in the analysis as long as the dose and schedule did not change and the medication was not discontinued during the ECG collection period.
Assessments
The primary objective of this study was to determine the change from time-matched baseline in QTc interval in the study population.
Holter monitoring for collection of digital 12-lead ECGs was performed during screening and the baseline ECG period, and was initiated on days 1, 8, 15, 22, and 29 prior to other scheduled procedures and discontinued after completion of the last resting ECG time point for the day. On each study day, resting ECGs were collected at the following time points relative to the start of the infusion of diphenhydramine: pre-dose, 1, 2, 3, and at 6 h on days 1 and 22 only. The primary ECG endpoint was the time-matched mean change from baseline (designated as “Δ”) in QTc at each ECG sampling time point. Similar endpoints were evaluated for PR interval, QRS interval, and ECG-derived heart rate (HR).
Cetuximab concentration-to-QTc relationship was also evaluated based on cetuximab serum samples obtained at the time of each ECG measurement to allow for accurate correlation between any observed QT/QTc changes and cetuximab serum concentration. A validated enzyme-linked immunosorbent assay (ELISA) was used for the quantification of cetuximab in human serum [28]. The ELISA method employed a recombinant human EGFR (full-length receptor) adsorbed onto a microtiter plate to capture cetuximab in 0.1 % human serum, which was then detected using a commercial rabbit anti-human IgGFC-HRP conjugate.
Adverse events (using terminology based on the Medical Dictionary for Regulatory Activities, or MedDRA, version 12.0) and other symptoms, with the exception of grade 3 or 4 infusion reactions, were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. A grade 3 infusion reaction was defined as symptomatic bronchospasms, requiring parenteral medication(s), with or without urticaria, or allergy-related edema or angioedema. A grade 4 infusion reaction was a life-threatening event characterized by the same symptomatology as a grade 3 reaction, but further complicated by symptomatic hypotension or oxygen saturation of 70 % or less. Assessment of magnesium levels on a weekly basis was incorporated into the study based on the historical observation of decreased levels in patients receiving cetuximab.
To assess adverse events of special interest based on clinical experience with cetuximab, composite categories of various MedDRA terms were pooled. Rash, rash pustular, rash erythematous, dermatitis acneiform, dermatitis exfoliative, rash papular, rash pruritic, rash generalized, rash macular, rash maculopapular, acne, acne pustular, skin desquamation, and dry skin were pooled into a composite event category called acneform rash. Infusion reaction comprised infusion-related reaction, hypersensitivity, anaphylactic reaction, anaphylactic shock, anaphylactoid reaction; dyspnea, pyrexia, and chills were included if onset occurred on the first day of study treatment. Cardiac events included coronary artery disorders, cardiac arrhythmias, heart failures, left ventricular failures, sudden cardiac death, cardiac death, and sudden death.
Assessments of efficacy parameters were not performed for this study. Disease response was documented at each evaluation visit (every 8 weeks) only for purposes of confirming eligibility for continuing therapy. The method of response assessment was as per the institution’s preference, and assessments were performed by the investigator at the institution.
Statistical analyses
This was a single-arm estimation trial, and thus, sample size was based on consideration of the confidence interval width. A time-matched mean ΔQTc was estimated at each ECG sampling time point with a one-sided upper 95 % confidence limit constructed for the population means of ΔQTc, based on the assumption that ΔQTc was normally distributed with a standard deviation of 20 ms. The study was to remain open to enrollment until at least 32 evaluable patients had completed the required study assessments. This sample size should have provided an upper 95 % confidence limit 5.815 ms higher than the point estimate.
QTc evaluable patients were all treated patients who met all inclusion criteria and none of the exclusion criteria and who were not considered unevaluable due to any of the following: required a cetuximab dose reduction before day 29, had an interruption of cetuximab infusions on day 1 or 22 for any reason, missed any ECG time points (baseline, day 1 or day 22), began taking any prohibited medication prior to day 22, discontinued or modified dose of a scheduled medication known to prolong the QT/Qc interval between screening and day 29, received any other cancer therapy prior to day 29, or withdrew informed consent for any reason prior to day 23.
