Abstract
Central nervous system (CNS) relapse in patients with diffuse large B cell lymphoma (DLBCL) is an uncommon event, and the outcome of patients with CNS relapse is poor. However, no reliable prediction models for CNS relapse have been developed. We retrospectively analyzed consecutive de novo DLBCL patients referred to our department between September 2004 and August 2015 and treated with R-CHOP or R-CHOP-like regimens. Of 413 patients analyzed in this study, a total of 27 patients (6.5 %) eventually developed CNS relapse. The 5-year probability of CNS relapse was 8.4 %. The median time from diagnosis of DLBCL to CNS relapse was 15 months, and the median survival after CNS relapse was 7 months. In univariate analysis, the risk factors significantly associated with CNS relapse were Ann Arbor stage 3 or 4, albumin level <3.2 mg/L, number of extranodal sites >1, and involvement of retroperitoneal lymph node. We developed a new prognostic model consisting of these four factors. The 5-year probability of CNS relapse was significantly higher in patients with at least three of these four factors than in those with two or fewer factors (26.4 vs. 3.0 %, P < 0.001). Using this model, we evaluated the incidence and the risk factors of CNS relapse in DLBCL patients. The new risk model consisting of the four factors demonstrated good risk stratification for CNS relapse, and could help to identify high-risk patients for whom CNS prophylaxis is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B cell lymphoma (DLBCL) is the most common subgroup of non-Hodgkin lymphoma [1]. The survival outcome of patients with DLBCL has improved dramatically with addition of rituximab to CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy [2–5].
Central nervous system (CNS) relapse in patients with DLBCL is an uncommon event and carries an extremely poor prognosis even in the rituximab era, with median survival after CNS relapse of less than 6 months [6–10]. Although CNS-directed prophylaxis is often administered in attempt to reduce the incidence of CNS relapse, this treatment may increase the toxicities of the systemic chemotherapy. There is a lack of consensus about which patients should receive CNS-directed prophylaxis, as well as the actual effectiveness of prophylaxis in the rituximab era.
There are several risk models for predicting CNS relapse, including models proposed by Hollender et al. in the pre-rituximab era [11] and by Schmitz et al. in the rituximab era [12]. Although both models can effectively select high-risk patients to a certain extent, the prognostic values have not been extensively validated. Furthermore, the incidence of CNS relapse in the high-risk patients defined by these models was not so high that CNS prophylaxis was warranted in all of these patients. Therefore, a new model is needed to more effectively identify patients at high risk for CNS relapse.
In this retrospective study, we evaluated the incidence and the risk factors of CNS relapse in DLBCL patients in the rituximab era using a database of our hospital. Additionally, we proposed a new prognostic model for CNS relapse and compared the prognostic value of this model with that of the previously proposed models.
Methods
Patients
We reviewed the records of 472 consecutive patients with de novo DLBCL who were newly diagnosed between September 2004 and August 2015 and treated in our hospital. The data of patients who were treated with R-CHOP or R-CHOP-like regimen were analyzed. We excluded patients with primary CNS lymphoma, intravascular B cell lymphoma, and with CNS involvement at diagnosis from this analysis. Patients were also excluded if they had human immunodeficiency virus-associated lymphoma or transformed lymphoma from a prior indolent B cell lymphoma. None of these patients had previously been treated for DLBCL.
Evaluation of the CNS at diagnosis by computed tomography/magnetic resonance imaging or by lumbar puncture with cerebrospinal fluid analysis was carried out at the discretion of the treating physician. Diagnosis of CNS relapse was established by the presence of intracranial or spinal masses detected by neurological imaging (parenchymal type), or by malignant cells detected by cerebrospinal fluid cytology (leptomeningeal type).
