Abstract
The development of tyrosine kinase inhibitors (TKI) represents a major milestone in oncology. However, their use has been found to be associated with serious toxicities that impinge on various vital organs including the heart. Sixteen TKIs have been approved for use in oncology as of 30 September 2012, and a large number of others are in development or under regulatory review. Cardiovascular safety of medicinal products is a major public health issue that has concerned all the stakeholders. This review focuses on three specific cardiovascular safety aspects of TKIs, namely their propensity to induce QT interval prolongation, left ventricular (LV) dysfunction and hypertension (both systemic and pulmonary). Analyses of information in drug labels, the data submitted to the regulatory authorities and the published literature show that a number of TKIs are associated with these undesirable effects. Whereas LV dysfunction and systemic hypertension are on-target effects related to the inhibition of ligand-related signalling pathways, QT interval prolongation appears to be an off-target class III electrophysiologic effect, possibly related to the presence of a fluorine-based pharmacophore. If not adequately managed, these cardiovascular effects significantly increase the morbidity and mortality in a population already at high risk. Hitherto, the QT effect of most QT-prolonging TKIs (except lapatinib, nilotinib, sunitinib and vandetanib) is relatively mild at clinical doses and has not led to appreciable morbidity clinically. In contrast, LV dysfunction and untreated hypertension have resulted in significant morbidity. Inevitably, dilemmas arise in determining the risk/benefit of a TKI therapy in an individual patient who develops any of these effects following the treatment of the TKI-sensitive cancer. QT interval prolongation, hypertension and LV dysfunction can be managed effectively by using reliable methods of measurement and careful monitoring of patients whose clinical management requires optimisation by a close collaboration between an oncologist and a cardiologist, an evolving subspecialty referred to as cardio-oncology. Despite their potential adverse clinical impact, the effects of TKIs on hypertension and LV function are generally inadequately characterised during their development. As has been the case with QT liability of drugs, there is now a persuasive case for a regulatory requirement to study TKIs systematically for these effects. Furthermore, since most of these novel drugs are studied in trials with relatively small sample sizes and approved on an expedited basis, there is also a compelling case for their effective pharmacovigilance and on-going reassessment of their risk/benefit after approval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The focus of drug development in oncology over the last decade has shifted markedly from development of nonspecific cytotoxic drugs to molecularly targeted agents. In this regard, protein kinases have emerged as key pharmacological targets [1]. These enzymes regulate the majority of cellular biochemical pathways involved in transduction of extracellular signal, thus regulating cellular functions and responses including differentiation, proliferation and survival. These responses are fundamental to oncogenesis. Activity of up to 30 % of all human proteins may be modified by kinases. Protein kinases fall into two broad classes, depending on their substrate specificity: those catalysing phosphorylation of tyrosine and those that phosphorylate serine/threonine. A small minority phosphorylate all three. Since most of the agents at present target tyrosine kinases, we will for convenience refer to all protein kinases henceforth as tyrosine kinases.
The past decade has witnessed the approval of a number of tyrosine kinase inhibitors (TKI) for the treatment of a variety of cancers. These include a number of monoclonal antibodies and small-molecule TKIs approved for clinical use in oncology. Monoclonal antibodies act extracellularly by blocking the ligand from binding to its receptor and thereby interrupt downstream signalling. This review is concerned exclusively with the small-molecule TKIs, which act by intracellular inhibition of phosphorylation.
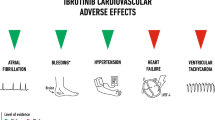
Concurrently, the safety of this new class of oncology drugs has also emerged as one of the major safety concerns, especially their cardiac safety [2, 3]. Following experience with drugs in a number of other pharmaco-therapeutic classes [4–6], regulatory authorities have routinely required all new drugs during their development to be characterised for their effect on cardiac repolarisation as reflected by changes in the duration of QTc interval (“QT liability”) of the surface electrocardiogram (ECG) and the risk of potentially fatal proarrhythmias [7, 8]. TKIs are no exception [9, 10] and also require their QT liability to be characterised during their development. Many TKIs have also been found to induce hypertension and impairment of left ventricular (LV) function, both major risk factors for cardiac safety [11, 12]. TKIs are labelled to be associated with a number of other serious cardiovascular adverse effects such as haemorrhage and arterial and venous thromboembolic events (Table 1). Two recent meta-analyses failed to confirm an increased risk of venous thromboembolism in association with VEGFR-related TKIs [13–16].
The purpose of this review is to provide a broad overview of the potential of the 16 antineoplastic TKIs approved as of 30 September 2012 to prolong the QT interval, induce hypertension or impair left ventricular (LV) function. Since the special focus of this review is their potential to prolong the QT interval, it is also our objective to discuss regulatory approaches to the assessment and communication of QT-related risk. To place the cardiac safety in the correct pharmacological and clinical context and better appreciate the impact of TKIs in oncology today, we provide a brief background of their pharmacological properties as well as the importance attached to this class of agents by the drug developers and regulatory authorities.
2 Data Sources
The information discussed in this review is derived from a variety of sources at the time of writing (31 October 2012). In particular, these include:
-
Assessment reports (“Reviews”) and the prescribing information (drug labels) posted on the Food and Drug Administration (FDA) website [17].
-
Briefing documents, presentations and discussions at the FDA Oncologic Drugs Advisory Committee (ODAC) [18].
-
Assessment reports (“European Public Assessment Report”) from the European Union’s (EU) Committee for Medicinal Products for Human Use (CHMP) and the EU prescribing information (Summary of Product Characteristics, SmPC), both posted on the European Medicines Agency (EMA) website [19].
-
Summary basis of decisions and product monographs issued by Health Canada [20].
-
Published literature, especially post-marketing studies and meta-analyses.
-
Available clinical study reports and results posted on the websites of the marketing authorisation holders.
-
National Cancer Institute (US) website [21].
In the prescribing information (the US label or the EU SmPC), the section most closely analysed was the section on “Warnings and precautions” in order to correlate its contents with the data reviewed by regulatory authorities or available elsewhere.
3 Molecular Biochemistry and Pharmacology of Small-Molecule Tyrosine Kinase Inhibitors
3.1 Molecular Biochemistry of Protein Kinases
The molecular biochemistry of protein kinases and their role in oncogenesis have been reviewed by other authors [1, 22]. The biochemical activity of a protein kinase involves enzymatic phosphorylation of one of three protein amino acids that have a free non-carboxyl hydroxyl group, namely tyrosine, serine or threonine [22]. Phosphorylation of proteins by these kinases is an important activating mechanism in the communication of signals within a cell and regulation of cellular activity and function [1, 22]. Kinase-mediated activation of proteins is known to regulate the majority of cellular biochemical pathways involved in the transduction of extracellular signals and thereby regulate cellular responses including differentiation, proliferation and survival. Thus, each kinase functions as an “on” switch in many cellular functions. Each protein kinase has a corresponding phosphatase to remove the phosphate group transferred and thus deactivate it. These phosphatases therefore serve as a “switch off” mechanism for terminating the signal.
Amongst the protein kinases, tyrosine kinases have attracted the greatest attention so far and are divided into two main families [22]. One family consists of transmembrane receptor-linked kinases (referred to as receptor tyrosine kinases or RTK) with a high affinity for many polypeptide growth factors, cytokines and hormones, which act as ligands of these receptors. Among the major RTKs in oncology are those linked to ligands such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF or c-MET), fibroblast growth factor (FGF) and insulin. These receptors phosphorylate themselves auto-catalytically upon ligand binding. The other family of tyrosine kinases consists of cytoplasmic proteins (referred to as non-receptor tyrosine kinases or NRTK). These are found in the cytosol, the nucleus and the inner surface of the plasma membrane. Major NRTKs in oncology at present are those linked to sarcoma (SRC), Janus-associated kinase (JAK) and tyrosine kinase from oncogenic transcript that results from fusion of the Abelson1 gene and breakpoint cluster region gene (BCR-ABL). Among the major serine/threonine receptor kinases are the BRAF (a member of rapidly accelerated fibrosarcoma) and protein kinase B (AKT) families. Except vemurafenib, all currently approved small-molecule kinase inhibitors are primarily TKIs. Vemurafenib is an inhibitor of a serine/threonine kinase but the development of other agents targeting this family of kinases is also gathering pace. Such is the pace of development in this area of oncology that Force [23] has estimated that there are more than 1,000 kinase inhibitors presently in development, and tens of thousands of patients, including children, are receiving these agents as part of clinical trials. According to our unpublished survey of the literature, no less than 50 of these kinase inhibitors are in fairly advanced stages of drug development.
The activities of tyrosine kinases are very tightly controlled and regulated but can also be overexpressed or the kinase become mutated and get stuck in the “on” position, resulting in a “gain-of-function” hyperactive kinase. This causes unregulated growth of the cell, a step necessary in oncogenesis. Not surprisingly, therefore, development of agents that inhibit responsiveness of these kinases is a major focus of pharmaceutical oncopharmacology. Efforts to identify TKIs have resulted in a diverse range of chemical structures but most of the TKIs in clinical use today are substituted quinazolines, pyrimidines and pyridines [24, 25].
