Abstract
Purpose
The aim of this study was to prospectively compare the MIFAP protocol, which had been shown to be effective in patients with relapsed and refractory Hodgkin’s lymphoma (HL) or aggressive non-Hodgkin’s lymphoma (NHL), to an established regimen like Dexa-BEAM.
Methods
Seventy-three adult patients with HL (N = 25) or aggressive NHL (N = 48) suffering from relapse or refractory disease were randomly allocated to receive two cycles of Dexa-BEAM (dexamethasone, carmustine, etoposide, cytarabine, melphalan; N = 37) or MIFAP (mitoxantrone, fludarabine, cytarabine, cisplatin; N = 36) prior to a consolidating high-dose therapy and hematopoietic cell transplantation (HCT). Primary endpoint was the overall response rate (ORR) [complete response (CR) and partial response (PR)] after two courses of salvage chemotherapy.
Results
The ORR was 51% (CR 38%) and 53% (CR 36%) in the Dexa-BEAM arm and in the MIFAP arm (both not significant), respectively. There was a significantly higher grade 3–4 toxicity after MIFAP compared to Dexa-BEAM. Thirty-five patients were consolidated by autologous (N = 29), allogeneic (N = 1) or sequential autologous/allogeneic (N = 5) HCT. No significant differences were found in progression-free survival (PFS) and overall survival (OS) between the Dexa-BEAM and the MIFAP arms.
Conclusion
Compared to Dexa-BEAM, MIFAP is associated with a higher toxicity and does not improve the outcome of patients with recurrent HL or aggressive NHL. For those patients, innovative treatment concepts like recently developed immunotherapies are necessary.
Trial registration number
EudraCT number 2021-001937-38.
Date of registration
7 April 2021, retrospectively registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 20–25 years, outcome of patients with newly diagnosed Hodgkin’s lymphoma (HL) and aggressive non-Hodgkin’s lymphoma (NHL) has significantly improved. Contributing factors have been risk-adapted treatment strategies using prognostic indices (Diehl and Fuchs 2007; Hasenclever and Diehl 1998; International Non-Hodgkin’s Lymphoma Prognostic Factors Project 1993; Sehn et al. 2007), the assessment of metabolic responses by positron emission tomography (PET) using fluorodeoxyglucose ([18F] FDG) (Borchmann et al. 2017; Borchmann 2020) and the application of different dose-escalated or dose-dense protocols (Pfreundschuh et al. 2004, 2008; Schmitz et al. 2012; Tresckow et al. 2018). In addition, the use of the anti-CD20 monoclonal antibody rituximab (Coiffier et al. 2002; Pfreundschuh et al. 2006) or the anti-CD30 antibody–drug conjugate (ADC) brentuximab vedotin (Connors et al. 2018) have improved the treatment results. However, the prognosis of patients with recurrent lymphomas is still poor. Especially primary refractory diseases or early relapses are associated with a worse outcome (Gisselbrecht et al. 2010; Josting et al. 2002, 2010). Since several studies investigating different platinum-containing protocols such as DHAP, ESHAP and ASHAP had shown encouraging results with response rates of 60–70% (Hänel et al. 2000; Rodriguez et al. 1999; Velasquez et al. 1988, 1993, 1994), the MIFAP regimen was developed. In a non-randomized phase II trial, 46 patients with very poor-risk recurrent lymphoma achieved results comparable to patients with prognostically more favorable diseases (Hänel et al. 2001). However, a comparison with an established protocol like Dexa-BEAM was still pending. Therefore, this prospective randomized trial was performed.
Patients and methods
Patient enrollment/course of study
Patients aged 18–65 years with HL or aggressive NHL suffering from up to three relapses, primary refractory diseases or refractory relapses were included. After randomization, all patients received the salvage therapy with either Dexa-BEAM or MIFAP. Restaging was performed after one cycle and patients with complete response (CR), partial response (PR) or minor response (MR) received a second course of Dexa-BEAM or MIFAP. Patients reaching at least PR after two chemotherapy cycles were consolidated by either high-dose chemotherapy (HDT) with autologous hematopoietic cell transplantation (HCT), allogeneic HCT or autologous HCT with subsequent allogeneic HCT after reduced intensity conditioning. Non-responders (less than MR after the first course or less than PR after the second course) dropped out from the trial. The applied remission criteria were in accordance with the recommendations valid at the time the study was initiated (Cheson et al. 1999); after the first course, MR was defined as a tumor size reduction between 25 and 49%.
