Abstract
Chemo-refractory NHL has a very poor outcome; the addiction of RIT to salvage regiment pre ASCT had recently demonstrated promising results.We performed a retrospective sequential study to determine the feasibility of standard Zevalin with BEAM in high-risk relapse/refractory NHL. A matched cohort analysis with a group treated with standard BEAM without Zevalin was performed as secondary endpoint. Between October 2006 and January 2013, 37 NHL patients at high risk for progression or early (< 1 year) or multiple relapses were treated with Z-BEAM and ASCT after R-DHAP or R-ICE as salvage therapy. Clinical characteristics were 19 refractory and 18 early or multiple relapse; 16 patients received 1, and 21 had 2 or more previous rituximab-containing chemotherapy. At the end of treatment, response was CR 22 (59%), PR 10 (27%), PD 4 (11%), and toxic death (TD) 1 (3%). With a median follow up of 61 months, 3-year PFS was 61% and OS 61%. Fifteen patients died, 12 of lymphoma. Comparison with 21 treated with BEAM alone showed a numerical higher 3-yr PFS rate in favor of Z-BEAM but not statistically significant (57 vs 48%). With the limitation of the small sample subgroup analysis, a significant benefit was observed in relapsed patients for PFS (78% Z-BEAM vs 22% BEAM p = 0.016) and OS (83% Z-BEAM vs 22% BEAM p = 0.001). In relapsed/refractory high-risk NHL, Z-BEAM+ASCT is able to achieve a good ORR. Three-year PFS is promising for early relapsed patients but is not satisfactory for those with refractory disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The combination of rituximab with an anthracycline-containing regimen as front-line treatment has significantly improved survival in patients with aggressive Non-Hodgkin Lymphoma (NHL). However, 20–50% of patients fail to achieve a complete response (CR) or relapse after first-line chemoimmunotherapy [1,2,3]. Salvage chemotherapy followed by high-dose chemotherapy with autologous stem cell transplantation (ASCT) is currently considered the standard of care in relapsed/refractory aggressive NHL [4].However, a number of studies demonstrated that the main benefit from ASCT may derive by chemo-sensitivity to salvage chemoimmunotherapy [5,6,7]. Chemo-refractory disease (either upfront or at relapse) has a very poor outcome, without an evident advantage by ASCT. More effective strategies have to be proposed for this particular group of patients. No differences have been showed for chemotherapy-based preparative regimens for ASCT and, since aggressive NHL is radiosensitive, total body irradiation (TBI) has been added to the conditioning regimens for ASCT. In the last years, to maximize the anti-tumor effect and reduce toxicities, TBI has been replaced by radio-immunotherapy (RIT) in the conditioning regimen for ASCT [8]. Radio-labeled anti-CD20 monoclonal antibody 90Y-ibritumomab tiuxetan (Zevalin) has shown a significant activity against both, indolent and aggressive B-cell non-Hodgkin lymphomas [9]. Recently, RIT has been successfully added to preparative regimens for ASCT in patients with relapsed diffuse large B cell lymphoma (DLBCL) in order to increase the anti-lymphoma effect and to improve the outcome of relapsed/refractory aggressive NHL [10,11,12]. Here, we present the data from a single center retrospective sequential trial focusing on the safety and efficacy of a standard dose of 90Y-ibritumomab tiuxetan followed by high-dose conditioning regimen BEAM (carmustine, etoposide, citarabine, melphalan) chemotherapy and ASCT in patients with aggressive NHL relapsed or refractory to a rituximab-containing chemotherapy.
Patients and methods
Eligibility
This study included patients with different histology: DLBCL, de novo or transformed, mantle cell (MCL), primary mediastinal lymphoma (PML), follicular (FL), or indolent lymphoma. Patients were considered at high risk and eligible for the study if they showed a progressive disease or an early relapse (< 1 year) from previous therapy or those with multiple relapses. Other eligibility criteria included age 18 to 65, Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–1 at relapse, and adequate cardiac, renal, and respiratory function. Central nervous system (CNS) lymphoma at the time of relapse, history of HIV infection, or previous ASCT were considered as exclusion criteria. All patients were required to be previously treated with rituximab.
Study design, treatment, and response assessment
This is a retrospective study conducted in a single Italian center in Turin, approved by the local Ethic Committee, and conducted in accordance with the Declaration of Helsinki. Signed informed consent was obtained from all patients prior to the start of salvage chemoimmunotherapy. The study was registered under the number NCT02992223 in clinicaltrial.gov.