3.2 Selectivity of TKIs
In contrast to the monoclonal antibodies, small-molecule TKIs interrupt downstream signalling by inserting into the ATP ‘pocket’ in the tyrosine kinase and blocking its activity. Because of their mode of action, TKIs are inherently less selective than the monoclonal antibodies and typically inhibit several kinases, some known and others hitherto unknown. The principal known pharmacological targets of the currently available TKIs are summarised in Table 2. Only a few of the currently approved agents such as axitinib, bosutinib, erlotinib, gefitinib, ruxolitinib and vemurafenib are selective enough for one or two receptor types. Most of the TKIs target a variety of receptors and the receptors most frequently targeted are VEGFR, EGFR, HGFR, PDGFR and KIT. When a ligand binds to its tyrosine kinase receptor, its phosphorylation is followed by activation of specific intracellular biochemical pathways downstream, such as the phosphoinositide 3-kinases (PI3K), AKT and MAPK pathways. It is therefore self-evident that the efficacy and effectiveness of TKIs also depend on the activity and responsiveness of further pathways downstream. For example, mutations in the downstream PI3K pathway are frequent in breast cancer, causing resistance to otherwise effective EGFR-2-targeted therapy. In general terms, the therapeutic outcome of EGFR-targeted therapy is suboptimal compared to VEGFR-targeted therapy. Studies using isolated cell lines have revealed that different cell lines can exhibit dramatically different responses to a given inhibitor. Therefore, it is important to evaluate an inhibitor for its selectivity of inhibition of kinase-mediated phosphorylation at the level of a whole organism to enable a more accurate evaluation of its efficacy and safety since toxicity could result from remote on- and off-target effects [26].
3.3 Toxicity of TKIs: On-Target and Off-Target Effects
As with most other small chemical molecules, some toxic effects of TKIs are unrelated to their primary pharmacodynamic activities and are therefore “off-target” effects resulting from the presence of unexpected secondary pharmacological properties. However, tyrosine kinases activate an array of proteins in virtually all organ systems, thus exerting unwanted effects at sites remote from the intended sites. Therefore, it is not surprising that the safety and efficacy of many TKIs are intricately linked to each other, both related to the inhibition of a primary pharmacological target. Thus, a number of major toxic effects are “on-target” effects. For example, TKIs that target angiogenesis (VEGFR) are typically associated with hypertension, hypothyroidism, haemorrhage and/or thrombosis [27–29]. In contrast, agents that target EGFR are more prone to induce diarrhoea or skin rash [30]. Appreciation of ligand-linked effects has prompted many investigators to evaluate some of these on-target side effects as potential biomarkers of effective pharmacological inhibition and therefore their efficacy [29, 31].
Lu et al. [32] exposed canine heart muscle cells to TKIs and other drugs known to prolong the QT interval and reported that drug-induced QT prolongation was actually due to inhibition of the PI3K signalling pathway, which affects multiple ion channels, not just the potassium channels. Drug-induced PI3K inhibition resulted in prolongation of action potential duration (APD) and the addition of a second messenger produced by the PI3K pathway normalised the APD. They also confirmed the finding by showing that mice bred to have reduced PI3K signalling showed QT prolongation. These findings have important implications in terms of QT liability being potentially an on-target class effect of any drug that downregulates PI3K and may call for a more refined approach to characterising drugs generally for their QT liability.
3.4 Pharmacokinetics of TKIs
The pharmacokinetic profile of a few of the currently available TKIs has been reviewed by others [24, 25]. Table 3 summarises the basic pharmacokinetics of the 16 TKIs approved at the time of writing this review. Most TKIs have poor absolute bioavailability (frequently unknown), are metabolised extensively and primarily by CYP3A4 and have a long elimination half-life permitting once a day administration but taking longer to attain steady state concentrations (and therefore the full therapeutic or toxic effects). These very long half-lives are double-edged swords since not only do they permit once a day administration and thereby improve compliance, but also require much longer periods for the TKI to clear from the body. Thus, patients require monitoring for an extended period after the drug has been discontinued as a result of a toxic effect. Although the metabolites of many TKIs are pharmacologically active, they do not circulate in high enough concentrations or are not potent enough (with the possible exception of imatinib, regorafenib and sorafenib) to be clinically relevant. Since the activity of CYP3A4 enzyme is highly susceptible to significant liver disease or to inhibition or induction by a number of co-medications, CYP3A4-mediated metabolism of TKIs predisposes the patient to major drug interactions and warrants caution and/or dose reduction in such patients. Some of the TKIs also have the potential to inhibit other drug-metabolising enzymes, thus further aggravating their drug interaction potential.
4 Regulatory Approval of Tyrosine Kinase Inhibitors
Because these new oncologic agents are highly targeted, they are perceived to be safer than the traditional cytotoxic agents used in cancer chemotherapy. In a study comparing three groups of antineoplastic agents, the clinical benefit derived from recently approved antineoplastic drugs was also found to be greater for targeted anticancer agents than for other chemotherapeutic agents [33]. As of 30 September 2012, 16 TKIs have been approved by the FDA, 14 of which are also approved for marketing by the European Commission, with bosutinib and regorafenib still under review by CHMP at that date. Regulatory and clinical interest in antineoplastic agents in this pharmacological class is illustrated by the fact that the first TKI, imatinib, was approved in 2001 and yet 7 of these 16 TKIs have been approved during the 18-month period from April 2011 to September 2012.
The majority of the approved TKIs are quinolone (bosutinib), quinazoline (erlotinib, gefitinib. lapatinib and vandetanib), pyrimidine (dasatinib, imatinib, nilotinib, pazopanib, ruxolitinib), pyridine (crizotinib, vemurafenib) or carboxamide (regorafenib, sorafenib, sunitinib) derivatives. Regulatory authorities have attached great importance to expedited review of many of these novel agents for a variety of cancers [34]. For example, the FDA had granted priority review to 12 and granted accelerated approvals to 6 (38 %) of the 16 TKIs approved in the US. By comparison, the CHMP granted conditional approvals to 4 (29 %) of the 14 TKIs approved in the EU. At least six others (afatinib, cabozantinib, dabrafenib, masitinib, ponatinib and tivozanib) were under regulatory review for marketing approval as of 30 September 2012. Another TKI, tofacitinib was approved by the FDA in November 2012 for use as an immunosuppressant in rheumatoid arthritis.
The clinical benefit of TKIs is considered sufficiently valuable that apart from six of them (bosutinib, gefitinib, lapatinib, nilotinib, sorafenib and vandetanib), none are absolutely contraindicated in the US despite having pharmacological properties and clinical effects that would otherwise have required certain contraindications. The contraindications applied to these drugs are modest and typically concern hypersensitivity to the drug (bosutinib, gefitinib, lapatinib and sorafenib), long QT syndrome (nilotinib and vandetanib) and uncorrected low serum potassium or magnesium (nilotinib). In the EU, all TKIs are contraindicated in patients with hypersensitivity to the drug but there are also other specific contraindications such as severe hepatic impairment (crizotinib), breast feeding (gefitinib, ruxolitinib and vandetanib), pregnancy (ruxolitinib) and patients with certain QT-related risk factors (vandetanib).
5 QT Liability of Tyrosine Kinase Inhibitors
5.1 Approaches to Investigating the QT Liability
Regulatory authorities have long been concerned with the potential of many drugs to delay cardiac repolarisation. This effect is reflected on the surface ECG by a prolonged heart rate-corrected QTc interval. In a vast majority of cases, drugs prolong the QTc interval by inhibiting the hERG (human ether-a-go-go) subunit of the channel conducting major ventricular repolarising potassium current (IKr) during phases 2–3 of the action potential [35, 36]. This manifests as prolongation of ventricular APD and its surface counterpart, the QTc interval. Although a prolonged QTc interval per se is not immediately harmful, it can induce potentially fatal ventricular tachyarrhythmias when it is prolonged excessively or in the presence of appropriate risk factors. The ventricular tachyarrhythmia most typically triggered is of a unique form known as torsade de pointes (TdP). This arrhythmia is most often transient but when sustained, it can give rise to symptoms of impaired cerebral circulation. In about 10–17 % of cases, it can degenerate into ventricular fibrillation, usually with a fatal outcome [37–39]. Regulatory authorities therefore require all new drugs, including oncology agents, to be investigated for their QT liability [7, 8]. Indeed, the FDA has established a QT Interdisciplinary Review Team (QT-IRT) whose remit is to assess the QT-related study protocols and data of all new drugs and, when appropriate, established drugs. In April 2010, the European Union’s Committee on Medicinal Products for Human Use also set up a QT Subgroup, reporting to its Cardiovascular Working Party.