Treatment plan
Enrolled patients were randomly assigned at a 1:1 allocation ratio to receive either two cycles Dexa-BEAM (standard arm (Table 1a) or two cycles MIFAP (experimental arm (Table 1b) as salvage therapy. In responding patients (at least PR after the second course), a subsequent consolidation by HCT (autologous, allogeneic or autologous-allogeneic) was planned, as shown in Fig. 1.
Trial course. Out of 76 patients assessed for eligibility, 73 were assigned to the Dexa-BEAM arm (N = 37) or the MIFAP arm (N = 36). After the first course 26 patients of the Dexa-BEAM arm and 23 patients of the MIFAP arm were classified as responders (CR complete response, PR partial response, MR minor response). Non-responders (NR) were assigned to crossover or leave the study. After the second course, both treatment arms had 19 responders each. Thereafter, the patients received autologous (auto-HCT), allogeneic (allo-HCT), autologous and allogeneic (auto-allo-HCT), or no hematopoietic cell transplantation (no-HCT)
Statistical analysis
The study design aimed at the detection of a promising response rate of 60%, in contrast to 40% considered to be futile with regard to data on several existing salvage therapies for the treatment of relapsed/refractory NHL/HL. According to a one-stage phase II design by Fleming (1982), the recruitment of 38 patients for the experimental arm was required to achieve 80% power with a one-sided type-I error of 0.05. A similar number was allocated to a randomized reference group, to verify the historical assumptions, and thus, control for selection bias.
The statistical survival analyses and multivariate Cox Proportional Hazard Models were performed using R version 3.1.3 (R Core Team 2020), including the packages ggplot2 1.0.1 (Wickham 2016), Hmisc 3.15-0 (Harrell Jr et al. 2015) and survival 2.38-1 (Therneau 2015; Therneau and Grambsch 2000). Overall survival (OS) and progression-free survival (PFS) were analyzed using the Kaplan–Meier method (KM estimator). Overall survival time was calculated from the first day of first cycle (Dexa-BEAM or MIFAP) until death from any cause or last contact (= censored observation). Patients were counted as events in case of relapse, disease progression, or death from any cause (PFS), or were censored with their last observation date. P values were calculated using two-sided Log-rank tests. The statistical analysis of the response rates, the toxicities according World Health Organization (WHO) as well as the supportive therapy were performed using R version 4.0.3 (R Core Team 2020) and the packages exactRankTests 0.8-31 (Hothorn and Hornik 2019), MASS 7.3.53 (Venables and Ripley 2002), car 3.0.10 (Fox and Weisberg 2019), DescTools 0.99.38 (Signorell et al. 2020), lubridate 1.7.9.2 (Grolemund and Wickham 2011), and binom 1.1.1 (Dorai-Raj 2014). The overall response rate (ORR) was calculated on the square matrix of patients’ best response after every stage including both induction cycles as well as the HCT. CR and PR were classified as response, whereas MR, stable disease (SD), progressive disease (PD), and death from any cause were summarized to non-response (NR). Fisher’s exact test was used to determine the P value. All P values are two-sided and considered explorative. Explorative multivariate analysis was performed with all factors listed in Table 3 using generalized linear models for overall and complete response rates after two cycles Dexa-BEAM or MIFAP (logit transformation). Toxicities were analyzed using two-sided Fisher’s exact tests on categorical variables and Welch tests or Wilcoxon rank sum tests on continuous variables if prior Shapiro–Wilk tests pointed to other than normal data distribution. P values were adjusted for multiple comparisons by calculation of false discovery rates (FDR). The treatment-related toxicities were compared using Fisher’s exact tests (WHO toxicity grades 0–2 and 3–4 were subsumed, respectively).
Results
Patient characteristics
Between 2000 and 2006, 76 patients from 10 institutions were screened for the study. Table 2 displays the patients’ characteristics and the trial course is depicted in Fig. 1. Out of 76 patients assessed for eligibility, 73 were assigned either to the Dexa-BEAM standard arm (N = 37) or the MIFAP experimental arm (N = 36). Overall, the patients included in this study comprised a population with a very unfavorable prognosis. Fifty-eight patients (79%) had early and/or multiple relapses or had been refractory to the previous therapy. In patients with relapsed diseases, the median duration of the last remission (before study entry) was only 8 months.