The pathological diagnosis was made according to the WHO 2008 classification. Clinical staging was performed according to the Ann Arbor classification. Computed tomography (CT) scans were performed in all patients and positron emission tomography (PET) scans were performed in some of the patients for staging. Performance status (PS) was evaluated based on Eastern Cooperative Oncology Group criteria. The International Prognostic Index (IPI) and the National Comprehensive Cancer Network (NCCN)-IPI were calculated based on age, serum lactate dehydrogenase (LDH), PS, Ann Arbor stage, and extranodal involvements at diagnosis [13, 14]. The clinical tumor response was assessed by CT and/or PET/CT scanning after completion of the initial therapy according to the International Workshop Criteria [15].
This study was approved by the institutional ethics review boards of our hospital and informed consent for retrospective analysis was obtained from all patients.
Treatment
All patients in this study were treated with at least once cycle of an R-CHOP or an R-THP-COP. In the R-THP-COP regimen, terahydropyranyladriamycin was used instead of doxorubicin [16]. CNS prophylaxis was carried out in 62 patients, who were considered as high risk for CNS relapse because of extranodal involvements such as testis, breast, paranasal sinuses, or bone marrow. These patients received intrathecal (IT) injection of methotrexate (MTX) with or without cytarabine (Ara-C) at least once. No patient received systemic MTX for CNS prophylaxis.
Prognostic models for CNS relapse
The risk of CNS relapse was stratified according to the new risk model, as well as according to previous models. The model by Hollender et al. was composed of LDH >institutional upper limit of normal, albumin level <3.2 mg/L, age >60 years, retroperitoneal lymph node involvement, and number of extranodal sites >1 [11]. Based on this model, we stratified the patients into two groups: a low-risk (0–3 factors) and a high-risk (4–5 factors) group. The model by Schmitz et al. was based on the five IPI factors and kidney and/or adrenal grand involvement, assigning patients into low-risk (0–1 factor), intermediate-risk (2–3 factors), or high-risk (4–6 factors) groups [12]. These risk models are compared in Table 1.
Statistical analysis
Time to CNS relapse (TTCNS) was defined as the period from the date of diagnosis of DLBCL to the date of CNS relapse. Patients who died without CNS relapse were censored in analysis of TTCNS. Overall survival (OS) was defined as the period from the date of diagnosis of DLBCL (or from the date of CNS relapse, where applicable) to the date of last follow-up or death from any cause. TTCNS and OS were estimated using the Kaplan-Meier method. Differences between survival curves were tested for significance using the log-rank test. Baseline characteristics of the patients with and without CNS relapse were compared using Fisher’s exact test for categorical data. All P values were two sided, and P values of 0.05 or less were considered significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics [17].
Results
Patient characteristics
We analyzed the data of 413 patients who met the inclusion criteria. The median age was 68 years (range, 27–97 years). The median number of cycles of chemotherapy was 6 (range, 1–8). The median follow-up time of surviving patients was 38 months. A complete response was achieved in 300 patients (72.6 %), a partial response was achieved in 98 patients (23.7 %), and 15 patients (3.6 %) showed progressive disease after the initial therapy. The characteristics of the patients with and without CNS relapse are presented in Table 2. Overall, baseline characteristics were similar between the two groups, although there were a greater proportion of patients in the CNS relapse group with advanced stage, low serum albumin level, the presence of extranodal diseases, involvement of retroperitoneal lymph node and bone marrow, and high-intermediate (HI)- and high (H)-risk patients by the IPI and the NCCN-IPI. Fourteen patients (22.6 %) in the CNS relapse group and 48 patients (13.9 %) in the non-CNS relapse group received IT prophylaxis, with no significant difference between the two groups.
CNS relapse
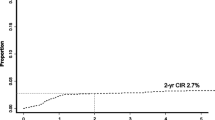
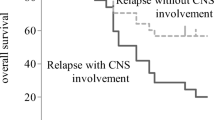
In total, 27 patients (6.5 %) eventually developed CNS relapse. The 5-year probability of CNS relapse was 8.4 % (95 % confidence interval, 5.6–12.4 %). The median time from the diagnosis of DLBCL to CNS relapse was 15 months (Fig. 1). More than half of the patients with CNS relapse had leptomeningeal diseases (59.3 %), while nine had parenchymal diseases (33.3 %) and two had both (7.4 %). Sixteen patients (59.3 %) developed isolated CNS relapse and 11 (40.7 %) had CNS relapse in the context of systemic relapse. The median survival after CNS relapse was 7 months (Fig. 2).