Preclinically, the most frequent approaches to characterising a drug for its QT liability include in vitro studies on hERG-transfected expression systems and APD and in vivo studies, usually in dogs [7] but not uncommonly in monkeys. Clinically, the QT liability is typically investigated in a specially designed study, referred to as the thorough QT (TQT) study, in healthy volunteers who are administered a placebo, an active control and therapeutic and supratherapeutic doses of the investigational drug to determine if the effect of the drug breaches a preset threshold of regulatory concern [8]. The relationship between drug concentrations and changes in QT interval is also determined since this relationship provides important information for interpreting TQT studies [40]. Following experience with the drugs in other therapeutic classes, regulatory authorities have adopted a conservative risk-averse approach in mitigation of QT-related risk.
5.2 Investigating the QT Liability of Oncology Drugs
A TQT study may not be feasible or ethical with many oncology drugs if they are cytotoxic or genotoxic or not otherwise tolerated at clinical doses by healthy volunteers. Therefore, these drugs are studied intensively in patients by monitoring ECGs and plasma concentrations during routine clinical trials or, if needed, in a dedicated ECG study in the target population with appropriately designed protocols [41–43]. The regulatory guideline (ICH E14) suggests that until the effects of the drug on the QT/QTc interval have been characterised, patients with a baseline prolongation of QT/QTc interval (e.g. repeated demonstration of a QTc interval >450 ms) should be excluded from clinical trials [8]. However, the appropriate reference values of QTc interval for adult cancer patients were evaluated from two phase II studies [44, 45] and the data indicated that the distribution of QTc interval duration was greater in these patients when compared with results from a trial with similar ECG methods conducted in healthy volunteers and that an exclusion criteria of a QTc interval >450 ms for oncology trials would exclude more than 10 % of patients phase I or phase II studies because of marginally prolonged QTc interval at baseline. Given that different risk-benefit considerations apply in oncology, an exclusion criterion that may be more appropriate is a baseline QTc interval >470 ms.
5.3 Prescribing Information of TKIs and QT-Related Warnings
The description of the QT effect and its consequences for prescribing information also needs to be very realistic. Prescribing information of three TKIs (erlotinib, imatinib and regorafenib) has no information concerning their QT liability. Nine of the other 13 TKIs carry a standard set of warnings and cautions with respect to their potential to prolong the QTc interval and recommendations or restrictions during their clinical use. Prescribing information for the remaining four TKIs is either cautiously non-committal (axitinib and gefitinib) or simply describes the results of a negative study (bosutinib and ruxolitinib. In pre-approval trials, pazopanib and vandetanib were each associated with two cases of TdP, although the causal association with pazopanib in one case appeared uncertain since the patient was receiving amiodarone. Sudden deaths have been reported with nilotinib. Post-approval, sunitinib too was reported to induce TdP. Not surprisingly, the prescribing information of nilotinib (US) and vandetanib (US and EU) carries a boxed warning that includes contraindications for their use in patients who may be particularly susceptible to QT prolongation (the EU prescribing information being much more restrictive than the US). Periodic on-treatment ECGs are advised as a requirement for use of nilotinib, vandetanib and vemurafenib.
5.4 Evaluation of QT Liability of TKIs
All 15 TKIs approved since 2003 (that is, except imatinib, which was first approved in 2001) have undergone pre-approval regulatory scrutiny of their ECG effects with focus on their QT-liability. We gathered the QT-related preclinical and clinical data from the regulatory reviews of these TKIs, especially the pharmacology, medical and QT-IRT reviews of the data submitted to the FDA, and the prescribing information [17–20]. These preclinical and clinical data are summarised in Tables 4 and 5, respectively. We then reviewed the above data for an overall determination on whether a TKI had a QT liability.
We evaluated preclinical data by individual study and then collectively to determine whether a TKI has preclinical evidence of a QT liability on the basis of its IC50 value for the hERG channel, potency to prolong APD, and the magnitude and the nature of the effect in vivo studies. Values of hERG IC50 below 1 μM were regarded as worrisome whereas values higher than 3 μM were generally regarded as reassuring. Other parameters of particular relevance were the dose–response relationship and reverse-use dependency. The clinical data were also evaluated individually by data source and collectively. Clinical data were considered to indicate a TKI to have a QT liability if the upper bound of 95 % confidence interval around its study population-based mean maximum effect exceeded the regulatory threshold of 10 ms and there was evidence of a positive exposure–response relationship. Account was also taken of the proportion of patients who exhibited an absolute on-treatment QTc interval >500 ms or an increase of >60 ms from baseline. The decision on what proportion of patients with these outlier responses is clinically worrisome was a judgement based on our experience in this field and the frequency of outliers with other drugs known to be QT prolongers and/or torsadogenic as a reference.
5.5 Evaluation of Label Warnings on QT Liability in Relation to the QT Data
Our overall assessment of whether a TKI prolongs the QT interval was made on synthesis of the collective evaluations of preclinical and clinical data and is shown in Table 6. This suggests that the effect of TKIs that prolong the QT interval is relatively mild (95 % upper bound around their mean effects being around 15 ms) except for that of sunitinib, lapatinib, nilotinib and vandetanib (95 % upper bound around the mean effect being 22.4, 23.4, 25.8 and 36.4 ms respectively). It is worth stressing that QT interval prolongation per se is not immediately harmful and is not considered to be a good quantitative surrogate marker of the real risk, namely proarrhythmias.
Although there were no pre-approval studies on QT liability of imatinib, QT interval prolongation has not emerged as a safety issue during its extensive post-marketing use. A recent study also suggests that imatinib is unlikely to block hERG channels at therapeutic concentrations. It is reported to have an IC50 value of 19.51 and 44.76 μM/L in hERG expression systems using HEK-293 cells and Xenopus oocytes, respectively [46]. Despite a relatively large effect observed in clinical trials, QT interval prolongation has not proved to be a significant safety issue during post-approval clinical use of lapatinib or nilotinib [47–49]. Although widely used for over 6 years now, there are hardly any reports of sunitinib-induced QT interval prolongation in contrast to the number of reports of its effect on cardiac function, kidneys and thyroid gland. We appreciate that as a condition for its approval of vandetanib, the FDA has required a risk evaluation and mitigation strategy, which necessitates educating prescribers about the risk, appropriate monitoring and management of QT prolongation to help minimise the occurrence of TdP and sudden death. Only prescribers and pharmacies certified through the Vandetanib Risk Evaluation Mitigation Strategy Program, a restricted distribution programme, are able to prescribe and dispense vandetanib [50]. However, in one relatively small study of vandetanib in 73 patients with thyroid cancer, 10 (14 %) developed grade 3 QTc prolongation [51]. A larger meta-analysis of nine trials with 2,188 patients treated with vandetanib revealed that the overall incidence of all-grade and high-grade QTc interval prolongation was 16.4 % (95 % CI 8.1–30.4 %) and 3.7 % (8.1–30.4 %), respectively, among non-thyroid cancer patients, and 18.0 % (10.7–28.6 %) and 12.0 % (4.5–28.0 %), respectively, among thyroid cancer patients. Patients with thyroid cancer who had longer treatment duration also had a higher incidence of high-grade events, with a relative risk of 3.24 (1.57–6.71) compared to patients with non-thyroid cancer [52].
Our review of the data suggests to us that the current labelling fully reflects the currently available data for 11 of the 16 TKIs but the remaining 5 labels (axitinib, dasatinib, gefitinib, pazopanib and sorafenib) could have reflected the available data somewhat differently. Since the QT effect is a concentration-driven effect, lack of an exposure–response relationship is a persuasive argument against a drug having a QT liability (as in the cases of dasatinib, erlotinib and pazopanib). Therefore, given the nature of the indications for these drugs and the rarity of TdP, we conclude from the available data that the labels for (1) axitinib and dasatinib could be free from any QT-related warnings, (2) gefitinib should reflect a degree of uncertainty in this regard and (3) pazopanib and sorafenib should have less restrictive warnings than are currently included in terms of their QT liability. We acknowledge that the FDA has approved regorafenib on interim data only and has required the sponsor to complete a clinical trial evaluating its potential to prolong the QTc interval in an adequate number of patients. Review of the pre-approval data available suggests that the QT-prolonging potential of regorafenib is likely to be negligible (that is, it is a non-QT-prolonger).
5.6 QT Liability of TKIs: Is it Class-Related?
Inevitably, a question arises as to whether the QT liability of TKIs may be a pharmacological effect linked to inhibition of one or more tyrosine kinases, which may regulate hERG function (on-target effect) or an effect related to a particular chemical class (off-target effect). Alternatively, it may be an incidental secondary pharmacological off-target effect that is associated with approximately 200 other drugs scattered across a range of other pharmaco-therapeutic classes.
Although the evidence summarised below may suggest an on-target effect, we believe that probably not to be the case. With regard to a relationship between QT liability and inhibition of a tyrosine kinase, a number of previous studies have suggested interactions between the activity of protein kinases and functions of ion channels, especially the hERG channel. Mechanisms that modulate hERG channel activity are now being elucidated, in particular its regulation by serine/threonine phosphorylation [53, 54]. Protein kinase A (PKA) has been reported to be involved in the regulation of IKr in guinea pig cardiac myocytes and hERG channels expressed heterologously in Xenopus oocytes [55]. A detailed discussion of these interactions between protein kinases and ion channels is beyond the scope of this review, and we refer the interested reader to a comprehensive review by Davis et al. [56] on how ion channels may be regulated by protein phosphorylation and dephosphorylation of serine, threonine and tyrosine residues by their specific kinases and phosphatases. Since that review, the complexities of these interactions have been further unravelled and we summarise below some key observations that we consider relevant:
-
Cyclic AMP-dependent protein kinase A (PKA) is a key enzyme for numerous regulatory processes in almost all types of cells. This serine/threonine kinase can be stimulated by extracellular signals that elevate intracellular cAMP concentrations [54]. β-Adrenergic stimulation increases IKs via channel activation by direct phosphorylation by PKA [57].