Fridericia’s correction formula [8] was used to calculate the heart rate-corrected QT interval (QTcF). For each ECG parameter (QT, QTc, RR, QRS, PR, and HR), the mean of the triplicate measurements at each time point was considered for all analyses. ECG measurements collected on the baseline day were used to compute time-matched change from baseline for each ECG parameter. Individual ECG parameter measurements and corresponding changes from baseline were listed, and values outside a normal range were flagged. ECG abnormalities identified by the ECG core laboratory were listed. Adverse events and laboratory tests were summarized for all treated patients.
The effect of cetuximab on the QTc interval was assessed by summary statistics for QTc and time-matched ΔQTc, and examination of plots of mean (one-sided upper 95 % confidence limit) QTc and time-matched ΔQTc versus time since dosing. At each ECG sampling time point, the mean time-matched ΔQTc was estimated and a one-sided upper 95 % confidence limit was constructed for the population means of ΔQTc at the time point.
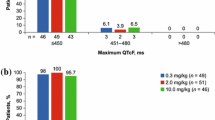
The effect of cetuximab on QTc interval was also assessed by frequency distributions for patients meeting or exceeding predefined values for maximum QTc (≤450, >450–480, >480–500, >500 ms) and maximum time-matched ΔQTc (≤30, >30–60, >60 ms).
Cetuximab concentration-QTc response was explored graphically using double y-axis plots of mean time-matched ΔQTc and mean drug concentration versus time since dosing and scatter plots of individual patients’ QTc and time-matched ΔQTc values versus the nearest corresponding serum drug concentrations. For the plot of time-matched ΔQTc versus drug concentrations, the estimated linear regression, taken from the results of fitting a random intercept and slope model with cetuximab concentration as the only covariate to the data, was included in the plot.
The effect of cetuximab on QRS and PR intervals and HR was assessed by summary statistics for reported values and the corresponding time-matched changes from baseline, and examination of plots of mean (+standard error) reported values and time-matched mean changes from baseline versus time since dosing. Cetuximab concentration–response was explored graphically as described above for QTc, but regression analysis was not performed.
Results
Patient demographics
A total of 79 patients were enrolled on study between February 2009 and February 2010 across 20 sites in the United States. Of these, 51 patients received treatment with cetuximab and were included in safety analyses; 28 were screening failures who never received treatment. Thirty-seven patients were evaluable for the assessment of QTc interval, comprising the “QTc evaluable” patients as described in “Methods”. Thirty-three patients completed 8 weeks of on-study treatment. Of the 18 patients who discontinued prior to the first scheduled tumor assessment at week 8, the most common reasons were disease progression (7 patients) and adverse events unrelated to study drug (3 patients).
Baseline demographics and characteristics for the 51 patients included in the safety analysis are summarized in Table 1. Patients were predominantly white with 59 % males and 41 % females. Mean age was 61 with a range of 38–84 years. More than 50 % of the patients had colorectal cancer (8, 16 %), lung cancer (14, 28 %), or head and neck cancer (5, 10 %), and 90 % had an ECOG performance status of 0 or 1. All 51 treated patients received concomitant medications while on-study for either management of symptoms or treatment for adverse events. One patient initiated use of amitriptyline, a medication known to prolong the QT/QTc interval, during the ECG collection period and was thus excluded from the QTc analysis.
Effect of cetuximab on QTc
Table 2 summarizes the time-matched mean change in QTcF (QT interval corrected for heart rate using the Fridericia’s formula [8]) at each ECG time point from day 1 to day 29. No trends were apparent in terms of time-matched mean ΔQTcF relative to the time that cetuximab was administered or in relation to repeated cetuximab infusions across all study days. The maximum change in QTcF was 4.5 ms on day 8 at the 2-h time point.