Treatment response
After the first course, 26 patients (70%) of the standard arm and 23 patients of the experimental arm (64%) achieved at least MR. Non-responders were assigned to crossover to the other treatment regime or to leave the study. Table 3 shows the response rates after the second treatment course for each treatment arm. The ORR was 51% in the Dexa-BEAM group and 53% in the MIFAP arm and the CR rate was 38% in the Dexa-BEAM arm and 36% in the MIFAP arm (both not significant). Patients with refractory diseases had a significant higher risk for non-response than patients with relapse (P < 0.001, OR = 14.1, 95% CI 4.0–69.1). In addition, elevated lactate dehydrogenase (LDH) levels increased the risk for not reaching a CR (P = 0.064, OR = 2.9, 95% CI 1.0–9.5). Successful mobilization and harvesting of autologous stem cells was observed in 65 (89%) patients. A poor mobilization was documented in only three cases (two patients in the Dexa-BEAM and one patient in the MIFAP group). Stem cell collection was not performed in five patients due to toxicity (n = 1) or disease progression (n = 4). With regard to stem cell harvest, there were no significant differences between the two treatment arms (Table 1S).
Toxicity
After the first treatment course, only the median duration of G-CSF support (12 days MIFAP vs. 9 days Dexa-BEAM, P = 0.016) was of significance. After the second course, however, the increased hematological toxicity observed in the MIFAP arm was much more pronounced. This included both the duration of grade 4 leukocytopenia (P < 0.001) or thrombocytopenia (P < 0.001) as well as of febrile neutropenia (P = 0.004). In addition, significantly more transfusions of red blood cells (P < 0.001) or platelets (P < 0.001) were necessary. Patients treated in the MIFAP arm needed more G-CSF support (P = 0.021), their hospitalization lasted longer (P = 0.002). Table 2S summarizes hematological toxicities and supportive therapy. Regarding the non-hematological toxicities, no major differences were observed between both groups after the first treatment course. After the second treatment cycle infections (P = 0.001), performance status (P = 0.007), and pain (P = 0.018) were significantly shifted towards higher WHO toxicity grades in the experimental MIFAP arm (Table 3S).
Survival
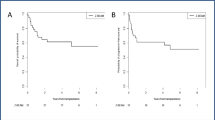
After a median follow-up of 14.4 years (inverse Kaplan–Meier method), the 15-year rates of PFS and OS of the entire group are 26% and 29% (median 5.6 and 26.6 months), respectively. There were no significant differences between Dexa-BEAM and MIFAP in PFS (median 5.6 vs. 5.4 months, P = 0.812) and OS (median 25.5 vs. 26.6 months, P = 0.440). In addition, in patients with relapsed or with refractory disease, no significant differences for PFS and OS were found between both regimens. Thirty-five of the 38 responders (with at least a PR after two courses) were consolidated by autologous (N = 29), allogeneic (N = 1) or sequential autologous/allogeneic (N = 5) HCT. Responding patients with subsequent autologous HCT achieved 15-year rates in PFS and OS of 59% and 50% (median not available and 121.8 months, respectively). Survival curves for patients suffering from HL or NHL are presented in Fig. 2, and the outcome of responders with subsequent autologous HCT is shown in Fig. 1S. In a univariate as well as in a multivariate analysis including treatment arm, lymphoma subtype, disease status, Ann Arbor stage as well as LDH level at randomization, we found no influence of treatment regimens (Dexa-BEAM vs. MIFAP) on PFS and OS (Table 4S).