Risk factors for CNS relapse
The influence of the following variables on TTCNS was evaluated: age >60 years; male sex; the presence of B symptoms; PS >1; Ann Arbor stage 3 or 4; LDH >institutional upper limit of normal; albumin level <3.2 mg/L; number of extranodal sites >1; involvement of testes, breast, paranasal sinus, kidneys, adrenal glands, retroperitoneal lymph node, and bone marrow; high-intermediate (HI) or high (H) risk by IPI and NCCN-IPI; and the administration of IT prophylaxis. Univariate analysis results are shown in Table 3. The following risk factors were significantly associated with CNS relapse: Ann Arbor stage, albumin level, number of extranodal sites, involvement of retroperitoneal lymph node, and HI or H risk by IPI and NCCN-IPI.
New risk model for CNS relapse
We developed a new prognostic model consisting of four factors: Ann Arbor stage 3 or 4, albumin level <3.2 mg/L, number of extranodal sites >1, and involvement of retroperitoneal lymph node. Two distinct risk groups were formed based on Kaplan-Meier survival curves: a low-risk group with 0–2 factors (n = 301, 72.9 %); and a high-risk group with 3–4 factors (n = 112, 27.1 %) (Table 1). This model distinguished two groups with a significantly different relapse rate; the 5-year probability of CNS relapse was 3.0 and 26.4 % for patients in the low- and high-risk groups, respectively (P < 0.001) (Fig. 3). We also adapted this model to the analysis of isolated CNS relapse. The 5-year probability of isolated CNS relapse was 2.5 and 11.7 % for patients in the low- and high-risk groups, respectively (P < 0.001) (Fig. 4).
Comparison of the prognostic values of the new model with those of other models
We compared the prognostic powers of the new model for predicting CNS relapse with those of the models reported by Hollender and by Schmitz [11, 12]. The estimated 5-year probability of CNS relapse in the low-risk group of the new model was lower than that of the Hollender and Schmitz models (3.0 % vs. 5.6 and 3.5 %, respectively), whereas the 5-year probability of CNS relapse in the high-risk group of the new model was higher than that of the other models (26.4 % vs. 18.3 and 17.6 %, respectively) (Fig. 3).
Discussion
In this study cohort, the incidence of CNS relapse was 8.4 %. Previous studies reported that the incidence of CNS relapse in DLBCL patients was between 1.1 and 10.4 % [6–8, 11, 18–26]. This wide range in the reported rate may be due to the heterogeneity of the study populations, associated risk factors, accuracy of diagnostic procedures, and the type of systemic therapy and CNS-directed prophylaxis.
There is controversy regarding whether rituximab has a protective effect against CNS relapse or not. The randomized prospective RICOVER-60 trial of 1222 patients with aggressive B cell lymphoma on CHOP-14 with or without rituximab suggested that rituximab decreased the risk of CNS relapse; the relative risk decreased to 0.58 (95 % CI 0.3–1.0, P = 0.046) [7]. On the other hand, a study conducted by the Groupe d’Etude des Lymphomes de l’Adulte (GELA) on 399 elderly patients with DLBCL treated with CHOP with or without rituximab could not demonstrate any effect of rituximab on the risk of CNS relapse [6]. Conflicting results have been reported from several other studies with some demonstrating a reduction in risk [8, 25, 27, 28] and others demonstrating no reduction [9, 20, 21, 29]. As reported in a recent meta-analysis, the effect of rituximab on CNS relapse remains controversial [30, 31].