In Fig. 2, the time-matched mean (one-sided upper 95 % confidence limit) ΔQTcF versus time since dosing for each study day is presented, with a threshold of 10 ms change from baseline depicted by a horizontal line in each panel. The one-sided upper 95 % confidence limit for the time-matched mean ΔQTcF was <10 ms at all time points throughout the study. This was consistent with a lack of effect of cetuximab on QTcF. A categorical analysis of the frequency distribution of subjects with a maximum QTcF or time-matched ΔQTcF meeting pre-specified criteria yielded no QTcF values >500 ms and no time-matched ΔQTcF values >60 ms. There was one patient with a history of hypertension and evidence of prior inferior myocardial infarction on baseline ECGs who had mean QTcF interval readings of 481 and 483 ms at 1 and 2 h, respectively, on day 22. Thirteen patients had a maximum time-matched ΔQTcF >30 and ≤60 ms at one or more time points during the study, but no trends were observed over time in the incidence of these outliers.
Scatter plots of time-matched ΔQTcF versus serum cetuximab concentrations for each study day (Fig. 3) show no apparent concentration-related trends in QTcF or ΔQTcF. Regression analysis on these plots for ΔQTcF yielded point estimates for the slope of the regression line ranging from −0.018 to +0.026 ms per ng/mL with two-sided 90 % confidence intervals encompassing zero, consistent with the lack of a trend in time-matched ΔQTcF with increasing concentration of cetuximab.
Effect of cetuximab on PR and QRS interval and heart rate
Eight patients had PR interval measurements that were >200 ms while on-study; each of these patients also had baseline PR interval measurements >200 ms. There were no patients with a QRS interval measurement >120 ms during the study. Heart rate measurements of >100 bpm at baseline and/or on-study were reported for 15 patients with no apparent trends in changes in heart rate observed during the study for these patients. There were no cetuximab concentration-related trends in prolonging PR interval, QRS interval, or increasing heart rate.
ECG results
Twelve-lead continuous digital ECG data were collected at screening, baseline, and on days 1, 8, 15, 22, and 29. All 37 QTc evaluable patients had at least one abnormal ECG during screening and/or the ECG period (inclusive of the baseline and on-treatment days). The most frequently reported ECG conduction abnormality was a non-specific intraventricular conduction defect, and the most frequently reported ECG rhythm abnormality was sinus tachycardia.
There were 22 patients who had 1 or more abnormal ECGs that were assessed as potentially clinically significant by a consulting cardiologist, including 10 with abnormal ECGs of potential clinical significance at screening or baseline only. Among the remaining 12 patients, abnormalities included evidence of previous myocardial infarction and/or conduction defects such as inverted T waves, ST wave depression, and first-degree atrioventricular block.
Twelve (32 %) patients had 1 or more ECGs with an abnormal finding of prolonged or borderline prolonged QTc interval, including 7 patients with QTc interval abnormalities reported after initiation of treatment with cetuximab. However, only 1 patient had QTc abnormalities reported on an ECG that was assessed by the cardiologist as potentially clinically significant while on-treatment. This patient had ECGs of potential clinical significance throughout the study, with findings of borderline prolonged QTc and inverted T waves at week 1 (2 time points) and week 3 (1 time point) and a consistent finding of inverted T waves and occasional sinus tachycardia on all other abnormal ECGs. None of the QTc interval abnormalities identified was reported by the investigator as an adverse event. There were no cases of torsade de pointes reported for the patients in this study.
Safety
Adverse events were evaluated in all treated patients (n = 51) and are summarized in Table 3. The majority of events were grade 1 or 2. Most common were rash (43 %), headache (24 %), dermatitis acneiform (18 %), nausea (12 %), and fatigue (10 %). Most common grade 3 events included headache (8 %) and fatigue and anemia (4 % each); the rest of the reported grade 3 events were all 2 % incidence. There was a single grade 4 event (pain) reported.