-
hERG channels can be subject to acute regulation by changes in cAMP and cAMP-dependent PKA. Sustained elevation of cAMP, and therefore of PKA activity, profoundly affects hERG protein abundance by a mechanism that includes enhanced protein translation, current density and rates of synthesis [58].
-
PKA phosphorylation of hERG protein is also believed to regulate the rate of channel synthesis [58], a process that is believed to occur at the endoplasmic reticulum surface through a highly complex process [59, 60].
-
Normal hERG function in HEK293 cells requires basal activity of protein kinase B (PKB, also known as AKT), another serine/threonine protein kinase [61]. Activation of PKB occurs downstream of PI3K (the PI3K/AKT/mTOR pathway and other signalling pathways).
-
hERG is modulated not only by the non-receptor SRC family of tyrosine kinases, but also by the EGF family of receptor tyrosine kinases [62]. Regulation of hERG channels by tyrosine kinases modifies the channel activity and thus likely alters the electrophysiological properties, including action potential duration and cell excitability, in human heart and neurons.
-
As stated earlier, Lu et al. [32] have recently shown that direct downregulation of PI3K signalling, or indirectly via tyrosine kinase inhibition, prolongs the QT interval by affecting multiple ion channels.
Whilst these complex interactions may at first suggest a class-related effect of TKIs that acts on certain kinase(s) linked to a specific ligand and inhibition of PI3K, there is no indication at present of a consistent or predictable link. No pattern emerges on reviewing the QT liability of the 16 drugs. TKIs with and TKIs without a QT liability inhibit tyrosine kinases that are frequently linked to the same ligand or set of ligands. For example, VEGFR is inhibited by sorafenib and vandetanib, both of which are QT prolongers, but also by axitinib, which is devoid of this effect. Similarly, PDGFR, KIT and BCR-ABL are inhibited by nilotinib, which has a potent QT effect, as well as by dasatinib, which seems to be without a similar QT effect. TQT studies have also been performed with three other TKIs (neratinib, which is a pan-ERB inhibitor; lenvatinib, which is a VEGFR inhibitor; tofacitinib, which targets JAK). These studies have also shown these three drugs to lack a QT liability. To complicate the matter further, one of the kinase inhibitors (BAY-79) was discontinued from further development because, at plasma concentrations slightly higher than those predicted to be therapeutically efficacious in humans, it was associated with QTc interval shortening in dogs, although there was no known mechanistic explanation for this [63], and this finding may have had no clinical relevance.
A QT liability arising from the chemical class of the TKI would also seem unlikely since TKIs from all chemical classes seem to be affected. One anilinoquinazoline (vandetanib) has an effect potent enough to require a boxed warning, while two others (gefitinib and erlotinib) have only questionable effect at worst. Similarly, one aminopyrimidine (nilotinib) carries a boxed warning while another (dasatinib) seems to be devoid of this effect.
5.7 QT Liability of TKIs and a Putative Class III Pharmacophore
In common with a number of QT-prolonging drugs in other therapeutic classes, a structural feature that distinguished TKIs that we consider to be QT prolongers from the non-prolongers is the presence of a fluorophenyl or fluoromethyl-phenyl ring. The correlation between the QT liability and the presence or absence of this structural feature is summarised in Table 7, which also includes 8 TKIs other than the 16 we have reviewed in this article. Morgan and Sullivan [64] suggested long ago that the 4-fluorophenyl group may play a role in determining a class III electrophysiologic profile of some compounds. It is interesting to speculate whether for TKIs as a class, a fluorinated phenyl ring (not necessarily only 4-fluorophenyl) is also a potential QT-prolonging pharmacophore that confers class III electrophysiological properties. If this were so, the only exception among the 16 TKIs reviewed here seems to be regorafenib (in addition to afatinib, which is not yet approved). Structurally, regorafenib is almost indistinguishable from sorafenib, including the presence of a trifluoromethyl-phenyl ring. Since regorafenib is sorafenib with an extra fluorine group, it is sometimes referred to as fluoro-sorafenib. Therefore, until more rigorous data are available, its structure suggests that careful surveillance be maintained for its QT liability notwithstanding the preliminary reassuring data. We acknowledge that absence of this structure does not imply the lack of a potential QT liability since there are other known class III pharmacophores [64] and other substituents that modify class III activity. Therefore, the presence of a fluorinated phenyl ring should serve as a structural alert leading to a more diligent evaluation of the drug concerned and assessment of its QT liability.
Regarding the pharmacokinetic parameters, a number of functional groups in the chemical structure of a drug can influence its metabolic fate and therefore the half-life. Fluorination is believed to protect against metabolic attack and increase the half-life of a drug [65, 66]. Without explicitly suggesting a causal relationship, we note with interest that the half-lives of the 11 fluorinated drugs in Table 7 that prolong the QT interval are longer compared to the 11 non-fluorinated drugs that do not (mean values approximately 75 versus 25 h).
6 Non-QT Cardiovascular Safety of Tyrosine Kinase Inhibitors
As summarised in Table 1, TKIs are also associated with a number of other serious cardiovascular adverse effects unrelated to QT interval. Available evidence suggests that some of these cardiovascular effects arise as a result of remote and wider on-target effects of TKIs and therefore may be intricately linked to their efficacy. However, there appear to be inconsistencies concerning the incidence and interval to onset of various on-target effects.
Hypertension appears to be a downstream consequence of disruption or inhibition of VEGFR-mediated angiogenesis but the data are not that clear cut regarding haemorrhage and thromboembolism. Although the occurrence of haemorrhage and thromboembolism from the same drug may seem paradoxical, they are both believed to be underpinned by the same mechanisms. Within the microvasculature, there is an extremely tightly regulated balance of pro- and anticoagulant proteins, platelet-activating and -inhibiting factors, and pro- and anti-fibrinolytic products [67]. Disruption of this intricate balance could tip the system either way, promoting thromboembolism or haemorrhage.
We provide an overview of three serious cardiovascular adverse effects of TKIs (systemic and pulmonary hypertension and LV dysfunction) and refer the interested reader to other detailed reviews for further information [67–72].
6.1 Systemic Hypertension
6.1.1 Incidence
Hypertension is the most frequently observed toxicity associated with inhibitors of VEGFR such as axitinib, pazopanib, regorafenib, sorafenib, sunitinib and vandetanib. Its incidence is typically in the order of 20–30 % but may be higher with some agents and it often varies with the indication. Mir et al. [73] have discussed the problems of quantifying the effect of TKIs on blood pressure. Foremost are the criteria used to diagnose hypertension and the frequency with which blood pressure is measured. Furthermore, there may be increases in mean blood pressure without it reaching any predefined threshold for diagnosing hypertension. In pre-approval clinical trials in patients with RCC, the incidence of hypertension was 40 % (16 % was grade 3–4) with axitinib and 29 % (11 % was grade 3–4) with sorafenib, the comparator used. In a study of 75 patients, treatment with sunitinib induced significant increases in mean systolic (21 mmHg) and diastolic (14 mmHg) blood pressures, and 47 % (35/75) of these patients developed hypertension (>150/100 mmHg) [74]. Mir et al. [73] summarised data indicating a relative risk for all-grade hypertension of 6.11 with sorafenib and 21.6 with sunitinib. The incidence also varies depending on the indication (tumour type), being higher in renal than in hepatocellular (sorafenib) and gastrointestinal stromal tumours (sunitinib) [70]. This may be a reflection of different doses, co-medication or duration of therapy. For example, a meta-analysis of 10 trials that included 3,154 patients (majority with thyroid and lung cancers) treated with vandetanib reported summary incidences of all-grade and high-grade hypertension of 24.2 and 6.4 % respectively, but the incidence of all-grade hypertension across these 10 trials ranged from 4.2 to 39.6 % [75]. The incidences of all-grade and high-grade hypertension were higher in patients with medullary thyroid cancer than with non-small-cell lung cancer, and higher following longer duration of treatment in medullary thyroid cancer [75]. Hypertensive crisis has also been reported on occasions with axitinib, pazopanib, sorafenib and vandetanib. The current consensus is that provided TKI-induced hypertension is adequately treated, patients can continue to receive the TKI therapy.