Standard dose DHAP (dexamethasone, cytarabine, cysplatin) or ICE (etoposide, ifosfamide, carboplatin) plus rituximab for 2–3 cycles were used as salvage chemotherapy and mobilizing regimen. On day − 21, patients were given rituximab 250 mg/m2; on day − 14, patients received 250 mg/m2 rituximab followed by 90Y-ibritumomab tiuxetan at a fixed dose of 0.4 mCi/kg (with a maximum total dose of 32 mCi) in an outpatient setting. One week later, patients were given BEAM or FEAM chemotherapy (carmustine 300 mg/m2 or fotemustine 300 mg/m2 on day − 6, etoposide 200 mg/m2 on days − 5 to − 2, cytarabine 200 mg/m2 twice a day on days − 5 to − 2, and melphalan 140 mg/m2 on day − 1) [13]. Autologous stem cells were re-infused on day 0. GCSF 5 μg/kg/d was started on day + 1 after ASCT until neutrophil recovery. Adverse events were recorded and graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0.
Early treatment-related mortality (TRM) was defined as death due to any transplantation related-cause other than disease relapse within 100 days from ASCT.
Baseline assessment, response evaluation, and follow-up
Before treatment complete baseline staging, including physical examination, blood count, and serum biochemistry determinations, bone marrow biopsy, echocardiography, and a whole-body evaluation with CT scan and PET/CT was done. Intermediate response was planned before Z-BEAM and ASCT with whole-body CT scan, PET/CT, and bone marrow biopsy (if positive at baseline); final response was evaluated 3 months after ASCT, according to 2007 Cheson criteria with CT scan and total body PET/CT [14]. Follow-up procedures were done every 3 months for the first-year post-transplant, and every 6 months thereafter for the next 2 years.
Statistical analysis
The primary endpoints were overall response rate (ORR) and progression-free survival (PFS) after Z-BEAM and ASCT. PFS was defined as the time from ASCT to progression/death as a result of any cause. Overall survival (OS) was defined as the time from ASCT to death as a result of any cause. Patients who were still progression-free/alive were censored at the date of last contact. Probabilities of PFS and OS were estimated using the Kaplan-Meier method, comparing the two arms by the log rank test. The Cox proportional hazard model was used to compare the following risk factors by the Wald test: age (> 60 vs ≤ 60 years), gender (female vs male), arm (Z-BEAM vs BEAM), status at accrual (relapse vs refractory), International Prognostic Index (IPI) score (3–5 vs 0–2), number of previous chemotherapy lines (> 2 vs 0–1), bulky disease/B symptoms/bone marrow involvement (any vs none), disease status before, after ASCT, and at last follow-up. The effect of the same risk factors on PFS and OS was assessed by the bivariate Cox model: as in the univariate ones, disease status at ASCT, after ASCT, and at the last follo- up were treated as time-dependent variables. Patient characteristics were tested using the Fisher’s exact test for categorical variables and the Mann-Whitney test for continuous ones. All reported p values were two-sided, at the conventional 5% significance level. Data were analyzed by R 3.1.1 (The R Foundation for Statistical Computing, Vienna-A; www.R-project.org).
As secondary endpoint, an historical population of high-risk relapsed/refractory NHL patients with the same characteristics treated with BEAM alone was compared as control group to the patients treated with Z-BEAM.
Results
Patient characteristics
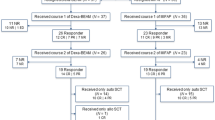
Thirty-seven consecutive patients were enrolled into the study between January 2006 and January 2013. Median age was 50 years (range18–68). Histology included de novo DLBCL/ PML in 23 patients, follicular grade I–II in 9, MCL in 3 and indolent lymphoma in 2 patients. At the time of inclusion, 28 patients were at stage III/IV disease, 14 patients had bulky disease (maximum diameter > 10 cm) and 17 had bone marrow involvement by lymphoma. All patients were pre-treated with rituximab-containing regimens.
Twenty-one patients (57%) received more than two previous rituximab-containing lines of treatment (range 2–5).
Nineteen patients were considered refractory to previous lines of treatment while 18 showed a relapse.
All patients received salvage therapy as follows: 2–3 courses of R-DHAP or 3 cycles of R-ICE. All patients had measurable disease at the time of inclusion in the trial, as defined by PET/CT.