Two cardiac events occurred in this study, both considered unrelated to study treatment. One patient received their week 4 (final) dose on day 22 and was then hospitalized due to a dislocated right shoulder and fracture after a fall. Upon admission, she was found to be tachycardic and an ECG revealed mild ST interval depression although she did not report any chest pain or shortness of breath. A cardiology consult was obtained, and a non-ST elevation myocardial infarction (MI) was diagnosed. The treating physician assessed the MI as grade 2 and not related to study therapy. The patient’s history included coronary artery disease and a prior MI in 2009 which was noted on the screening ECG as an old anteroseptal MI. The patient was subsequently discontinued from the study on day 68 following an event of right shoulder separation. In the second case, the patient received two doses of cetuximab at weeks 1 (day 1) and 2 (day 8). On day 10, the patient experienced symptoms of acute coronary syndrome and was admitted to the hospital; the event was assessed by the treating physician as grade 3, not related to study therapy. The patient’s history included diabetes, hypertension, and hyperlipidemia. The day 8 ECG report (prior to dosing) noted atrial fibrillation and an old inferior MI. The patient was discharged from the hospital on day 14 and discontinued from the study on day 19 without receiving any additional study therapy.
One or more serious adverse events (SAEs) were reported for 16 (31 %) patients, with disease progression as the most frequent event (8 %). There were 9 patients who reported grade 3 SAEs and 1 with a grade 4 SAE (intractable pain). Only two SAEs were considered related to study therapy, both of which were related to infusion reactions during administration of the first infusion of cetuximab and resulted in discontinuation of treatment. Six patients died while on-study; cause of death in each case was underlying disease and was unrelated to study drug.
No new safety concerns related to laboratory data were identified during the present study. Based on observations of decreased magnesium levels in patients receiving cetuximab, a class effect of anti-EGFR monoclonal antibody therapies [29], the study protocol was amended prior to enrollment of the first patient to include collection of magnesium levels during the portion of the study when ECGs were performed. Serum chemistry changes from baseline are summarized in Table 4. Hypomagnesemia was reported for 11 (29 %, n = 38) patients while on cetuximab therapy; the majority were grade 1 and 2 with only a single grade 4 case. The most common serum chemistry abnormalities were low albumin (60 %) and elevations in glucose (82 %), alkaline phosphatase (56 %), and aspartate transaminase (42 %).
Discussion
Since its approval for colorectal cancer in both the USA and European Union in 2004, cetuximab has demonstrated clinical benefit in multiple tumor indications as a single agent or in combination with chemotherapy [19–25]. However, the effect of cetuximab on QT/QTc in humans was not rigorously characterized prior to initial approval, as is currently recommended for new pharmaceutical agents in the 2005 ICH E14 Guidelines [4]. Therefore, the primary goal of this study was to determine whether cetuximab showed any effect on QTc interval in patients with advanced solid tumors.
Cetuximab at the approved dose and dosing regimen (initial dose of 400 mg/m2 administered over 120 min on day 1 followed by a maintenance dose of 250 mg/m2 administered over 60 min weekly thereafter) did not result in a clinically meaningful prolongation of the QTc interval in this study. The ΔQTcF was small, and the one-sided upper 95 % confidence limit for the time-matched mean ΔQTcF was <10 ms at all time points studied. There were no QTcF values >500 ms and no time-matched ΔQTcF values ≥60 ms. No QTc abnormality was reported as an AE by the investigators. No serum concentration-dependent effect for cetuximab on QTcF or ΔQTcF was apparent. In addition, no effect of cetuximab treatment or its serum concentration was apparent on the PR interval, QRS interval, or heart rate.
Adverse events reported for patients on-study were consistent with the known safety profile for cetuximab. Additionally, the laboratory data did not reveal any new safety concerns for cetuximab. Given the propensity for cancer patients toward electrolyte imbalances which can exacerbate cardiac events, the added potential of cetuximab to cause decreased magnesium levels was of interest. The lack of any QTc prolongation in cancer patients with a variety of co-morbidities, susceptibility to hypomagnesemia, and receiving a variety of concomitant medications suggests that cetuximab can be administered as a single agent at the approved dose and schedule without risk of a clinically meaningful effect on the QTc interval.
Agents that target the HER receptor family exhibit differences in their potential for cardiotoxicity. Trastuzumab, a humanized monoclonal antibody targeting the HER2 receptor used in treatment for metastatic breast cancer, can cause decreased left ventricular ejection fraction, especially when administered with an anthracycline [30, 31]. QTc prolongation is not a reported event associated with trastuzumab. However, lapatinib, a tyrosine kinase inhibitor also targeting HER2, has been associated with QTc prolongation [4]. Given the differences observed between trastuzumab and lapatinib, both of which target HER2, the different spectrum of cardiotoxicities does not appear to be associated with the drugs’ target.