6.1.2 Interval to Onset
The median time to onset of hypertension in clinical trials with axitinib therapy was within the first month of the start of treatment. Hypertension occurs early following treatment with pazopanib (40 % of cases occurred by day 9 and 90 % of cases occurred in the first 18 weeks). However, increases in blood pressure reaching pre-defined thresholds for diagnosing hypertension have been observed as early as 3 days on cediranib [76] and 4 days on axitinib [17]. Lesser increases may occur even earlier. Maitland et al. [77] reported a mean increase of 8.2 mmHg systolic and 6.5 mmHg diastolic pressures within the first 24 h of sorafenib therapy. In the sunitinib study referred to above [74], significant elevation in blood pressure occurred within the first 4 weeks of therapy. Veronese et al. [78] reported that 75 % of 20 sorafenib-treated patients developed an increase in systolic pressure >10 mmHg and 60 % exhibited an increase >20 mmHg after 3 weeks of therapy. Patients with greater increases in VEGF levels during treatment tended to have less increase, but the correlation was not statistically significant.
6.1.3 Mechanism
The exact molecular mechanisms and pathophysiology underpinning hypertension induced by VEGFR inhibitors are not fully understood. It is known, however, that vascular endothelium is physiologically highly active, secreting vasodilators such as nitric oxide and prostacyclin, and stimulation of VEGFR results in reduction of blood pressure. The onset of hypertension, as early as 3–4 days, suggests acute inhibition of VEGFR. It is therefore not surprising that hypertension is the most frequently observed toxicity associated with TKIs that potently inhibit VEGFR, especially the VEGFR2. In support of this notion is the finding that in patients with renal cell cancer (RCC), the VEGF-634CC genotype was associated with substantially decreased frequency and duration of sunitinib-induced hypertension compared to the VEGF-634GG genotype (8.9 versus 27.2 %) [79]. Vascular rarefaction from impaired angiogenesis, resulting in an increase in peripheral resistance, has been proposed as an alternative mechanism for hypertension associated with TKIs, but this does not explain the rapid onset of hypertension so often observed, and its amelioration on discontinuation of treatment, with a VEGFR inhibitor. Other studies, however, have suggested that a contributory role of vascular rarefaction cannot be discounted [80]. According to data published by GlaxoSmithKline on its website, the cumulative incidence of hypertension (systolic pressure ≥160 mmHg or diastolic pressure ≥100 mmHg) was 28, 44 and 57 % by days 9, 22 and 29, respectively, following treatment with pazopanib [81]. This time-dependent increase in frequency also supports a role of vascular rarefaction in induction of TKI-associated hypertension.
6.2 Pulmonary Arterial Hypertension
Among the TKIs approved so far, dasatinib is the only one well documented to induce pulmonary arterial hypertension (PAH). Quintás-Cardama et al. [82] have reported a dose-dependent increase in right ventricular systolic pressure in a subgroup of 18 patients receiving dasatinib therapy and this normalised in 10 of them following cessation of therapy. Dasatinib-induced PAH may have a long period of latency, as much as a year, before it becomes manifest and can be severe enough to give rise to cor pulmonale. Since the complication is typically reversible, it is recommended that patients are evaluated for any underlying cardiopulmonary disease prior to initiating dasatinib therapy and during treatment and that the drug should be discontinued if PAH develops. According to their FDA labels, there are isolated reports of PAH in association with bosutinib and nilotinib. The quality of these reports is unknown and the literature contains only very rare cases of this complication in association with nilotinib [49].
6.2.1 Mechanism
The molecular mechanism responsible for pathogenesis of dasatinib-induced PAH is unknown [83]. A major problem has been that none of the experimental models currently used entirely replicates the PAH observed in patients. However, progression of PAH is associated with increased proliferation and migration of pulmonary vascular smooth muscle cells. PDGF is a potent mitogen involved in this process. PDGF alone or multiple growth factors are able to stimulate pulmonary arterial smooth muscle cell proliferation and migration in vitro [84]. Thus, the available experimental evidence strongly implicates PDGF in the pathogenesis of PAH. Apart from inhibiting BCR-ABL, imatinib also inhibits PDGFR. There is compelling evidence suggesting that imatinib has a beneficial effect in experimental animal models of PAH [85]. Case reports of patients with severe PAH have also suggested that imatinib can improves their clinical condition [86–91]. This anecdotal evidence from case reports has been replicated in a controlled clinical study. In 42 patients who completed the study, there was a significant decrease in pulmonary vascular resistance and increase in cardiac output on imatinib when compared to placebo [92]. PDGF-BB is the ligand with the highest affinity for PDGFRβ and upregulated levels of this ligand in patients with pulmonary hypertension have been shown to be suppressed by imatinib [93]. In pulmonary artery endothelial cells, tryptophan hydroxylase (TPH1) regulates the synthesis of serotonin, which is a potent pulmonary vasoconstrictor. Further evidence on the role of PDGFR-β comes from chronic hypoxia/SU5416 murine model of PAH, which is believed to recapitulate many of the hallmarks of the human disease [94]. In this model, imatinib has been reported to induce downregulation of TPH1 via inhibition of PDGFR-β signalling [95]. These investigators also report that in post hoc subgroup analysis, patients with PAH with greater haemodynamic impairment showed significantly reduced serotonin plasma levels after imatinib treatment compared with placebo. This finding highlights a complex interplay between PDGF and other pathways in PAH. Sorafenib and sunitinib are currently under investigation for their use in PAH because of their inhibitory effects on PDGF, VEGF and other pro-proliferative signalling pathways [96].
6.3 Left Ventricular Dysfunction
LV dysfunction is now a well-recognised toxicity of a number of TKIs. It can range from asymptomatic ECG changes (in QRS and T-waves and ST-segment) through decrease in LV function (detectable noninvasively by echocardiography or radionuclide techniques) to severe cardiac failure and fluid retention (including pleural and pericardial effusions and pulmonary oedema). Diastolic dysfunction, cardiomyopathy and pericarditis are other clinical manifestations of cardiotoxicity reported with TKIs. It is suggested that LV dysfunction induced by TKIs is generally reversible except in patients who have only a marginal reserve where a transient decrease in contractility produces sufficient stress to result in secondary cell death. Many patients who recover may go on to tolerate further re-exposure to the TKI concerned for longer periods. This is in contrast to LV dysfunction because of anthracyclines that are directly cytotoxic to the cardiac myocytes.
6.3.1 Incidence
The TKIs most frequently reported to induce these effects are bosutinib, dasatinib, lapatinib, nilotinib, pazopanib, sorafenib, sunitinib and vandetanib. Predisposing factors are previous anthracycline therapy and TKI-induced hypertension. The incidence appears highest with sunitinib and pazopanib (5–8 %). In a study by Chu et al. [74], 11 % of 75 sunitinib-treated patients suffered a cardiovascular event with congestive heart failure occurring in 8 % (6/75) of the population. Of the 36 patients who had received FDA-approved doses of sunitinib, 28 % (10/36) had LVEF declines of ≥10, and 19 % (7/36) experienced LVEF declines of ≥15 %. Congestive heart failure and LV dysfunction in these patients responded to withholding drug and instituting medical management. The likely scale of this toxicity is illustrated by a study by Schmidinger et al. [97]. Following a prospective study of 74 consecutive eligible patients treated with sunitinib or sorafenib for metastatic RCC, these investigators reported that 25 (34 %) patients (11 on sunitinib and 14 on sorafenib) had a cardiovascular event. ECG changes were present in 12 of the 25 patients, creatine phosphokinase and troponin T were elevated in 17 and 9 patients, respectively, and echocardiographic findings were abnormal in 10 patients. No statistically significant differences were found between sunitinib and sorafenib patients in terms of cardiovascular events. All patients recovered after cardiovascular management and were considered eligible for TKI continuation. Thus, patients who recover may go on to tolerate re-exposure for long periods of time. In a longitudinal study of 81 patients treated with nilotinib, cardiac function, as assessed by measurement of LVEF, did not change significantly from baseline at any time-point during a median follow-up period of 26 (1–72) months [49]. Imatinib can also give rise to severe LV dysfunction in patients with hypereosinophilic syndromes. Although Kerkelä et al. [98] described ten imatinib-treated patients in whom LVEF declined markedly (from 56 ± 7 % pre-treatment to 25 ± 8 %), post-marketing safety surveillance of imatinib suggests that its potential for cardiotoxicity is relatively low.
In routine clinical oncology, the scale of the problem may be much higher. Patients with cardiac disease who are the most at risk of cardiotoxicity have traditionally been excluded from clinical trials. Lenihan et al. [99] have reported that at the European Institute of Oncology, inaugurated in May 1994, the percentage of cancer patients admitted who had a cardiac consultation was 7 % at the end of 1st year in June 1995 and this had increased to 77 % at the end of 15th year in December 2008. Thus, the extent of cardiotoxicity from TKI treatment in routine oncology practice may be a largely underestimated toxicity.