IT chemotherapy, including MTX, Ara-C, and steroids, has been largely used as a strategy for CNS prophylaxis. The RICOVER-60 trial, described above, showed that IT MTX failed to reduce the risk of CNS relapse in elderly patients with DLBCL treated with R-CHOP14. Further subgroup analysis suggested that IT prophylaxis may benefit patients treated without rituximab, but also suggested that this benefit was lost when rituximab was added to CHOP [7]. Other studies in the rituximab era also did not demonstrate a significant benefit of IT MTX [8, 21, 28, 32]. However, IT MTX is an important part of the treatment of patients with primary testicular DLBCL [33]. Due to the poor penetration of IT chemotherapy, it is unlikely to have a protective effect on parenchymal relapses. As an alternative method of reducing CNS relapse, CNS-penetrating doses of antimetabolites such as MTX and Ara-C have been recently used to prevent CNS relapse. Although there have been several studies that support the efficacy of systemic MTX in the rituximab era [10, 27, 34, 35], the optimal dose, infusion rate, number of cycles, and the need for concomitant IT chemotherapy remain unclear.
Even though several studies have attempted to identify patients with a high rate of CNS relapse, there remains no consensus regarding how to define high-risk patients. The involvement of many specific anatomic sites has been proposed as predictors of increased risk of CNS relapse. In particular, testicular involvement is consistently correlated with a high relapse rate [36]. Breast, kidney, adrenal glands, paranasal sinuses, epidural space, bone, and bone marrow involvement have also been reported as high-risk sites [37]. Other clinical factors, such as elevated LDH level and more than one extranodal involvement, have been identified as risk factors [6, 7, 25, 29]. However, other studies have demonstrated no significant increase in CNS relapse in patients with these clinical factors [8, 9, 20, 21, 38], and no consistent conclusion regarding this matter has yet been reached.
Several biological risk factors associated with CNS relapse have been reported. Patients harboring MYC gene rearrangement or a combination of MYC and BCL2 translocations have a significantly higher risk of CNS relapse, ranging between 9 and 50 % [39–45]. A recent study suggested that dual expression of MYC and BCL2 is associated with an increased risk of CNS relapse, with an estimated 2-year risk of 9.7 % [46]. Patients with DLBCL whose tumors are CD5 positive have been reported to have increased risk of CNS relapse; the 2-year CNS relapse rate was 12.7 % [47].
Although no single risk factor has been reported to have sufficient predictive value, specific combinations of such factors are expected to be more highly associated with the risk of CNS relapse. Hollender et al. analyzed 1220 patients with high grade non-Hodgkin lymphoma and proposed a prognostic model composed of LDH level, albumin level, age, retroperitoneal lymph node involvement, and number of extranodal sites >1. The risk of CNS relapse was 6.2 % or less at 5 years with up to three of these risk factors, whereas it rose to 25.3 % with four of these factors and to 32.7 % with all of the factors [11]. Schmitz et al. analyzed 2164 patients with DLBCL in the German High-Grade Lymphoma Study Group (DSHNHL) prospective study and identified IPI factors and kidney and/or adrenal grand involvement as independent risk factors for CNS relapse. Low- (0–1 factor), intermediate- (2–3 factors), and high-risk (4–6 factors) groups had a 2-year actuarial CNS relapse rate of 0.6, 4.1, and 17.0 %, respectively [12]. Although those two risk models could distinguish two or three patient groups with significantly different relapse rates, the new model proposed in this study demonstrated better risk stratification than those models.
HI or H risk by IPI, which was not included in the new model, was also associated with CNS relapse (Table 3). We investigated the effect of the model consisting of the five factors, HI or H risk by IPI and the four factors in the new risk model. Two distinct risk groups were formed based on Kaplan-Meier survival curves: a low-risk group with 0–3 factors (n = 316, 76.5 %); and a high-risk group with 4–5 factors (n = 97, 23.5 %). The 5-year probability of CNS relapse was 5.4 and 18.8 % for patients in the low- and high-risk groups, respectively (P < 0.001) (Fig. 5). Although this model could also stratify the risk of CNS relapse, the prognostic value of the four-factor model was suggested to be better than that of this five-factor model.