Cetuximab and panitumumab are both monoclonal antibodies, like trastuzumab, but their target is the EGF receptor (HER1). Panitumumab is a fully humanized monoclonal antibody with side effects that are similar to cetuximab, including rash, infusion reaction, and hypomagnesemia; cardiotoxicity has not been reported [32]. An ongoing phase 3 trial of panitumumab versus best supportive care in patients with metastatic colorectal cancer includes endpoints investigating the potential QT effects of panitumumab [33].
The present study of cetuximab was designed and conducted based on the parameters around QT interval length in the 2005 ICH E14 Guidelines. Specifically, we used as the limit for concern a QTc interval exceeding 500 ms during treatment or an increase >60 ms from baseline [15]. Applying those boundaries, the results of the present study do not indicate a concern for single-agent cetuximab at the approved dose. Generally, a QTc interval exceeding 500 ms is the point at which the risk of developing torsade de pointes increases significantly. However, there has yet to be an established threshold for QTc prolongation under which there is no longer any concern for arrhythmias to develop [34]. Likewise, there is no consensus on what constitutes a prolonged QTc interval other than the use of the NCI CTCAE Guidelines for grading severity of QTc prolongation. This leaves treating physicians with the dilemma of having no clear-cut parameters on which to base the decision of when to discontinue drugs that prolong the QTc interval. Until such a consensus is reached and guidelines are updated, a multidisciplinary approach may be best, in which cardiac risk factors are thoroughly explored in cancer patients prior to deciding upon a course of therapy, and patients are then closely monitored during that therapy [1, 2]. Indeed, while the results of this study address single-agent therapy with cetuximab, patients treated with cetuximab in combination with other drugs still need to be monitored due to the potential for new cardiac issues to arise from such combinations.
References
Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM (2010) Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. JNCI 102:14–25
Ederhy S, Izzedine H, Massard C, Dufaitre G, Spano JP, Milano G et al (2011) Cardiac side effects of molecular targeted therapies: towards a better dialogue between oncologists and cardiologists. Crit Rev Oncol/Hematol 80:369–379
Brana I, Taberno J (2010) Cardiotoxicity. Ann Oncol 21(Suppl 7):vii173–vii179
Yeh ETH, Bickford CL (2009) Cardiovascular complications of cancer therapy. J Am Coll Cardiol 53:2231–2247
Mirvis DM, Goldberger AL (2005) Electrocardiography. In: Zipes DP, Libby P, Braunwald E, Bonow RO (eds) Braunwald’s heart disease: a textbook of cardiovascular medicine, 7th edn. Saunders, Philadelphia, pp 107–151
Belardinelli L, Antzelevitch C, Vos MA (2003) Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol Sci 24:619–625
Bazett HC (1920) An analysis of the time relationship of electrocardiograms. Heart 7:353–370
Fridericia LS (1920) Die Systolendauer im Elektrokardiogramm bei normalen menschen und bei herzkranken [letter]. Acta Med Scand 53:489
Hodges M, Salerno Q, Erlien D (1983) Bazett’s QT correction reviewed. Evidence that a linear QT correction for heart rate is better (abstract). J Am Coll Cardiol 1:694
Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D (1992) An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 70:797–801
Strevel EL, Ing DJ, Siu LL (2007) Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 25:3362–3371
Bagnes C, Panchuk PN, Recondo G (2010) Antineoplastic chemotherapy induced QTc prolongation. Curr Drug Safety 5:93–96
Raschi E, Vasina V, Ursino MG, Boriani G, Martoni A, De Ponti F (2010) Anticancer drugs and cardiotoxicity: insights and perspectives in the era of targeted therapy. Pharmacol Ther 125:196–218
Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH (2002) Timing of new black box warnings and withdrawals for prescription medications. JAMA 287:2215–2220
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Steering Committee: ICH Harmonized Tripartite Guideline: The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, E14. Geneva, Switzerland, ICH 2005. Available at: http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html