Discussion
Although the benefit of consolidating high-dose therapy followed by autologous HCT has been demonstrated in patients with recurrent lymphomas (Philip et al. 1995; Schmitz et al. 2002), this option is typically limited to patients having chemosensitive disease. Therefore, it is very important to induce at least a partial remission by an effective salvage regimen. In this trial report, the platinum-containing MIFAP protocol, which had shown encouraging results in a phase II study (Hänel et al. 2001), was tested within a randomized phase II trial against the Dexa-BEAM regimen. No significant differences between the two treatment arms regarding response and survival were documented. In general, the achieved response rates were about 10–15% lower than reported in other trials (Gisselbrecht et al. 2010; Josting et al. 2010; Schmitz et al. 2002). However, the extremely high proportion (79%) of prognostically unfavorable patients with refractory disease or early and/or multiple relapses in our study must be considered. For comparison, the proportion of these patients in the HDR2 study (Josting et al. 2010) and the CORAL study (Gisselbrecht et al. 2010) was only 43% (120/279 patients) and 54% (228/396 patients), respectively. Since no superiority of the experimental arm was observed in response and survival, but the MIFAP regimen was significantly more toxic than Dexa-BEAM (particularly after the second course of treatment), the primary hypothesis of the study could not be confirmed. Still, the results for responders with autologous HCT are comparable to that of other studies (Gisselbrecht et al. 2010; Josting et al. 2010; Schmitz et al. 2002). The main problem remains the achievement of confirmed remission (at least PR) as a prerequisite to perform autologous HCT, especially in patients with refractory or early relapsed diseases (Gisselbrecht et al. 2010; Josting et al. 2010; Schmitz et al. 2002). So far, there is no optimal and favorable salvage protocol available. The DHAP regimen is routinely used as the standard salvage protocol for recurrent HL and aggressive NHL, although—to our best knowledge—its superiority to other protocols has not been demonstrated in a prospective randomized study. Furthermore, allogeneic HCT has not become a standard option for the treatment of Hodgkin’s lymphomas, and remains under debate in non-Hodgkin lymphomas (Shanbhag et al. 2019; Sureda et al. 2014). Nevertheless, allogeneic HCT for high-risk patients (defined as primary refractory/refractory relapse/recurrence after autologous HCT) is still a treatment option for non-Hodgkin lymphoma (Glass et al. 2014). For Hodgkin’s lymphomas, brentuximab vedotin is administered for maintenance therapy in high-risk patients after autologous HCT (Moskowitz et al. 2019). Polatuzumab vedotin (Sehn et al. 2020) has recently demonstrated very good results as salvage therapy without autologous HCT in DLBCL patients and could possibly be investigated as maintenance therapy in high-risk patients after autologous HCT. In addition, the checkpoint inhibitors nivolumab and pembrolizumab are options in the treatment of relapsed or refractory Hodgkin’s lymphoma (Chen et al. 2017; Younes et al. 2016) and are also intensively investigated for the treatment of NHL (Merryman et al. 2017).
Autologous genetically modified T cells, expressing chimeric antigen receptors (CARs, CAR T cells) directed against CD19 are a novel innovative treatment modality for patients with relapsed or refractory DLBCL and PMBCL from the 3rd line onwards. The ZUMA-1 study investigating the efficacy of axicabtagene ciloleucel demonstrated an overall response rate of 83% and a CR rate of 58% (Locke et al. 2019). Subsequently, a 4-year overall survival rate of 44% at a median follow-up of 51.1 months was reported (Jacobson et al. 2020). In the JULIET trial, 52% of patients responded to treatment with tisagenlecleucel and 40% achieved CR (Schuster et al. 2019b). After a median follow-up of 40.3 months, a 3-year overall survival rate of 36% was reported (Jaeger et al. 2020). Recently, a 5-year progression-free survival of 31% at a median follow-up of 60.7 months has been described (Chong et al. 2021). In clinical practice, the survival outcomes were consistent with the ZUMA-1 results (Nastoupil et al. 2020). In addition, Neelapu et al. showed that axicabtagene ciloleucel improved survival to conventional treatment based on standardized analyses. In the ZUMA-1 study, over 50% of the patients were alive at 2 years (Neelapu et al. 2019), compared to 12% of the SCHOLAR-1 study, the largest patient-level pooled retrospective analysis to characterize response rates and survival for a population of patients with refractory DLBCL (Crump et al. 2017).
Bispecific antibodies and T cell engagers (BiTE) bind both the target antigen of malignant lymphoma cells and that of CD3-positive T cells. On one hand, this leads to cell-mediated cytotoxicity, and on the other hand to the activation of various cellular and humoral immune reactions (Huehls et al. 2015). In heavily pretreated patients, various anti-CD3/anti-CD20 bispecific antibodies lead to overall response rates of up to 60% (Bannerji et al. 2020; Coyle et al. 2020; Lugtenburg et al. 2019; Schuster et al. 2019a). In patients after previous CAR T cell therapy, an overall response rate of up to 39% was reported (Bannerji et al. 2020; Schuster et al. 2019a).