Peripheral blood stem cells (PBSC) were collected in all cases with the use of G-CSF after salvage chemotherapy with a median yield of 6.45 × 106/kg CD34+ cells (range, 3.46–10.65).
A historical control of 21 patients treated with BEAM or FEAM followed by ASCT without Zevalin was considered for comparison with Z-BEAM group. The historical group was selected with the same high-risk characteristics and treatment, included rituximab, was performed between December 1999 and February 2006.
Comparative baseline clinical features between Z-BEAM vs BEAM are shown in Table 1; no significant differences were shown in the two groups, except for higher number of previous lines of treatment in those treated with RIT (57% Z-BEAM vs 9% BEAM) and lower CR rate before ASCT (43% Z-BEAM vs 86% BEAM).
Early toxicity, TRM, and engraftment
No infusion reactions or hematological toxicities related to 90Y-ibritumomab tiuxetan before ASCT were recorded. All patients engrafted. The median time from ASCT to neutrophil engraftment (ANC > 500/mm3) was 10 days (range 9–12), and median time to platelet engraftment (plts > 20.000/mm3 without platelet support) was 9 days (range 5–15). Fever of unexplained origin was recorded in eight cases, and sepsis was documented in four patients during aplastic phase, all resolved with antibiotic treatment. Grades 3–4 mucositis was shown in ten patients and non-infectious enteritis in two cases and one case of intestinal perforation due to intestinal mucositis during neutropenia were recorded. One toxic death (TD) was recorded after 30 days from ASCT due to aspergillosis associated with H1N1 pneumonia (100 days TRM 2.7%); this patient was in CR and had reached engraftment at death.
Response and survival
Response was evaluated on an intent-to treat basis. The overall response rate after R-DHAP/R-ICE (ORR) was 78%. CR with a negative PET-CT was recorded in 16 patients (43%), PR in 13 cases (35%). Progressive disease (PD) was documented in eight cases (22%). At the end of treatment with Z-BEAM and ASCT, ORR was 87% and response status was CR 22 (59%), PR 10 (27%), PD 4 (11%), and TD 1 (3%). With a median follow-up of 61 (range 17–114) months from transplant among surviving patients, death was documented in 15 patients, 12 of them for progressive disease; 1 patient died early for aspergillosis associated with H1N1 pneumonia, 2 patients died later as detailed in section “late effects”. The estimated 3-year OS and PFS were both 61% (Fig. 1a, b). A significant worse 3-year-OS was shown in patients with refractory disease (47% refractory vs 83% relapsed, p = 0.006) and impaired performance status (ECOG > 1 33% vs ECOG 0–1 80%, p = 0.006). Only bad performance status significantly worsened PFS (ECOG > 1 33% vs ECOG < 1 84%, p < 0.001).
No differences in 3-year-OS and 3-year-PFS were found between patients according to different histology.
The comparative analysis with the control group treated with BEAM alone showed a marginal advantage for Z-BEAM in 3 years. PFS (61% Z-BEAM vs 48% BEAM 95% CI 0.54–2.50 p = 0.708), without differences in terms of OS (60% Z-BEAM vs 57% BEAM 95% CI 0.43–2.24, p = 0.965). A significantly different 3-year-PFS (78% Z-BEAM vs 22% BEAM p = 0.016) and OS (83% Z-BEAM vs 22% BEAM p = 0.001) in favor of RIT were shown in those with early relapsed disease at presentation (Fig. 2a, b). Conversely, the addition of RIT failed to demonstrate a difference in terms of 3-year PFS (63% Z-BEAM vs 67% BEAM p = 0.164) and OS (47% Z-BEAM vs 83% BEAM p = 0.04) in patients with primary refractory disease (Fig. 2c–d).
Late effects
One patient developed a refractory anemia with excess of blasts (RAEB) 40 months after Z-BEAM, and one patient in Z-BEAM group died from progressive multifocal leukoencephalopathy by John Cunningham (JC) virus 1 year after ASCT. No other significant late effects were recorded.
Discussion
The purpose of our study was to evaluate the efficacy and safety of the combination of standard dose radioimmunotherapy with high dose chemotherapy and autologous stem cell transplantation in patients at high risk of further relapse/progression. Our data showed that Z-BEAM is a safe and effective treatment, with a 3-year PFS and OS both of 61%. Time to engraftment and overall adverse events were comparable to those usually reported with BEAM alone. In our study, we enrolled only patients with high risk relapsed/refractory lymphoma defined as those with multiple relapses or relapse within 12 months after first line chemotherapy or who did not achieve a CR pre-ASCT.