FDA Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs 2006. Available at: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM129357.pdf
National Cancer Institute: Cancer therapy evaluation program, Common terminology for adverse events, version 3.0 DCTD, NCI, NIH, DHHS, 2006. Available from: http://ctep.cancer.gov/forms/CTCAEv3.pdf
Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J (1995) Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res 1:1311–1318
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Jonker DJ, O’Callaghan CJ, Karapetis CS et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Karapetis CS, Khambata-Ford S, Jonker DJ et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Van Cutsem E, Kohne C-H, Lang I et al (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29:2011–2019
Bonner JA, Harai PM, Giralt J et al (2006) Radiotherapy plus cetuximab for squamous cell carcinoma of the head and neck. N Engl J Med 345:567–578
Burtness G, Goldwasser MA, Flood W, Mattar B, Forastiere AA (2005) Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer. An Eastern Cooperative Oncology Group study. J Clin Oncol 23:8646–8654
Vermorkin JB, Mesia R, Rivera F et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127
Guidance for industry, E14 Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs, 2005. Available at http://www.fda.gov/cder/Guidance/6922fnl.pdf
Garnett CE, Beasley N, Bhattaram VA et al (2007) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18
Fracasso PM, Howard Burris III, Arquette MA et al (2007) Pharmacodynamic rationale for dosing study of cetuximab: pharmacokinetic and a phase 1 escalating single-dose and weekly fixed-dose. Clin Cancer Res 13:986–993
Pettrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Barni S (2012) Risk of anti-EGFR monoclonal antibody-related hypomagnesemia: systematic review and pooled analysis of randomized studies. Expert Opin Drug Saf 11(Suppl 1):S9–S19
Raschi E, De Ponti F (2012) Cardiovascular toxicity of anticancer-targeted therapy: emerging issues in the era of cardio-oncology. Intern Emerg Med 7:113–131
Albini A, Cesana E, Dontaelli F, Cammarota R, Bucci EO, Baravelli M et al (2011) Cardio-oncology in targeting the HER receptor family: the puzzle of different cardiotoxicities of HER2 inhibitors. Futur Cardiol 7:693–704
Keating GM (2010) Panitumumab: a review of its use in metastatic colorectal cancer. Drugs 70:1059–1078
NCT01412957: A Phase 3, Multicenter, Randomized, Open-label Trial to Evaluate the Survival Benefit of Panitumumab and Best Supportive Care, Compared to Best Supportive Care Alone, in Subjects with Chemorefractory Wild-type KRAS Metastatic Colorectal Cancer. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01412957?id=NCT01412957&rank=1. Accessed 12 July 2012
Al-Khatib SM, Allen La Pointe NM, Kramer JM, Califf RM (2003) What clinicians should know about the QT interval. JAMA 30:2120–2127
Acknowledgments
This study (NCT00698841) was sponsored by Bristol-Myers Squibb. The authors would like to acknowledge all the investigators, and their sites, participating in this study: Richy Agajanian, Vincent A. Armenio, John Deeken, Lourdes J. Feliciano, Troy H. Guthrie Jr, Jayne Gurtler, Donald W. Hill, Charles W. Hollen, Haresh S. Jhangjiani, Lawrence P. Leichman, Andre K.D. Liem, Hima Bindu Lingham, An D. Nguyen, Craig H. Reynolds, and. Brian J. Shimkus. The authors would also like to thank the patients as well as their families and caregivers. Medical writing and editorial support were provided by Jamie H. Zhang, PhD, an employee of Bristol-Myers Squibb.
Conflict of interest
Authors JFD, BS, AL, DH, and JG declared no conflict of interest. Authors EB, LT, and HL are employed by Bristol-Myers Squibb (BMS) and may own stock. Authors OT and SZ were both employed by BMS at time of study conduct and during early stages of manuscript preparation, but have since left that employment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deeken, J.F., Shimkus, B., Liem, A. et al. Evaluation of the relationship between cetuximab therapy and corrected QT interval changes in patients with advanced malignancies from solid tumors. Cancer Chemother Pharmacol 71, 1473–1483 (2013). https://doi.org/10.1007/s00280-013-2146-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2146-5