There were some limitations to this study. First, this was a retrospective study conducted in a single institute, and had some biases. In particular, there were inconsistencies in the indication, timing, dose, and number of times of CNS prophylaxis. Although the efficacy of IT prophylaxis was not proven in this study, this may be due to the small number of the patients with CNS relapse and it is difficult to estimate whether IT prophylaxis had some impact on the risk of CNS relapse. Second, the prognostic influences of immunohistochemical markers or biologic markers such as translocation involving MYC and BCL2 were not taken into account. Third, there was a small number of CNS relapses and the analysis of each risk factor had poor statistical power. Among the involved sites, involvement of retroperitoneal lymph node was the only factor to reach statistical significance for CNS relapse. This was likely to be because of the larger number of patients with retroperitoneal lymph node involvement. Clinicians should still consider possible site-specific indications for CNS prophylaxis in addition to the new prognostic model. Finally, this new model was derived from a dataset from a single institution, and needs to be validated using independent datasets.
Conclusion
We analyzed the incidence and the risk factors of CNS relapse in patients with DLBCL in the rituximab era. A new prognostic model consisting of the four factors: Ann Arbor stage 3 or 4, albumin level <3.2 mg/L, number of extranodal sites >1, and involvement of retroperitoneal lymph node, demonstrated better risk stratification than the previously reported models. This model may help to identify high-risk patients who would benefit from CNS-directed prophylaxis.
References
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (2008) World Health Organization classification of tumours of haematopoietic and lymphoid tissues, 4th edn. France IARC Press, Lyon, pp 233–237
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346(4):235–242. doi:10.1056/NEJMoa011795
Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA, Horning SJ (2006) Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 24(19):3121–3127. doi:10.1200/jco.2005.05.1003
Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann R, Trumper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass B, Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M (2008) Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 9(2):105–116. doi:10.1016/s1470-2045(08)70002-0
Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, Gill DS, Walewski J, Pettengell R, Jaeger U, Zinzani PL, Shpilberg O, Kvaloy S, de Nully BP, Stahel R, Milpied N, Lopez-Guillermo A, Poeschel V, Grass S, Loeffler M, Murawski N (2011) CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 12(11):1013–1022. doi:10.1016/s1470-2045(11)70235-2
Feugier P, Virion JM, Tilly H, Haioun C, Marit G, Macro M, Bordessoule D, Recher C, Blanc M, Molina T, Lederlin P, Coiffier B (2004) Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol 15(1):129–133
Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M (2009) CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 113(17):3896–3902. doi:10.1182/blood-2008-10-182253
Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ (2010) Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol 21(5):1046–1052. doi:10.1093/annonc/mdp432