6.3.2 Interval to Onset
For most agents, this information is poorly documented. The interval to onset of LV dysfunction from initiation of therapy is about 12 weeks with lapatinib. In one of the pazopanib studies submitted to support a marketing approval, none of the 74 patients had a LVEF <40 % following a median treatment exposure of 2.9 (0.2–15.3) months [17]. However, one patient had a 10 % decrease in LVEF to a level below the institute lower limit of normal. One additional patient had a decline from baseline LVEF of 55 to 45 % and pazopanib was discontinued by the investigator. It is unclear whether longer exposure may be associated with a significant risk of decreasing LVEF with pazopanib. In the study by Chu et al. [74], 6 of the 75 patients treated with sunitinib developed congestive heart failure with a mean interval to onset of 27 weeks. In the study by Schmidinger et al. [97] referred to earlier, the median duration of therapy with sunitinib or sorafenib at the time of cardiovascular event was 8 weeks. In the ten cases with imatinib-induced decline in LVEF reported by Kerkelä et al. [98], the mean follow-up period was 7 months.
It is recommended that the ejection fraction be measured at baseline and regularly while the patient is receiving pazopanib or lapatinib, but this would seem prudent with all TKIs known to be potentially associated with this effect. Therapy should only be started if the baseline ejection fraction is normal and discontinued or dose adjusted depending on the intensity of the effect observed during treatment. One practical consequence of this requirement for a normal pre-treatment LVEF is the dilemma regarding how to treat patients with pre-existing cardiac disease who have a malignancy that is potentially TKI-sensitive. The risk/benefit in these patients is unclear and needs careful evaluation.
6.3.3 Mechanism
The mechanism underlying this effect is not well understood either and the reader is referred to other reviews for further details on this subject [11, 12, 22, 68, 71, 100–102]. Sunitinib is well known to cause cardiotoxicity, but of the several targets of sunitinib, only PDGF receptor is expressed in cardiac myocytes. PDGFβ signalling is known to be important in the development of heart [103], and recently, PDGF has been demonstrated to have some cardio-protective effects. It seems possible that inhibition of PDGFR in the heart may be a contributory factor in the cardiotoxicity associated with the use of some of these TKIs [100]. Cheng et al. [104] have also provided preliminary evidence that suggests that downstream inhibition of Raf/MEK/ERK pathway probably mediates, at least in part, the cardiotoxicity associated with sorafenib. Histological studies of the affected ventricle suggest the possibility of an adverse effect on mitochondrial function [74] and/or inhibition of AMP-activated protein kinase (AMPK) [105, 106]. The latter is an important regulator of cellular energy metabolism and is required to maintain normal cardiac contractility. These findings have led these investigators to suggest that sunitinib-induced cardiotoxicity results from an off-target effect. Hasinoff has shown that damage to myocytes gave a good rank order correlation with clinically observed cardiotoxicity [107]. Following a study of 18 kinase inhibitors (canertinib, dasatinib, dovitinib, erlotinib, flavopiridol, gefitinib, imatinib, lapatinib, midostaurin, motesanib, pazopanib, sorafenib, staurosporine, sunitinib, tandutinib, tozasertib, vandetanib and vatalanib), Hasinoff and Patel [108, 109] suggested that the combined tyrosine kinase and serine-threonine kinase selectivity scores were highly correlated with the myocyte-damaging effects of the kinase inhibitors studied and that myocyte damage was due to a lack of target selectivity to binding of both tyrosine kinases and serine-threonine kinases and was not due to binding to either group specifically. Kinase inhibitor binding was significantly correlated with myocyte damage for 12 kinases, leading them to conclude that myocyte damage may be multifactorial in nature with the inhibition of a number of kinases involved in producing kinase inhibitor-induced myocyte damage.
7 Characterising and Managing the Cardiac Safety of TKIs
A number of TKIs are under regulatory review and many more are under development. The next advance in vasculature-targeted drug development is expected to come from the development of tumour vascular-disrupting agents (VDAs) and other novel mechanisms such as inhibition of farnesyl protein transferase (FPTase). In contrast to VEGF-based angiogenesis inhibitors, VDAs selectively disrupt existing tumour vasculature with minimal impact on the inhibition of angiogenesis or normal tissue vasculature. FPTase inhibitors selectively inhibit post-translational farnesylation of Ras proteins and other cell-signalling pathways, thereby inducing antitumour effects. Early indications suggest that these novel classes of drugs share many of the adverse cardiovascular effects, including QT liability, known to be associated with TKIs already approved [9, 110].
The strategy for investigating the QT liability is already described in regulatory guidelines [7, 8]. There is now a need for similar initiatives with regard to LV dysfunction. Force and Kerkela [111] have described some preclinical and clinical approaches to identify early the potential of a TKI to induce LV dysfunction. Given the extent of the concern on the effect of TKIs on LV function, it is not inconceivable that, in future, regulatory recommendations may also include characterisation of their effect on cardiac function. Yang and Papoian [112] from the FDA have recently commented that reports of cardiac toxicity following treatment with TKIs were unexpected and not well predicted by preclinical studies and that clinical findings of cardiotoxicity have exposed gaps in current preclinical drug testing for predicting the development of cardiac toxicities in humans. They recommend better preclinical investigation of the cardiotoxic potential of TKIs and have suggested some preclinical strategies they consider appropriate.
In order to achieve the challenging goals of achieving anticancer efficacy and optimising risk/benefit at an individual patient level, an International CardiOncology Society was established in January 2009 to facilitate a partnership between the two specialties [99] and learned societies have also begun to issue guidelines on the management of cardiac toxicity in cancer patients [113, 114].
8 Conclusions
In addition to their adverse cardiovascular effects, TKIs are also associated with other serious adverse effects such as hepatotoxicity, pneumonitis, skin and hypersensitivity reactions, hypothyroidism, nephrotoxicity and gastric perforation. Although the safety profile of each TKI varies in terms of the frequency and severity of these effects, the use of TKIs clearly carries significant risks. Arising from the common mechanisms involved, the efficacy of many TKIs is de facto associated with treatment-related morbidity and mortality. Compared to patients in clinical trials, patients in routine oncologic practice have an increased likelihood of toxicity and lower probability of benefit. As the number of these agents approved increase, and their indications expand in scope, their use is expected to increase markedly with the attendant increase in the frequency of toxicity in these patients. It is therefore necessary to better characterise the safety profile, including cardiovascular effects, of these agents. Our key conclusions concerning cardiovascular toxicity of TKIs can be summarised under four headings.
8.1 Regulatory and Drug Development Issues
The data we have reviewed suggests that the effect of TKIs on LV dysfunction may be associated with morbidity far greater than that associated with their QT liability. The incidence, severity and reversibility of LV dysfunction in association with TKIs are not sufficiently well characterised. As is the case with QT liability, LV dysfunction and hypertension need to be systematically characterised during the development of TKIs. Common standards are required for characterising these effects. The exclusion criteria (baseline QT interval of LV ejection fraction) that eliminate patients with cardiovascular morbidity from clinical trials may need to be relaxed as long as the patients are carefully monitored. Given the nature of the indication, a more pragmatic and less risk-averse approach to communicating the risk may be appropriate such as a more realistic emphasis on QT effects of supratherapeutic doses. The therapeutic doses may be quite safe in the absence of concurrent medications that increase plasma concentrations.
8.2 Clinical Practice Issues
Cardio-oncologists need to be better informed of the risk factors for cardiac toxicity. Diarrhoea and vomiting are among the most frequent, and often severe, effects of almost all TKIs. The resulting electrolyte imbalance may well aggravate the QT-prolonging effects of the agents concerned. In routine clinical practice, patients may have cardiovascular comorbidities and been prescribed a range of co-medications, including CYP3A4 inhibitors such as antiemetics and antibacterial and antifungal agents and those that prolong QT interval. CYP3A4-mediated metabolism of all these TKIs also exposes the patient to the risks of clinically relevant drug-drug interactions. Monitoring ECG waveforms and intervals may be very challenging in elderly cancer patients who have cardiac comorbidities. It is also vital that, as in healthy volunteers, reliable methods are used to measure this interval and other ECG parameters.
8.3 Management of Patients by Cardio-Oncologists
Hypertension and LV dysfunction can be readily managed whereas PAH has not proved to be a significant clinical issue with TKIs. Provided the patients are carefully monitored using reliable methods and correctly managed, it should be possible to achieve anticancer efficacy and optimise risk/benefit at an individual patient level. Patients treated with TKIs should ideally be managed in collaboration with other appropriate specialists such as cardiologists. Oncologists should lead a multi-specialty team when managing cancer patients.
8.4 Pharmacovigilance
Patients with risk factors for TKI-induced cardiovascular morbidity or mortality account for a substantial proportion of patients in oncology practice. Exclusion of these patients from clinical trials, coupled with the approval of TKIs on priority basis with studies of relatively small sample size may further aggravate the problem of adequate risk/benefit assessment in routine clinical practice. Therefore, we believe that the post-marketing safety of TKIs should be carefully monitored through diligent pharmacovigilance and their efficacy, safety and risk/benefit analysis requires on-going reassessment.
References
Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–87.
Strevel EL, Siu LL. Cardiovascular toxicity of molecularly targeted agents. Eur J Cancer. 2009;45(Suppl 1):318–31.
des Guetz G, Uzzan B, Chouahnia K, et al. Cardiovascular toxicity of anti-angiogenic drugs. Target Oncol. 2011;6:197–202.