In summary, treatment results of patients with recurrent lymphomas have been stagnant for about 20–25 years. This is especially true for unfavorable refractory diseases and early relapses. Despite different salvage regimes, autologous and allogeneic HCT, the results have not significantly improved. In the future, new treatment strategies using CAR T cells, BiTE and ADC may improve the outcome of patients with recurrent lymphomas.
Data availability
Data and study documentation are located in the respective study centers and are stored in accordance with legal requirements. However, the datasets generated during and/or analyzed during the current study are not publicly available due to individual privacy rights, but are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Change history
09 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00432-022-04324-3
References
Bannerji R, Allan JN, Arnason JE, Brown JR, Advani R, Ansell SM, O’Brien SM, Duell J, Martin P, Joyce RM, Li J, Flink DM, Zhu M, Weinreich DM, Yancopoulos GD, Sirulnik A, Chaudhry A, Ambati SR, Topp MS (2020) Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy. Blood 136:42–43. https://doi.org/10.1182/blood-2020-136659
Borchmann P (2020) Positron emission tomography guided omission of radiotherapy in early-stage unfavorable Hodgkin lymphoma: final results of the international, randomized phase III HD17 trial by the GHSG. EHA Library:S101
Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, Zijlstra JM, Markova J, Meissner J, Feuring-Buske M, Hüttmann A, Dierlamm J, Soekler M, Beck H-J, Willenbacher W, Ludwig W-D, Pabst T, Topp MS, Hitz F, Bentz M, Keller UB, Kühnhardt D, Ostermann H, Schmitz N, Hertenstein B, Aulitzky W, Maschmeyer G, Vieler T, Eich H, Baues C, Stein H, Fuchs M, Kuhnert G, Diehl V, Dietlein M, Engert A (2017) PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 390:2790–2802. https://doi.org/10.1016/S0140-6736(17)32134-7
Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, Tomita A, von Tresckow B, Shipp MA, Zhang Y, Ricart AD, Balakumaran A, Moskowitz CH (2017) Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 35:2125–2132. https://doi.org/10.1200/JCO.2016.72.1316
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244. https://doi.org/10.1200/JCO.1999.17.4.1244
Chong EA, Ruella M, Schuster SJ (2021) Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med 384:673–674. https://doi.org/10.1056/NEJMc2030164
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, van den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235–242. https://doi.org/10.1056/NEJMoa011795
Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, Younes A, Alekseev S, Illés Á, Picardi M, Lech-Maranda E, Oki Y, Feldman T, Smolewski P, Savage KJ, Bartlett NL, Walewski J, Chen R, Ramchandren R, Zinzani PL, Cunningham D, Rosta A, Josephson NC, Song E, Sachs J, Liu R, Jolin HA, Huebner D, Radford J (2018) Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med 378:331–344. https://doi.org/10.1056/NEJMoa1708984
Coyle L, Morley NJ, Rambaldi A, Mason KD, Verhoef G, Furness C, Desai R, Mergen N (2020) Updated analysis of an open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Blood 136:14–15. https://doi.org/10.1182/blood-2020-141117
Crump M, Neelapu SS, Farooq U, van den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, Boussetta S, Feng L, Maurer MJ, Navale L, Wiezorek J, Go WY, Gisselbrecht C (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 130:1800–1808. https://doi.org/10.1182/blood-2017-03-769620Accessed 14 April 2021
Diehl V, Fuchs M (2007) Early, intermediate and advanced Hodgkin’s lymphoma: modern treatment strategies. Ann Oncol 18(Suppl 9):ix71–ix79. https://doi.org/10.1093/annonc/mdm297
Dorai-Raj S (2014) Binom: binomial confidence intervals for several parameterizations. https://CRAN.R-project.org/package=binom. Accessed 14 Apr 2021
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38:143–151
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. SAGE Publications Inc, Thousand Oaks
Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, Ma D, Brière J, Moskowitz CH, Schmitz N (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28:4184–4190. https://doi.org/10.1200/JCO.2010.28.1618
