Abstract
Fluoroquinolone (FQ) and fluconazole prophylaxis is recommended for patients undergoing allogeneic hematopoietic cell transplantation (alloHCT). However, due to an uncertain scientific basis and the increasing emergence of resistant germs, this policy should be questioned. Therefore, FQ and fluconazole prophylaxis was omitted in alloHCT at our center. In this retrospective analysis, all consecutive patients (n = 63) who underwent first alloHCT at our institution from September 2010 to September 2013 were included. Patients neither received FQ nor fluconazole prophylaxis. Day 100 mortality, incidence of febrile neutropenia, bacterial infections, and invasive fungal diseases (IFD) were assessed. Sixteen patients who started conditioning under antimicrobial treatment/prophylaxis due to pre-existing neutropenia (3/16), IFD (12/16), or aortic valve replacement (1/16) were excluded from the analysis. Finally, 47 patients were transplanted without prophylaxis as intended. Day 100 mortality was 9 %. Febrile neutropenia occurred in 62 % (29/47); 17/47 patients (36 %) experienced a blood stream infection (BSI) with detection of Gram-positive bacteria in 14 patients, Gram-negative bacteria in five patients, and candida in one patient, respectively. Coagulase-negative staphylococci were the most frequently isolated Gram-positive bacteria; 12/21 isolated Gram-positive and 3/6 Gram-negative bacteria were FQ resistant. In 21 % (10/47) of the patients, IFD (1x proven, 1x probable, and 8x possible) were diagnosed. To conclude, all three criteria, day 100 mortality, the incidence of IFD, and BSI, are in the range of published data for patients transplanted with FQ and fluconazole prophylaxis. These data demonstrate that alloHCT is feasible without FQ and fluconazole prophylaxis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial infections and invasive fungal diseases (IFD) remain major causes of morbidity and mortality in patients undergoing allogeneic hematopoietic cell transplantation (alloHCT). After disruption of physiological barriers, Gram-negative bacteria may cause infections by relocating from the gut into the bloodstream, while Gram-positive bacteria penetrate through the damaged mucosa or skin lesions. Moreover, Gram-positive bacteremia is facilitated by central venous access. Blood stream infections (BSI) in general lead to increased mortality [1–3]. Therefore, antibiotic prophylaxis by fluoroquinolones (FQ) is widely used to reduce the incidence of infections and mortality. According to German [4] and EBMT guidelines [5], FQ prophylaxis is recommended for patients undergoing alloHCT with A1- and B1-rating, respectively. However, the majority of corresponding prospective and retrospective studies on FQ prophylaxis included a heterogeneous cohort of patients with solid malignancies, leukemia, and patients who underwent autologous HCT, respectively. AlloHCT patients were either not included or in the vast minority. The increasing emergence of FQ resistance emerged in a debate about FQ prophylaxis [1, 6]. Thus, the common standard of FQ prophylaxis in alloHCT has to be considered critically.
Invasive mold infections are the most frequent and relevant IFD in alloHCT. Fluconazole is not effective against these infections. Nevertheless, according to German guidelines [4] and the updated ECIL 3–2009 guidelines [7], fluconazole is still the standard for antifungal prophylaxis at least until day 75 post transplantation. These recommendations are based on studies on patients undergoing bone marrow transplantation with myeloablative conditioning published more than twenty years ago.
Here, we report on a single center experience of patients after alloHCT without FQ and fluconazole prophylaxis being transplanted between September 2010 and September 2013.
Material and methods
Patients
In this retrospective study, all consecutive patients who underwent first alloHCT at our institution between September 2010 and September 2013 were included.
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Definitions
Blood stream infections (BSI) were defined according to Busca et al. [8] as an isolate of a bacterial or fungal pathogen from at least one blood culture, with the exception of coagulase-negative staphylococci (CoNS) and normal skin contaminants, which required two separate positive blood cultures with the same susceptibility testing.
BSI were classified polymicrobial if ≥2 pathogens were isolated in a single blood culture or in separate blood cultures obtained up to 48 h apart, respectively [8].
The diagnosis of catheter-related infections was made in accordance with the 2009 updated guidelines of the Infectious Diseases Society of America for the diagnosis and management of intravascular catheter-related infections [9].
Invasive fungal diseases (IFD) were defined either as proven, probable, or possible IFD according to the revised EORTC/MSG consensus criteria [10].
Febrile neutropenia was defined as fever ≥38 °C in grade IV neutropenia (ANC < 0.5/nl). Antithymocyte globulin (ATG)-induced fever was neither counted nor treated as febrile neutropenia.
Routine surveillance and transplant procedures
Patients were accommodated in isolation in single rooms with high-efficiency particulate air filtration from the beginning of conditioning until neutrophil recovery (ANC > 0.5/nl). They received a low microbial diet. As blood vessel access, a central venous catheter (CVC) was placed.