Tomita N, Yokoyama M, Yamamoto W, Watanabe R, Shimazu Y, Masaki Y, Tsunoda S, Hashimoto C, Murayama K, Yano T, Okamoto R, Kikuchi A, Tamura K, Sato K, Sunami K, Shibayama H, Takimoto R, Ohshima R, Hatta Y, Moriuchi Y, Kinoshita T, Yamamoto M, Numata A, Ishigatsubo Y, Takeuchi K (2012) Central nervous system event in patients with diffuse large B-cell lymphoma in the rituximab era. Cancer Sci 103(2):245–251. doi:10.1111/j.1349-7006.2011.02139.x
Ferreri AJ, Bruno-Ventre M, Donadoni G, Ponzoni M, Citterio G, Foppoli M, Vignati A, Scarfo L, Sassone M, Govi S, Caligaris-Cappio F (2015) Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br J Haematol 168(5):654–662. doi:10.1111/bjh.13194
Hollender A, Kvaloy S, Nome O, Skovlund E, Lote K, Holte H (2002) Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol 13(7):1099–1107
Schmitz N, Zeynalova S, Nickelsen M, Ziepert M, Pfreundschuh M, Glass B, Loeffler M (2013) A new prognostic model to assess the risk of CNS disease in patients with aggressive B-cell lymphoma. Hematol Oncol 31(S1):96–150
A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project (1993). N Eng J Med 329 (14):987-994. doi:10.1056/nejm199309303291402
Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, Nademanee A, Kaminski MS, Czuczman MS, Millenson M, Niland J, Gascoyne RD, Connors JM, Friedberg JW, Winter JN (2014) An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 123(6):837–842. doi:10.1182/blood-2013-09-524108
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586. doi:10.1200/jco.2006.09.2403
Tsurumi H, Hara T, Goto N, Kanemura N, Kasahara S, Sawada M, Yasuda I, Yamada T, Shimizu M, Takami T, Moriwaki H (2007) A phase II study of a THP-COP regimen for the treatment of elderly patients aged 70 years or older with diffuse large B-cell lymphoma. Hematol Oncol 25(3):107–114. doi:10.1002/hon.815
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. doi:10.1038/bmt.2012.244
Boehme V, Zeynalova S, Kloess M, Loeffler M, Kaiser U, Pfreundschuh M, Schmitz N (2007) Incidence and risk factors of central nervous system recurrence in aggressive lymphoma—a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol 18(1):149–157. doi:10.1093/annonc/mdl327
Arkenau HT, Chong G, Cunningham D, Watkins D, Agarwal R, Sirohi B, Trumper M, Norman A, Wotherspoon A, Horwich A (2007) The role of intrathecal chemotherapy prophylaxis in patients with diffuse large B-cell lymphoma. Ann Oncol 18(3):541–545. doi:10.1093/annonc/mdl434
Yamamoto W, Tomita N, Watanabe R, Hattori Y, Nakajima Y, Hyo R, Hashimoto C, Motomura S, Ishigatsubo Y (2010) Central nervous system involvement in diffuse large B-cell lymphoma. Eur J Haematol 85(1):6–10. doi:10.1111/j.1600-0609.2010.01438.x
Tai WM, Chung J, Tang PL, Koo YX, Hou X, Tay KW, Quek R, Tao M, Lim ST (2011) Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Ann Hematol 90(7):809–818. doi:10.1007/s00277-010-1150-7
Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI (2009) Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516—the Southwest Oncology Group. J Clin Oncol 27(1):114–119. doi:10.1200/jco.2008.16.8021
Bjorkholm M, Hagberg H, Holte H, Kvaloy S, Teerenhovi L, Anderson H, Cavallin-Stahl E, Myhre J, Pertovaara H, Ost A, Nilsson B, Osby E (2007) Central nervous system occurrence in elderly patients with aggressive lymphoma and a long-term follow-up. Ann Oncol 18(6):1085–1089. doi:10.1093/annonc/mdm073
Haioun C, Besson C, Lepage E, Thieblemont C, Simon D, Rose C, Tilly H, Sonet A, Lederlin P, Attal M, Briere J, Reyes F (2000) Incidence and risk factors of central nervous system relapse in histologically aggressive non-Hodgkin’s lymphoma uniformly treated and receiving intrathecal central nervous system prophylaxis: a GELA study on 974 patients. Groupe d’Etudes des Lymphomes de l’Adulte. Ann Oncol 11(6):685–690