Glass B, Hasenkamp J, Wulf G, Dreger P, Pfreundschuh M, Gramatzki M, Silling G, Wilhelm C, Zeis M, Görlitz A, Pfeiffer S, Hilgers R, Truemper L, Schmitz N (2014) Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol 15:757–766. https://doi.org/10.1016/S1470-2045(14)70161-5
Grolemund G, Wickham H (2011) Dates and times made easy with lubridate. J Stat Softw. https://doi.org/10.18637/jss.v040.i03
Hänel M, Kröger N, Hoffknecht MM, Peters SO, Metzner B, Fiedler F, Braumann D, Schubert JC, Illiger HJ, Hänel A, Krüger WH, Zeller W, Weh HJ, Hossfeld DK, Zander AR (2000) ASHAP–an effective salvage therapy for recurrent and refractory malignant lymphomas. Ann Hematol 79:304–311. https://doi.org/10.1007/s002779900150
Hänel M, Kröger N, Kroschinsky F, Birkmann J, Hänel A, Herbst R, Naumann R, Friedrichsen K, Ehninger G, Zander AR, Fiedler F (2001) Salvage chemotherapy with mitoxantrone, fludarabine, cytarabine, and cisplatin (MIFAP) in relapsing and refractory lymphoma. J Cancer Res Clin Oncol 127:387–395. https://doi.org/10.1007/s004320000226
Harrell Jr FE, with contributions from Charles Dupont, many others (2015) Hmisc: Harrell miscellaneous. https://CRAN.R-project.org/package=Hmisc. Accessed 14 Apr 2021
Hasenclever D, Diehl V (1998) A prognostic score for advanced Hodgkin’s disease. International prognostic factors project on advanced Hodgkin’s disease. N Engl J Med 339:1506–1514. https://doi.org/10.1056/NEJM199811193392104
Hothorn T, Hornik K (2019) exactRankTests: exact distributions for rank and permutation tests. https://CRAN.R-project.org/package=exactRankTests. Accessed 14 Apr 2021
Huehls AM, Coupet TA, Sentman CL (2015) Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol 93:290–296. https://doi.org/10.1038/icb.2014.93
International Non-Hodgkin’s Lymphoma Prognostic Factors Project (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 329:987–994. https://doi.org/10.1056/NEJM199309303291402
Jacobson C, Locke FL, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff PJ, Flinn IW, Farooq U, Goy AH, McSweeney PA, Munoz J, Siddiqi T, Rossi JM, Bot A, Zheng L, Vezan R, Bashir Z, Kim JJ, Chu R, Neelapu SS (2020) Long-term survival and gradual recovery of B cells in patients with refractory large B cell lymphoma treated with axicabtagene ciloleucel (Axi-Cel). Blood 136:40–42. https://doi.org/10.1182/blood-2020-134362
Jaeger U, Bishop MR, Salles G, Schuster SJ, Maziarz RT, Han X, Savchenko A, Roscoe N, Orlando E, Knoblock D, Tiwari R, Bubuteishvili Pacaud L, Corradini P (2020) Myc expression and tumor-infiltrating T cells are associated with response in patients (Pts) with relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL) treated with tisagenlecleucel in the juliet trial. Blood 136:48–49. https://doi.org/10.1182/blood-2020-137045
Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, Rudolph C, Diehl V, Engert A (2002) New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol 20:221–230. https://doi.org/10.1200/JCO.2002.20.1.221
Josting A, Müller H, Borchmann P, Baars JW, Metzner B, Döhner H, Aurer I, Smardova L, Fischer T, Niederwieser D, Schäfer-Eckart K, Schmitz N, Sureda A, Glossmann J, Diehl V, DeJong D, Hansmann M-L, Raemaekers J, Engert A (2010) Dose intensity of chemotherapy in patients with relapsed Hodgkin’s lymphoma. J Clin Oncol 28:5074–5080. https://doi.org/10.1200/JCO.2010.30.5771
Josting A, Müller H, Borchmann P, Baars JW, Metzner B, Döhner H, Aurer I, Smardova L, Fischer T, Niederwieser D, Schäfer-Eckart K, Schmitz N, Sureda A, Glossmann J, Diehl V, DeJong D, Hansmann M-L, Raemaekers J, Engert A (2010) Dose intensity of chemotherapy in patients with relapsed Hodgkin’s lymphoma. J Clin Oncol 28:5074–5080. https://doi.org/10.1200/JCO.2010.30.5771
Lugtenburg P, Mous R, Clausen MR, Chamuleau ME, Johnson P, Linton K, Rule S, Oliveri RS, DeMarco D, Hiemstra IH, Chen G, Azaryan A, Gupta M, Ahmadi T, Hutchings M (2019) First-in-human, phase 1/2 trial to assess the safety and clinical activity of subcutaneous GEN3013 (DuoBody®-CD3×CD20) in B-cell non-Hodgkin lymphomas. Blood 134:758. https://doi.org/10.1182/blood-2019-121460