Standard dose of yttrium-90 ibritumomab tiuxetan is approved in Europe without the necessity of imaging studies or a specific expertise, is considered safe and feasible with an ambulatory approach and reduced radiation risk to the patients’ family and to health care providers. In our retrospective study, primary endpoint was the evaluation of ORR, PFS, and OS in high-risk patients treated with Z-BEAM. With a median follow up of 61 months, Z-BEAM showed a 3-year PFS of 61% and an OS of 61%. Our results are similar to those reported by Shimoni et al. [8] in 21 patients with aggressive lymphoma with a 2-year estimated PFS and OS equal to 52 and 67%, respectively. Krishnan et al. [10] published also similar data in 41 patients with different hystologies (20 DLBCL, 13 MCL, 4 FL, and 4 transformed), treated with Z-BEAM, considered ineligible for total body irradiation because of older age or previous radiotherapy. With a median follow-up of 18 months, 2-year PFS and OS were 69 and 80%. Recently, Briones et al. [16] published a study on 30 patients with DLBCL de novo or transformed, primary refractory or resistant to salvage immune chemotherapy treated with RIT and high-dose chemotherapy BEAM followed by ASCT. Our results are superimposable to this study with 60% of patients in CR after ASCT and 3-year PFS and OS of 61 and 63% respectively.
Comparative Table 2 shows the results of all studies with Z-BEAM.
131-I Tositumomab, another radio-immunotherapeutic directed against non-Hodgkin lymphoma, had also shown promise in phases I and II trials. Despite the initial favorable results, the phase III trial comparing tositumomab plus BEAM with rituximab plus BEAM for patients with persistent or relapsed chemotherapy-sensitive DLBCL yielded similar 2-year PFS and OS rates [17]. Nonetheless, tositumomab and ibritumomab tiuxetan are not interchangeable; Zevalin is a pure beta emitter, whereas tositumomab emits gamma and beta radiation. Potential disadvantages of tositumomab include in vivo dehalogenation and a half-life (8 days) that is longer than the period of maximum tumor uptake. Zevalin, when chelated to antibodies exhibits relative in vivo stability, a half-life of 2.7 days, and a longer path length than tositumomab. Pathways of clearance of the isotope in the body and distribution also differ. The high energy radiation and long beta paths are advantageous for treating bulky, poorly vascularized tumors, as well as those with subpopulations lacking the targeted antigen, which potentially make Zevalin a more attractive agent.
In our study, even in poor risk patients, the addition of RIT to standard BEAM regimen showed a good safety profile: all patients engrafted with a median time to neutrophils and platelet recovery comparable to BEAM. Major toxicity was grades 3–4 mucositis, with one early death (2.7%) due to infectious toxicity. Our early toxicity can be considered superimposable to 2% TRM shown in study by Krishnan [18] and 4.1% shown by Vose et al. with Tositumomab [17]. Late toxicities were mild, with only one heavily pretreated patient showing a treatment-related myelodysplastic syndrome (t-MDS) and one with JCV infection. These results compare favorably with previous published studies [11, 19,20,21] showing feasibility, no increased late toxicity in comparison with standard BEAM, and with a crude incidence of t-MDS/t-AML of about 1–2%.
Secondary end-point of our study was the comparison of Z-BEAM arm with a control previous group treated with BEAM alone with same clinical presentation and pretreated with rituximab in terms of ORR, PFS, and OS.
A numerical higher 3-year-PFS rate in favor of Z-BEAM but not statistically significant was shown in the current study (57% Z-BEAM vs 48% BEAM p = ns), without any significant difference in terms of OS (60% Z-BEAM vs 57% BEAM p = ns). With the limits of a non-randomized comparative study, PFS results appear similar to those presented by Shimoni et al. [12] in 43 patients with different hystologies (15 DLBCL, 7 transformed by FL, and 1 MCL) aggressive relapsed/refractory CD20+ lymphoma randomized to Z-BEAM, or BEAM as consolidation treatment after salvage therapy. With a median follow-up of 29 months, the authors reported a trend in favor of Z-BEAM in PFS (59% Z-BEAM vs 37% BEAM p = 0.2). A better 2-year OS rate in the RIT arm (91% Z-BEAM vs 62% BEAM p = 0.05) was explained by the authors as related to better salvage responses after ASCT relapse. Only patients who failed to achieve a complete remission after first-line chemotherapy or relapsed after CR and chemosensitive to salvage treatment (with a maximum of two prior lines) were allowed in the study of Shimoni et al. Conversely, in our study, all patients included had poorer prognostic features as progressive disease (19/37 patients), or early or multiple relapses (21/37 patients). Pre-treatment with rituximab, considered as poor prognostic factor in the salvage setting [15], was primary inclusion criteria in our study.