Shah RR. The significance of QT interval in drug development. Br J Clin Pharmacol. 2002;54:188–202.
Shah RR. Cardiac repolarisation and drug regulation: assessing cardiac safety 10 years after the CPMP guidance. Drug Saf. 2007;30:1093–110.
Shah RR. Drug-induced QT interval prolongation: does ethnicity of the thorough QT study population matter? Br J Clin Pharmacol. 2013;75:347–58.
Committee for Medicinal Products for Human Use. ICH note for guidance: the nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals (ICH S7B) (CHMP/ICH/423/02). EMA, London (2005). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002841.pdf (Accessed Sep 22 2012).
Committee for Medicinal Products for Human Use. ICH note for guidance: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs (ICH E14) (CHMP/ICH/2/04). EMA, London (2005). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002879.pdf (Accessed Sep 22 2012).
Strevel EL, Ing DJ, Siu LL. Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol. 2007;25:3362–71.
Ederhy S, Cohen A, Dufaitre G, et al. QT interval prolongation among patients treated with angiogenesis inhibitors. Target Oncol. 2009;4:89–97.
Garcia-Alvarez A, Garcia-Albeniz X, Esteve J, Rovira M, et al. Cardiotoxicity of tyrosine-kinase-targeting drugs. Cardiovasc Hematol Agents Med Chem. 2010;8:11–21.
Force T, Kolaja KL. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat Rev Drug Discov. 2011;10:111–26.
Qi WX, Min DL, Shen Z, et al. Risk of venous thromboembolic events associated with VEGFR-TKIs: a systematic review and meta-analysis. Int J Cancer 2012 (Epub ahead of print).
Sonpavde G, Je Y, Schutz F, et al. Venous thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2013 (Epub ahead of print).
Choueiri TK, Schutz FA, Je Y, et al. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–5.
Schutz FA, Je Y, Richards CJ, et al. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol. 2012;30:871–7.
Food and Drug Administration Product Reviews and Labels. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm (Accessed Oct 28 2012).
Food and Drug Administration Oncologic Drugs Advisory Committee Documents. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/default.htm (Accessed Oct 28 2012).
European Medicines Agency European Public Assessment Reports Assessment History and Product Information. http://www.emea.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124 (Accessed Oct 28 2012).
Health Canada Summary Basis of Decision. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/index-eng.php (Accessed Oct 28 2012).
National Cancer Institute NCI Drug Dictionary. http://www.cancer.gov/drugdictionary (Accessed Oct 28 2012). doi:10.1007/s40264-013-0050-x
Chen MH, Kerkela R, Force T. Mechanisms of cardiomyopathy associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118:84–95.
Force T. Introduction to cardiotoxicity reviews. Circ Res. 2010;106:19–20.
Scheffler M, Di Gion P, Doroshyenko O, et al. Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on 4-anilinoquinazolines. Clin Pharmacokinet. 2011;50:371–403.
Di Gion P, Kanefendt F, Lindauer A, et al. Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on pyrimidines, pyridines and pyrroles. Clin Pharmacokinet. 2011;50:551–603.
Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39.
van Cruijsen H, van der Veldt A, Hoekman K. Tyrosine kinase inhibitors of VEGF receptors: clinical issues and remaining questions. Front Biosci. 2009;14:2248–68.
Roodhart JM, Langenberg MH, Witteveen E, et al. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol. 2008;3:132–43.
Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: Their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013 (in press).
Asnacios A, Naveau S, Perlemuter G. Gastrointestinal toxicities of novel agents in cancer therapy. Eur J Cancer. 2009;45(Suppl 1):332–42.
Dienstmann R, Braña I, Rodon J, et al. Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist. 2011;16:1729–40.
Lu Z, Wu CY, Jiang YP, et al. Suppression of phosphoinositide 3-kinase signalling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci Transl Med. 2012;4:131ra50.
Amir E, Seruga B, Martinez-Lopez J, et al. Oncogenic targets, magnitude of benefit, and market pricing of antineoplastic drugs. J Clin Oncol. 2011;29:2543–9.
Shah RR, Roberts SA, Shah DR. A fresh perspective on comparing the FDA and the CHMP/EMA: approval of antineoplastic tyrosine kinase inhibitors. Br J Clin Pharmacol. 2013. doi:10.1111/bcp.12085.
Sanguinetti MC, Jiang C, Curran ME, et al. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307.
Vandenberg JI, Perry MD, Perrin MJ, et al. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012;92:1393–478.
Milon D, Daubert JC, Saint-Marc C, et al. Torsade depointes. Apropos of 54 cases [Article in French]. Ann Fr Anesth Reanim. 1982;1:513–20.
Salle P, Rey JL, Bernasconi P, et al. Torsades de pointe. Apropos of 60 cases. Ann Cardiol Angeiol (Paris). 1985;34:381–8.
Fung MC, Hsiao-hui Wu H, Kwong K, et al. Evaluation of the profile of patients with QTc prolongation in spontaneous adverse event reporting over the past three decades—1969–98. Pharmacoepidemiol Drug Saf. 2000;9(Suppl 1):S24.
Garnett CE, Beasley N, Bhattaram VA, et al. Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol. 2008;48:13–8.
Rock EP, Finkle J, Fingert HJ, et al. Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J. 2009;157:827–36.
Morganroth J, Shah RR, Scott JW. Evaluation and management of cardiac safety using the electrocardiogram in oncology clinical trials: focus on cardiac repolarization (QTc interval). Clin Pharmacol Ther. 2010;87:166–74.
Shah RR, Morganroth J. Early investigation of QTc liability: the role of multiple ascending dose (MAD) study. Drug Saf. 2012;35:695–709.
Piekarz RL, Frye AR, Wright JJ, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–73.
Varterasian M, Meyer M, Fingert H, et al. Baseline heart rate-corrected QT and eligibility for clinical trials in oncology. J Clin Oncol. 2003;21:3378–9.
Dong Q, Fu XX, Du LL, et al. Blocking of the human ether-à-go-go-related gene channel by imatinib mesylate. Biol Pharm Bull. 2013;36:268–75.
Dogan E, Yorgun H, Petekkaya I, et al. Evaluation of cardiac safety of lapatinib therapy for ErbB2-positive metastatic breast cancer: a single center experience. Med Oncol. 2012;29:3232–9.
Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–40.
Kim TD, le Coutre P, Schwarz M, et al. Clinical cardiac safety profile of nilotinib. Haematologica. 2012;97:883–9.
AstraZeneca CAPRELSA REMS Program. http://www.caprelsarems.com/learn.aspx (Accessed Jan 20 2013).
Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897–905.
Zang J, Wu S, Tang L, et al. Incidence and risk of QTc interval prolongation among cancer patients treated with vandetanib: a systematic review and meta-analysis. PLoS One. 2012;7:e30353.
Barros F, Gomez-Varela D, Viloria CG, et al. Modulation of human erg K+ channel gating by activation of a G protein-coupled receptor and protein kinase C. J Physiol. 1998;511(Pt 2):333–46.
Thomas D, Zhang W, Karle CA, et al. Deletion of protein kinase A phosphorylation sites in the HERG potassium channel inhibits activation shift by protein kinase A. J Biol Chem. 1999;274:27457–62.
Kiehn J, Karle C, Thomas D, et al. HERG potassium channel activation is shifted by phorbol esters via protein kinase A-dependent pathways. J Biol Chem. 1998;273:25285–91.
Davis MJ, Wu X, Nurkiewicz TR, et al. Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol. 2001;281:H1835–62.
Marx SO, Kurokawa J, Reiken S, et al. Requirement of a macromolecular signalling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–9.
Chen J, Sroubek J, Krishnan Y, et al. PKA phosphorylation of HERG protein regulates the rate of channel synthesis. Am J Physiol Heart Circ Physiol. 2009;296:H1244–54.
Sroubek J, McDonald TV. Protein kinase A activity at the endoplasmic reticulum surface is responsible for augmentation of human ether-a-go-go-related gene product (HERG). J Biol Chem. 2011;286:21927–36.
Krishnan Y, Li Y, Zheng R, et al. Mechanisms underlying the protein-kinase mediated regulation of the HERG potassium channel synthesis. Biochim Biophys Acta. 2012;1823:1273–84.
Zhang Y, Wang H, Wang J, et al. Normal function of HERG K+ channels expressed in HEK293 cells requires basal protein kinase B activity. FEBS Lett. 2003;534:125–32.
Zhang DY, Wang Y, Lau CP, et al. Both EGFR kinase and Src-related tyrosine kinases regulate human ether-à-go-go-related gene potassium channels. Cell Signal. 2008;20:1815–21.
Himmel HM, Hoffmann M. QTc shortening with a new investigational cancer drug: a brief case study. J Pharmacol Toxicol Methods. 2010;62:72–81.
Morgan TK Jr, Sullivan ME. An overview of class III electrophysiological agents: a new generation of antiarrhythmic therapy. Prog Med Chem. 1992;29:65–108.
Park BK, Kitteringham NR. Effects of fluorine substitution on drug metabolism: pharmacological and toxicological implications. Drug Metab Rev. 1994;26:605–43.