Shimazu Y, Notohara K, Ueda Y (2009) Diffuse large B-cell lymphoma with central nervous system relapse: prognosis and risk factors according to retrospective analysis from a single-center experience. Int J Hematol 89(5):577–583. doi:10.1007/s12185-009-0289-2
van Besien K, Ha CS, Murphy S, McLaughlin P, Rodriguez A, Amin K, Forman A, Romaguera J, Hagemeister F, Younes A, Bachier C, Sarris A, Sobocinski KS, Cox JD, Cabanillas F (1998) Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood 91(4):1178–1184
Abramson JS, Hellmann M, Barnes JA, Hammerman P, Toomey C, Takvorian T, Muzikansky A, Hochberg EP (2010) Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 116(18):4283–4290. doi:10.1002/cncr.25278
Kumar A, Vanderplas A, LaCasce AS, Rodriguez MA, Crosby AL, Lepisto E, Czuczman MS, Nademanee A, Niland J, Gordon LI, Millenson M, Zelenetz AD, Friedberg JW, Abel GA (2012) Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: findings from a large national database. Cancer 118(11):2944–2951. doi:10.1002/cncr.26588
Chihara D, Oki Y, Matsuo K, Onoda H, Taji H, Yamamoto K, Morishima Y (2011) Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: analyses with competing risk regression model. Leuk Lymphoma 52(12):2270–2275. doi:10.3109/10428194.2011.596966
Ghose A, Elias HK, Guha G, Yellu M, Kundu R, Latif T (2015) Influence of rituximab on central nervous system relapse in diffuse large B-cell lymphoma and role of prophylaxis—a systematic review of prospective studies. Clin Lymphoma Myeloma Leuk 15(8):451–457. doi:10.1016/j.clml.2015.02.026
Zhang J, Chen B, Xu X (2014) Impact of rituximab on incidence of and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: a systematic review and meta-analysis. Leuk Lymphoma 55(3):509–514. doi:10.3109/10428194.2013.811239
Tomita N, Takasaki H, Ishiyama Y, Kishimoto K, Ishibashi D, Koyama S, Ishii Y, Takahashi H, Numata A, Watanabe R, Tachibana T, Ohshima R, Hagihara M, Hashimoto C, Takemura S, Taguchi J, Fujimaki K, Sakai R, Motomura S, Ishigatsubo Y (2015) Intrathecal methotrexate prophylaxis and central nervous system relapse in patients with diffuse large B-cell lymphoma following rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 56(3):725–729. doi:10.3109/10428194.2014.931953
Vitolo U, Chiappella A, Ferreri AJ, Martelli M, Baldi I, Balzarotti M, Bottelli C, Conconi A, Gomez H, Lopez-Guillermo A, Martinelli G, Merli F, Novero D, Orsucci L, Pavone V, Ricardi U, Storti S, Gospodarowicz MK, Cavalli F, Sarris AH, Zucca E (2011) First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol 29(20):2766–2772. doi:10.1200/jco.2010.31.4187
Cheah CY, Herbert KE, O’Rourke K, Kennedy GA, George A, Fedele PL, Gilbertson M, Tan SY, Ritchie DS, Opat SS, Prince HM, Dickinson M, Burbury K, Wolf M, Januszewicz EH, Tam CS, Westerman DA, Carney DA, Harrison SJ, Seymour JF (2014) A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer 111(6):1072–1079. doi:10.1038/bjc.2014.405
Holte H, Leppa S, Bjorkholm M, Fluge O, Jyrkkio S, Delabie J, Sundstrom C, Karjalainen-Lindsberg ML, Erlanson M, Kolstad A, Fossa A, Ostenstad B, Lofvenberg E, Nordstrom M, Janes R, Pedersen LM, Anderson H, Jerkeman M, Eriksson M (2013) Dose-densified chemoimmunotherapy followed by systemic central nervous system prophylaxis for younger high-risk diffuse large B-cell/follicular grade 3 lymphoma patients: results of a phase II Nordic Lymphoma Group study. Ann Oncol 24(5):1385–1392. doi:10.1093/annonc/mds621