Early or multiple relapses are considered an independent factor that adversely affect response in term of EFS, PFS, and OS in patients treated with high dose CHT and ASCT. In CORAL study, patients with relapsed/refractory chemo-sensitive disease proceeded with ASCT with a 3-year PFS and OS 53 and 39%. Early relapse and prior rituximab treatment defined a population with a poor response rate to the standard treatment with 3-year PFS only 23% [4]. Furthermore, an analysis from the European Bone Marrow Transplantation Registry (EBMT) in patients with relapsed DLBCL in CR after salvage CHT and treated with ASCT, showed a 5-year PFS and OS rates of 44 and 60% for the entire group and a 3-year PFS of 68% for patients pre-treated with rituximab [5]. In our study, a significant different 3-year-PFS (78% Z-BEAM vs 22% BEAM p = 0.016) and OS (83% Z-BEAM vs 22% BEAM p = 0.001) in favor of RIT were shown in those with early relapsed disease at presentation. Our results in BEAM cohort (3-year PFS and OS 48 and 57%) compare favorably with other pre-published data in high-risk patients with the limitation of the small number of cases.
Chemo-sensitivity after relapse is considered one of the most important factors for the success of an autologous stem cell transplant [22]. Several studies have shown that a positive PET/CT scan after salvage therapy and before ASCT has a negative impact on outcome [23,24,25]. In our trial, all patients received PET/CT scan as staging and response procedures pre and post ASCT, and some patients were transplanted with a positive pre-ASCT PET scan, underlying the unfavorable profile of our cohort.
In our study, the addition of RIT failed to demonstrate a difference in terms of 3-year- PFS (63% Z-BEAM vs 67% BEAM p = 0.164) and OS (47% Z-BEAM vs 83 BEAM p = 0.04) in patients with primary refractory disease. As previously documented in other published studies [26], patients with refractory disease remain a poor prognostic subset. SCHOLAR-1, an international, multi-cohort retrospective non-Hodgkin lymphoma research study from two-phase III clinical trials, retrospectively evaluated outcomes in patients with refractory DLBCL. Refractory status was defined as progressive disease or stable disease as best response at any point during chemotherapy (> 4 cycles of first-line or 2 cycles of later-line therapy). For patients with refractory DLBCL, the objective response rate was 26% (complete response rate, 7%) to the next line of therapy, and the median overall survival was 6.3 months. Twenty percent of patients were alive at 2 years. Outcomes were consistently poor across patient subgroups and study cohorts.
Observational studies may be affected by several and uncontrolled bias. In our study, the retrospective nature, the small sample size, different hystologies, and the non-randomized comparison with a historical series may represent some limitations. At the same time, we would like to underline that, in order to avoid the impact of these bias, we carefully checked the subjects who were given high dose chemotherapy and ASCT in the same time frame, and none patient with the same characteristics received BEAM without Zevalin. The historical control group was treated in a previous period of time with the same clinical characteristics of the study group. So, the two cohorts were treated in different, sequential years. Performing a full inferential analysis of the classical baseline characteristics, no critical unbalancing differences were shown, and patients with Z-BEAM had comparable risk factors than BEAM ones except for more lines of therapy and lower CR rate before ASCT in those treated with RIT. The selection of a more slightly favorable group in BEAM arm could explain the absent impact on outcome in all patients.
In summary, with the limits of a small series and a comparative non-randomized study, radio-immunotherapy can be safely added to BEAM regimen followed by ASCT in high-risk patients with rituximab-exposed, refractory, or early relapsed aggressive lymphoma. This approach may be associated with better response and PFS rates particularly in a chemo-sensitive relapsed group. No advantages seem to be shown for primary refractory patients, for whom alternative approaches are warranted.