Park BK, Kitteringham NR, O’Neill PM. Metabolism of fluorine-containing drugs. Annu Rev Pharmacol Toxicol. 2001;41:443–70.
Elice F, Rodeghiero F, Falanga A, et al. Thrombosis associated with angiogenesis inhibitors. Best Pract Res Clin Haematol. 2009;22:115–28.
Girardi F, Franceschi E, Brandes AA. Cardiovascular safety of VEGF-targeting therapies: current evidence and handling strategies. Oncologist. 2010;15:683–94.
Minami M, Matsumoto S, Horiuchi H. Cardiovascular side-effects of modern cancer therapy. Circ J. 2010;74:1779–86.
Keefe D, Bowen J, Gibson R, et al. Noncardiac vascular toxicities of vascular endothelial growth factor inhibitors in advanced cancer: a review. Oncologist. 2011;16:432–44.
Mellor HR, Bell AR, Valentin JP, et al. Cardiotoxicity associated with targeting kinase pathways in cancer. Toxicol Sci. 2011;120:14–32.
Sonpavde G, Bellmunt J, Schutz F, et al. The double edged sword of bleeding and clotting from VEGF inhibition in renal cancer patients. Curr Oncol Rep. 2012;14:295–306.
Mir O, Ropert S, Alexandre J, et al. Hypertension as a surrogate marker for the activity of anti-VEGF agents. Ann Oncol. 2009;20:967–70.
Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–9.
Qi WX, Shen Z, Lin F, et al. Incidence and risk of hypertension with vandetanib in cancer patients: a systematic review and meta-analysis of clinical trials. Br J Clin Pharmacol. 2012;75:919–30.
Robinson ES, Khankin EV, Karumanchi SA, et al. Hypertension induced by vascular endothelial growth factor signalling pathway inhibition: mechanism and potential use as a biomarker. Semin Nephrol. 2010;30:591–601.
Maitland ML, Kasza KE, Karrison T, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–7.
Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol. 2006;24:1363–9.
Kim JJ, Vaziri SA, Rini BI, et al. Association of VEGF and VEGFR2 single nucleotide polymorphisms with hypertension and clinical outcome in metastatic clear cell renal cell carcinoma patients treated with sunitinib. Cancer. 2012;118:1946–54.
Steeghs N, Gelderblom H, Roodt JO, et al. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14:3470–6.
GlaxoSmilthKline Clinical Study Register A meta-analysis of the cumulative incidence of hypertension in the first month of treatment with pazopanib across three RCC studies: VEG102616, VEG105192 and VEG107769 (Study number 115227). http://www.gsk-clinicalstudyregister.com/result_detail.jsp?protocolId=115227&studyId=7FE7FADD-D3FA-4BD6-9BF5-0845FF2A2C90&compound=pazopanib&type=Compound&letterrange=L-P (Accessed Oct 25 2012).
Quintás-Cardama A, Kantarjian H, O’brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25:3908–14.
Guignabert C, Montani D. Key roles of Src family tyrosine kinases in the integrity of the pulmonary vascular bed. Eur Respir J. 2013;41:3–4.
Pullamsetti SS, Berghausen EM, Dabral S, et al. Role of Src tyrosine kinases in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2012;32:1354–65.
Schermuly RT, Dony E, Ghofrani HA, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–21.
Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353:1412–3.
Tapper EB, Knowles D, Heffron T, et al. Portopulmonary hypertension: imatinib as a novel treatment and the Emory experience with this condition. Transplant Proc. 2009;41:1969–71.
ten Freyhaus H, Dumitrescu D, Bovenschulte H, et al. Significant improvement of right ventricular function by imatinib mesylate in scleroderma-associated pulmonary arterial hypertension. Clin Res Cardiol. 2009;98:265–7.
Chhina MK, Nargues W, Grant GM, et al. Evaluation of imatinib mesylate in the treatment of pulmonary arterial hypertension. Future Cardiol. 2010;6:19–35.
ten Freyhaus H, Dumitrescu D, Berghausen E, et al. Imatinib mesylate for the treatment of pulmonary arterial hypertension. Expert Opin Investig Drugs. 2012;21:119–34.
Antoniu SA. Targeting PDGF pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:1055–63.
Ghofrani HA, Morrell NW, Hoeper MM, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med. 2010;182:1171–7.
Hatano M, Yao A, Shiga T, et al. Imatinib mesylate has the potential to exert its efficacy by down-regulating the plasma concentration of platelet-derived growth factor in patients with pulmonary arterial hypertension. Int Heart J. 2010;51:272–6.
Ciuclan L, Bonneau O, Hussey M, et al. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:1171–82.
Ciuclan L, Hussey MJ, Burton V, et al. Imatinib attenuates hypoxia-induced pulmonary arterial hypertension pathology via reduction in 5-hydroxytryptamine through inhibition of tryptophan hydroxylase 1 expression. Am J Respir Crit Care Med. 2013;187:78–89.
Kojonazarov B, Sydykov A, Pullamsetti SS, et al. Effects of multikinase inhibitors on pressure overload-induced right ventricular remodeling. Int J Cardiol. 2012 (Epub ahead of print).
Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–12.
Kerkelä R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–16.
Lenihan DJ, Cardinale D, Cipolla CM. The compelling need for a cardiology and oncology partnership and the birth of the International CardiOncology Society. Prog Cardiovasc Dis. 2010;53:88–93.
Cheng H, Force T. Why do kinase inhibitors cause cardiotoxicity and what can be done about it? Prog Cardiovasc Dis. 2010;53:114–20.
Dasanu CA, Padmanabhan P, Clark BA 3rd, et al. Cardiovascular toxicity associated with small molecule tyrosine kinase inhibitors currently in clinical use. Expert Opin Drug Saf. 2012;11:445–57.
Montaigne D, Hurt C, Neviere R. Mitochondria death/survival signalling pathways in cardiotoxicity induced by anthracyclines and anticancer-targeted therapies. Biochem Res Int. 2012;Article ID 951539.
Van den Akker NM, Winkel LC, Nisancioglu MH, et al. PDGF-B signalling is important for murine cardiac development: its role in developing atrioventricular valves, coronaries, and cardiac innervation. Dev Dyn. 2008;237:494–503.
Cheng H, Kari G, Dicker AP, et al. A novel preclinical strategy for identifying cardiotoxic kinase inhibitors and mechanisms of cardiotoxicity. Circ Res. 2011;109:1401–9.
Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibitors. Nat Rev Cancer. 2007;7:332–44.
Kerkela R, Woulfe KC, Durand JB, et al. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin Transl Sci. 2009;2:15–25.
Hasinoff BB. The cardiotoxicity and myocyte damage caused by small molecule anticancer tyrosine kinase inhibitors is correlated with lack of target specificity. Toxicol Appl Pharmacol. 2010;244:190–5.
Hasinoff BB, Patel D. Mechanisms of myocyte cytotoxicity induced by the multikinase inhibitor sorafenib. Cardiovasc Toxicol. 2010;10:1–8.
Hasinoff BB, Patel D. The lack of target specificity of small molecule anticancer kinase inhibitors is correlated with their ability to damage myocytes in vitro. Toxicol Appl Pharmacol. 2010;249:132–9.
Subbiah IM, Lenihan DJ, Tsimberidou AM. Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist. 2011;16:1120–30.
Force T, Kerkela R. Cardiotoxicity of the new cancer therapeutics- mechanisms of, and approaches to, the problem. Drug Discov Today. 2008;13:778–84.
Yang B, Papoian T. Tyrosine kinase inhibitor (TKI)-induced cardiotoxicity: approaches to narrow the gaps between preclinical safety evaluation and clinical outcome. J Appl Toxicol. 2012 (Epub ahead of print).
Eschenhagen T, Force T, Ewer MS, et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10.
Steingart RM, Bakris GL, Chen HX, et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signalling pathway inhibitors. Am Heart J. 2012;163:156–63.
Conflict of interest
The authors have no conflicts of interest that are directly relevant to the content of this review and have not received any financial support for writing it. RRS was formerly a Senior Clinical Assessor at the Medicines and Healthcare products Regulatory Agency (MHRA), London, UK, and the ICH E14 Topic Leader, representing the EU. JM is the Chief Cardiac Consultant to eResearchTechnology Inc. (eRT), Philadelphia, PA, USA, which provides cardiac safety services to drug development companies. Both RRS and JM now provide expert consultancy services on QT liability of drugs and development of new drugs to a number of pharmaceutical companies. DRS is a first-year house officer at a district general hospital and has no consultancy relationships.
Author information
Authors and Affiliations
Corresponding author
Additional information
The views expressed in this article are those of the authors and do not necessarily reflect the views or opinions of their affiliates, any regulatory authorities or any of their advisory bodies.
Rights and permissions
About this article
Cite this article
Shah, R.R., Morganroth, J. & Shah, D.R. Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on Cardiac Repolarisation (QT Interval). Drug Saf 36, 295–316 (2013). https://doi.org/10.1007/s40264-013-0047-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-013-0047-5