Merryman RW, Armand P, Wright KT, Rodig SJ (2017) Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv 1:2643–2654. https://doi.org/10.1182/bloodadvances.2017012534
Moskowitz AJ, Herrera AF, Beaven AW (2019) Relapsed and refractory classical Hodgkin lymphoma: keeping pace with novel agents and new options for salvage therapy. Am Soc Clin Oncol Educ Book 39:477–486. https://doi.org/10.1200/EDBK_238799
Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, Dahiya S, Lunning M, Lekakis L, Reagan P, Oluwole O, McGuirk J, Deol A, Sehgal AR, Goy A, Hill BT, Vu K, Andreadis C, Munoz J, Westin J, Chavez JC, Cashen A, Bennani NN, Rapoport AP, Vose JM, Miklos DB, Neelapu SS, Locke FL (2020) Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol 38:3119–3128. https://doi.org/10.1200/JCO.19.02104
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Reagan PM, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Crump M, Kuruvilla J, van den Neste E, Farooq U, Navale L, DePuy V, Kim JJ, Gisselbrecht C (2019) A comparison of two-year outcomes in ZUMA-1 (axicabtagene ciloleucel) and SCHOLAR-1 in patients with refractory large B cell lymphoma. Blood 134:4095. https://doi.org/10.1182/blood-2019-125792
Pfreundschuh M, Trümper L, Kloess M, Schmits R, Feller AC, Rübe C, Rudolph C, Reiser M, Hossfeld DK, Eimermacher H, Hasenclever D, Schmitz N, Loeffler M (2004) Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood 104:634–641. https://doi.org/10.1182/blood-2003-06-2095
Pfreundschuh M, Trümper L, Österborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani P-L, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, López-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M (2006) CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 7:379–391. https://doi.org/10.1016/S1470-2045(06)70664-7
Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann R, Trümper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass B, Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M (2008) Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 9:105–116. https://doi.org/10.1016/S1470-2045(08)70002-0
Philip T, Guglielmi C, Hagenbeek A, Somers R, van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL (1995) Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med 333:1540–1545. https://doi.org/10.1056/NEJM199512073332305
R Core Team (2020) R: a language and environment for statistical computing. https://www.R-project.org/. Accessed 14 Apr 2021
Rodriguez J, Rodriguez MA, Fayad L, McLaughlin P, Swan F, Sarris A, Romaguera J, Andersson B, Cabanillas F, Hagemeister FB (1999) ASHAP: a regimen for cytoreduction of refractory or recurrent Hodgkin’s disease. Blood 93:3632–3636
Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, Boissevain F, Zschaber R, Müller P, Kirchner H, Lohri A, Decker S, Koch B, Hasenclever D, Goldstone AH, Diehl V (2002) Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet 359:2065–2071. https://doi.org/10.1016/S0140-6736(02)08938-9
Schmitz N, Nickelsen M, Ziepert M, Haenel M, Borchmann P, Schmidt C, Viardot A, Bentz M, Peter N, Ehninger G, Doelken G, Ruebe C, Truemper L, Rosenwald A, Pfreundschuh M, Loeffler M, Glass B (2012) Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002–1). Lancet Oncol 13:1250–1259. https://doi.org/10.1016/S1470-2045(12)70481-3
Schuster SJ, Bartlett NL, Assouline S, Yoon S-S, Bosch F, Sehn LH, Cheah CY, Shadman M, Gregory GP, Ku M, Wei MC, Yin S, Kwan A, Yousefi K, Hernandez G, Li C-C, O’Hear C, Budde LE (2019a) Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and is active in treatment through multiple lines. Blood 134:6. https://doi.org/10.1182/blood-2019-123742
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak Ö, Salles G, Maziarz RT (2019b) Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380:45–56. https://doi.org/10.1056/NEJMoa1804980
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, Gascoyne RD, Connors JM (2007) The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109:1857–1861. https://doi.org/10.1182/blood-2006-08-038257
Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, Assouline S, Kim TM, Kim WS, Ozcan M, Hirata J, Penuel E, Paulson JN, Cheng J, Ku G, Matasar MJ (2020) Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 38:155–165. https://doi.org/10.1200/JCO.19.00172