References
Coiffier B, Thieblemont C, Van Den Neste E et al (2010) Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’ Etudes des Lymphomes de l’Adulte. Blood 23(116):2040–2045
Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, Pocock C, Ardeshna KM, Radford JA, McMillan A, Davies J, Turner D, Kruger A, Johnson P, Gambell J, Linch D (2013) Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 381(9880):1817–1826
Schmitz N, Nickelsen M, Ziepert M, Haenel M, Borchmann P, Schmidt C, Viardot A, Bentz M, Peter N, Ehninger G, Doelken G, Ruebe C, Truemper L, Rosenwald A, Pfreundschuh M, Loeffler M, Glass B, German High-Grade Lymphoma Study Group (DSHNHL) (2012) Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol 13(12):1250–1911
Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, Ma D, Brière J, Moskowitz CH, Schmitz N (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28(27):4184–4190
Mounier N, Canals C, Gisselbrecth C (2012) High dose therapy and autologous stem cell transplantation in first relapse for diffuse large B cell lymphoma in the rituximab era: ana analysis base on data from the European Bloos and marrow transplantation registry. Biol Blood Marrow Transaplant 18:788–793
Vose JM, Zhang MJ, Rowlings PA, Lazarus HM, Bolwell BJ, Freytes CO, Pavlovsky S, Keating A, Yanes B, van Besien K, Armitage JO, Horowitz MM, Others of the Autologous Blood and Marrow Transplant Registry Lymphoma Working Committee (2001) Autologous transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the autologous blood and marrow transplant registry. J Clin Oncol 19(2):406–413
Caballero MD, Pérez-Simón JA, Iriondo A, Lahuerta JJ, Sierra J, Marín J, Gandarillas M, Arranz R, Zuazu J, Rubio V, Fernández de Sevilla A, Carreras E, García-Conde J, García-Laraña J, Grande C, Sureda A, Vidal MJ, Rifón J, Pérez-Equiza C, Varela R, Moraleda JM, García Ruíz JC, Albó C, Cabrera R, San Miguel JF, Conde E (2003) High dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish cooperative group. Ann Oncol 14(1):140–151
Shimoni A, Nagler A (2007) Radioimmunotherapy and stem-cell transplantation in the treatment of aggressive B-cell lymphoma. Leuk Lymphoma 48(11):2110–2120
Witzig TE, White CA, Gordon LI, Wiseman GA, Emmanouilides C, Murray JL, Lister J, Multani PS (2003) Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-hodgkin’s lymphoma. J Clin Oncol 21(7):1263–1270
Krishnan A, Nademanee A, Fung HC, Raubitschek AA, Molina A, Yamauchi D, Rodriguez R, Spielberger RT, Falk P, Palmer JM, Forman SJ (2008) Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and highdose chemotherapy in patients with non-Hodgkin's lymphoma. J Clin Oncol 26(1):90–95
Nademanee A, Forman S, Molina A et al (2005) A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood 106(8):2896–2902
Shimoni A, Avivi I, Rowe JM, Yeshurun M, Levi I, Or R, Patachenko P, Avigdor A, Zwas T, Nagler A (2012) A randomized study comparing yttrium-90 ibritumomab tiuxetan (Zevalin) and high-dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma. Cancer 118(19):4706–4714
Musso M, Messina G, Di Renzo N et al (2016) Improved outcome of patients with relapsed/refractory Hodgkin lymphoma with a new fotemustine-based high-dose chempotherapy regimen. Br J Heamatol 172:111–121
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on Lymphoma (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Shimoni A, Zwas ST, Oksman Y, Hardan I, Shem-Tov N, Yerushalmi R, Avigdor A, Ben-Bassat I, Nagler A (2007) Yttrium 90 Ibritumomab Tiuxetan (Zevalin) combined with high dose BEAM chemotherapy and autologous stem cell transplantation for chemorefractory aggressive non-Hodgkin’s lymphoma. Exp Hematol 35(4):534–540
Briones J, Novelli S, Garcia-Marco JA, Tomas JF, Bernal T, Grande C, Canales MA, Torres A, Moraleda JM, Panizo C, Jarque I, Palmero F, Hernandez M, Gonzalez-Barca E, Lopez D, Caballero D, on behalf of GELTAMO (2014) Autologous stem cell transplantation after conditioning with yttrium 90 Ibritumomab Tiuxetan plus BEAM in refractory non-Hodgkin’s diffuse large B cell lymphoma: results of a prospective, multicenter, phase II clinical trial. Haematologica 99(3):505–510