Zucca E, Conconi A, Mughal TI, Sarris AH, Seymour JF, Vitolo U, Klasa R, Ozsahin M, Mead GM, Gianni MA, Cortelazzo S, Ferreri AJ, Ambrosetti A, Martelli M, Thieblemont C, Moreno HG, Pinotti G, Martinelli G, Mozzana R, Grisanti S, Provencio M, Balzarotti M, Laveder F, Oltean G, Callea V, Roy P, Cavalli F, Gospodarowicz MK (2003) Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 21(1):20–27
Cheah CY, Seymour JF (2015) Central nervous system prophylaxis in non-Hodgkin lymphoma: who, what, and when? Curr Oncol Rep 17(6):25. doi:10.1007/s11912-015-0450-4
Guirguis HR, Cheung MC, Mahrous M, Piliotis E, Berinstein N, Imrie KR, Zhang L, Buckstein R (2012) Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: a single centre experience and review of the literature. Br J Haematol 159(1):39–49. doi:10.1111/j.1365-2141.2012.09247.x
Kanungo A, Medeiros LJ, Abruzzo LV, Lin P (2006) Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol 19(1):25–33. doi:10.1038/modpathol.3800500
Le Gouill S, Talmant P, Touzeau C, Moreau A, Garand R, Juge-Morineau N, Gaillard F, Gastinne T, Milpied N, Moreau P, Harousseau JL, Avet-Loiseau H (2007) The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica 92(10):1335–1342. doi:10.3324/haematol.11305
Niitsu N, Okamoto M, Miura I, Hirano M (2009) Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 23(4):777–783. doi:10.1038/leu.2008.344
Oki Y, Noorani M, Lin P, Davis RE, Neelapu SS, Ma L, Ahmed M, Rodriguez MA, Hagemeister FB, Fowler N, Wang M, Fanale MA, Nastoupil L, Samaniego F, Lee HJ, Dabaja BS, Pinnix CC, Medeiros LJ, Nieto Y, Khouri I, Kwak LW, Turturro F, Romaguera JE, Fayad LE, Westin JR (2014) Double hit lymphoma: the MD Anderson cancer center clinical experience. Br J Haematol 166(6):891–901. doi:10.1111/bjh.12982
Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, Horsman DE, Gascoyne RD (2009) MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 114(17):3533–3537. doi:10.1182/blood-2009-05-220095
Snuderl M, Kolman OK, Chen YB, Hsu JJ, Ackerman AM, Dal Cin P, Ferry JA, Harris NL, Hasserjian RP, Zukerberg LR, Abramson JS, Hochberg EP, Lee H, Lee AI, Toomey CE, Sohani AR (2010) B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol 34(3):327–340. doi:10.1097/PAS.0b013e3181cd3aeb
Tomita N, Tokunaka M, Nakamura N, Takeuchi K, Koike J, Motomura S, Miyamoto K, Kikuchi A, Hyo R, Yakushijin Y, Masaki Y, Fujii S, Hayashi T, Ishigatsubo Y, Miura I (2009) Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica 94(7):935–943. doi:10.3324/haematol.2008.005355
Savage KJ, Slack GW, Mottok A, Sehn LH, Villa D, Kansara R, Kridel R, Steidl C, Ennishi D, Tan KL, Ben-Neriah S, Johnson NA, Connors JM, Farinha P, Scott DW, Gascoyne RD (2016) The impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. doi:10.1182/blood-2015-10-676700
Miyazaki K, Yamaguchi M, Suzuki R, Kobayashi Y, Maeshima AM, Niitsu N, Ennishi D, Tamaru JI, Ishizawa K, Kashimura M, Kagami Y, Sunami K, Yamane H, Nishikori M, Kosugi H, Yujiri T, Hyo R, Katayama N, Kinoshita T, Nakamura S (2011) CD5-positive diffuse large B-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol 22(7):1601–1607. doi:10.1093/annonc/mdq627
Acknowledgments
We would like to thank all the patients who took part in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Kanemasa, Y., Shimoyama, T., Sasaki, Y. et al. Central nervous system relapse in patients with diffuse large B cell lymphoma: analysis of the risk factors and proposal of a new prognostic model. Ann Hematol 95, 1661–1669 (2016). https://doi.org/10.1007/s00277-016-2744-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2744-5