Shanbhag S, Wagner-Johnston N, Ambinder RF, Jones RJ (2019) Is it time to revisit the role of allogeneic transplantation in lymphoma? Curr Oncol Rep 21:65. https://doi.org/10.1007/s11912-019-0809-z
Signorell A et al (2020) DescTools: tools for descriptive statistics. https://cran.r-project.org/package=DescTools. Accessed 14 Apr 2021
Sureda A, Domenech E, Schmitz N, Dreger P (2014) The role of allogeneic stem cell transplantation in Hodgkin’s lymphoma. Curr Treat Options Oncol 15:238–247. https://doi.org/10.1007/s11864-014-0287-3
Therneau TM (2015) A package for survival analysis in R. https://CRAN.R-project.org/package=survival. Accessed 14 Apr 2021
Therneau TM, Grambsch PM (2000) Modeling survival data: extending the Cox model. Springer, New York
Velasquez WS, Cabanillas F, Salvador P, McLaughlin P, Fridrik M, Tucker S, Jagannath S, Hagemeister FB, Redman JR, Swan F (1988) Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood 71:117–122
Velasquez WS, Dunphy F, Santillana S, Adkins D, Bowers C, Broun GO Jr, Petruska PJ, Spitzer G (1993) ASHAP, an effective treatment for relapsing and refractory Hodgkin’s disease (HD) and non-Hodgkin’s lymphoma (NHL). Blood 82:138a (abstr 538)
Velasquez WS, Dunphy F, Santillana S, Adkins D, Bowers C, Broun GO Jr, Petruska PJ, Spitzer G (1993) ASHAP, an effective treatment for relapsing and refractory Hodgkin’s disease (HD) and non-Hodgkin’s lymphoma (NHL). Blood 82:138a (abstr 538)
Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, Romaguera J, Rubenstein E, Cabanillas F (1994) ESHAP–an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol 12:1169–1176. https://doi.org/10.1200/JCO.1994.12.6.1169
Venables WN, Ripley BD (2002) Modern applied statistics with S with 152 illustrations, 4th edn. Statistics and computing. Springer, New York. Accessed 14 Apr 2021
von Tresckow B, Kreissl S, Goergen H, Bröckelmann PJ, Pabst T, Fridrik M, Rummel M, Jung W, Thiemer J, Sasse S, Bürkle C, Baues C, Diehl V, Engert A, Borchmann P (2018) Intensive treatment strategies in advanced-stage Hodgkin’s lymphoma (HD9 and HD12): analysis of long-term survival in two randomised trials. Lancet Haematol 5:e462–e473. https://doi.org/10.1016/S2352-3026(18)30140-6
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V, Kuruvilla J, Cohen JB, Collins G, Savage KJ, Trneny M, Kato K, Farsaci B, Parker SM, Rodig S, Roemer MGM, Ligon AH, Engert A (2016) Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 17:1283–1294. https://doi.org/10.1016/S1470-2045(16)30167-X
Acknowledgements
We thank all patients and partners of the participating study centers. We greatly appreciate the support of Dr. S. Ibach from the X-act Cologne Clinical Research GmbH, Cologne, Germany for statistical analysis of the survival data.
Funding
The study was supported by the Carl Gustav Carus University Hospital of the Technical University Dresden and the Klinikum Chemnitz gGmbH.
Author information
Authors and Affiliations
Contributions
FK, GE, JB, FF and MH designed the study; RH, FK, JB, AH, KSE, GE, JB, FF and MH performed the study; SK, ARB, LS, FK, JB, MB, SF and MH analyzed the results; all the authors created the figures and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Eligible patients were registered at the Medical and Outpatient’s clinic I of the Carl Gustav Carus University Hospital of the Technical University Dresden, where the trial was designed and approved by the local ethics committee in 2001 (11 January 2001; number of registration: EK155102000).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study. In addition, all the authors consented the last version of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: To rectify the error in figure 2.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kürzel, S., Blaudszun, AR., Stahl, L. et al. Dexa-BEAM versus MIFAP as salvage regimen for recurrent lymphoma: a prospective randomized multicenter phase II trial with a median follow-up of 14.4 years. J Cancer Res Clin Oncol 148, 1171–1181 (2022). https://doi.org/10.1007/s00432-021-03702-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03702-7