Vose JM, Carter S, Burns LJ, Ayala E, Press OW, Moskowitz CH, Stadtmauer EA, Mineshi S, Ambinder R, Fenske T, Horowitz M, Fisher R, Tomblyn M (2013) Phase III randomized study of rituximab carmustine, etoposide cytarabine and melfalan (BEAM) compared with iodine-131 Tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B cell lymphoma: results from the BMT CTN 0401 trial. J Clin Oncol 31:1662–1668
Krishnan AY, Palmer J, Nademanee AP, Chen R, Popplewell LL, Tsai NC, Sanchez JF, Simpson J, Spielberger R, Yamauchi D, Forman SJ (2017) Phase II study of yttrium-90 ibritumomab tiuxetan plus high dose BCNU, etoposide, cytarabine and melphalan for non-Hodgkin lymphoma: the role of hystology. Biol Bone Marrow Transplant 23:922–929
Kewalramani T, Zelenetz AD, Hedrick EE, Donnelly GB, Hunte S, Priovolos AC, Qin J, Lyons NC, Yahalom J, Nimer SD, Moskowitz CH (2000) High-dose chemoradiotherapy and autologous stem cell transplantation for patients with primary refractory aggressive non-Hodgkin lymphoma: an intention-to-treat analysis. Blood 96(7):2399–2404
Czuczman MS, Emmanouilides C, Darif M, Witzig TE, Gordon LI, Revell S, Vo K, Molina A (2007) Treatment-related myelodysplastic syndrome and acute myelogenous leukemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol 25(27):4285–4292
Winter JN, Inwards DJ, Spies S, Wiseman G, Patton D, Erwin W, Rademaker AW, Weitner BB, Williams SF, Tallman MS, Micallef I, Mehta J, Singhal S, Evens AM, Zimmer M, Molina A, White CA, Gordon LI (2009) Yttrium-90 ibritumomab tiuxetan doses calculated to deliver up to 15 Gy to critical organs may be safely combined with high-dose BEAM and autologous transplantation in relapsed or refractory B-cell non- Hodgkin’s lymphoma. J Clin Oncol 27(10):1653–1659
Philip T, Guglielmi C, Hagenbeek A, Somers R, van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL, Coiffier B, Biron P, Mandelli F, Chauvin F (1995) Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med 333:1540–1545
Derenzini E, Musuraca G, Fanti S, Stefoni V, Tani M, Alinari L, Venturini F, Gandolfi L, Baccarani M, Zinzani PL (2008) Pretransplantation positron emission tomography scan is the main predictor of autologous stem cell transplantation out-come in aggressive B-cell non-Hodgkin lymphoma. Cancer 113(9):2496–2503
Poulou LS, Thanos L, Ziakas PD (2010) Unifying the predictive value of pretransplant FDG PET in patients with lymphoma: a review and meta-analysis of published trials. Eur J Nucl Med Mol Imaging 37:156–162
Schot BW, Zijlstra JM, Sluiter WJ, van Imhoff GW, Pruim J, Vaalburg W, Vellenga E (2007) Early FDG-PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood 109:486–491
Crump M, Neelapu SS, Farooq U, van den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, Boussetta S, Feng L, Maurer MJ, Navale L, Wiezorek J, Go WY, Gisselbrecht C (2017) Outcome in refractory diffuse large B-cell lymphoma: results from the international Scholar-1 study. Blood 130:1800–1808
Author information
Authors and Affiliations
Contributions
Chiara Ciochetto, Barbara Botto, and Umberto Vitolo designed the study; Chiara Ciochetto, Barbara Botto, and Umberto Vitolo supervised the study, analyzed the data, and wrote the manuscript. Roberto Passera analyzed and interpreted the data, and all authors enrolled patients and reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Umberto Vitolo was on the advisory committee of Roche, Janssen and received a lecture fee from Roche, Celgene, Janssen, Takeda, and Gilead.
The remaining authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ciochetto, C., Botto, B., Passera, R. et al. Yttrium-90 ibritumomab tiuxetan (Zevalin) followed by BEAM (Z-BEAM) conditioning regimen and autologous stem cell transplantation (ASCT) in relapsed or refractory high-risk B-cell non-Hodgkin lymphoma (NHL): a single institution Italian experience. Ann Hematol 97, 1619–1626 (2018). https://doi.org/10.1007/s00277-018-3328-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3328-3