Before commencing the conditioning, microbiological baseline investigations including cultures from stool and urine, serum aspergillus galactomannan testing, and swabs from the nose, throat, genital, and anal area were performed. Surveillance cultures from mouth wash and urine as well as serum galactomannan testing were performed weekly. Analysis of full blood counts and C-reactive protein were conducted on a daily routine. Prophylaxis with cotrimoxazole, colistin, metronidazole, and acyclovir was routinely administered orally. Mouth rinse with chlorhexidine and topical amphotericin B suspension several times a day was obligatory. Patients neither received additional antibiotic prophylaxis with FQ nor any systemic antifungal prophylaxis routinely. Patients presenting with pre-existing conditions in the medical history qualifying for an antimicrobial prophylaxis or therapy, i.e., (1) long-term neutropenia with primary antifungal prophylaxis, (2) antibiotic endocarditis prophylaxis after cardiac valve replacement, and (3) antifungal and antibiotic therapy for active fungal and bacterial infections, respectively, were excluded from the analysis.
Conditioning regimens included both, myeloablative and dose-reduced protocols, respectively. Antithymocyte globulin (ATG Fresenius® S, Neovii Biotech GmbH) was applied as part of the conditioning regimen for unrelated donor HCT or as a mandatory part of the FLAMSA-RIC regimen. Stem cell sources comprised peripheral blood stem cells (PBSC) or bone marrow (BM), respectively. Standard GvHD prophylaxis consisted of cyclosporine A in combination with either methotrexate (MTX) or mycophenolate mofetil (MMF) as per protocol.
Management of febrile neutropenia and infections
In case of febrile neutropenia ≥38 °C, empirical broad-spectrum intravenous antibiotic therapy with piperacillin/tazobactam or imipenem/cilastatin was started immediately. Simultaneously, microbiological diagnostic including cultures from blood, urine, and, if applicable, sputum was conducted. As second-line antimicrobial regimen, vancomycin, clindamycin, or linezolid was administered, respectively. Antibiotic therapy was adjusted according the microbiological findings. High-resolution computed tomography of the thorax was performed at latest after 72 h in case of persisting fever or the development of pulmonary symptoms. As pre-emptive antifungal therapy, either liposomale amphotericin B or voriconazole was initiated. The empirical therapeutic regimen included voriconazole, liposomale amphotericin B, or caspofungin, respectively.
Statistical analysis
Data were obtained starting from the first day of the conditioning until day 100 post transplantation. The primary study end point was day 100 mortality. Secondary objectives were the incidence of (1) febrile neutropenia, (2) BSI, (3) pulmonary infiltrates, (4) IFD, and (5) acute GvHD.
Results
Out of the 63 consecutive patients who underwent allogeneic transplantation from September 2010 until September 2013, 47 were eligible for this analysis. Despite the general internal policy to omit FQ and antifungal prophylaxis, 16 of 63 patients received antimicrobial treatment due to pre-existing medical conditions from the beginning of the conditioning phase. These patients have been excluded from the analysis. Figure 1 demonstrates the reasons for antimicrobial treatment and exclusion from the analysis.
Flow diagram on eligible and excluded patients. Forty-seven of 63 patients who received neither antifungal nor FQ treatment as intended were included in the analysis. Sixteen of 63 patients with antimicrobial treatment were excluded. One asterisk patient with aortic valve replacement, endocarditis prophylaxis with sultamicillin after insertion of CVC, two asterisk primary prophylaxis with voriconazole due to pre-existing long-term neutropenia, three asterisk antifungal therapy due to pre-existing IFD (n = 10): possible IFD (n = 8), probable IFD (n = 1), and proven IFD (n = 1), four asterisk antibiotic and antifungal therapy due to pre-existing concomitant bacterial pneumonia and IFD (n = 2): possible IFD (n = 1) and probable IFD (n = 1)
Patient characteristics
A total of 47 consecutively treated patients have been enrolled in this analysis. Table 1 shows the patient characteristics. At time of transplantation, patients’ median age was 54 years (range 19–74). The underlying diseases were AML (n = 23), myelodysplastic syndrome (MDS) (n = 8), ALL (n = 3), lymphoma (n = 8), and myeloproliferative neoplasms (MPN) (n = 5), respectively. Reduced intensity conditioning (RIC) regimen was used in 32 (68 %), myeloablative conditioning (MAC) in 15 (32 %) patients. Patients received either PBSC (n = 45) or bone marrow (n = 2); 13/47 patients were transplanted from a matched related donor (MRD), 27/47 from a matched unrelated donor (MUD), and 7/47 from a mismatched unrelated donor (MMUD), respectively.
Outcome
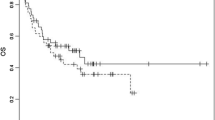
The outcome parameters are listed in Table 2. Day 100 mortality was 9 % (4/47). Causes of death were acute GvHD (n = 1), respiratory distress syndrome due to pneumonia (n = 2), and multi-organ failure due to sepsis (n = 1). Sixty-two percent (29/47) of the patients experienced febrile neutropenia. The latency until the occurrence of fever after the onset of neutropenia was in median seven days. The median duration of neutropenia (i.e., ANC < 0.5/nl) was 20.5 days (range 9–49).
The incidence of BSI was 36 % (17/47) with 14 of 17 Gram-positive, five of 17 Gram-negative, five of 17 polymicrobial BSI, and one of 17 with candidemia, respectively.
Altogether, 27 bacterial pathogens were isolated from blood cultures. A list of identified bacterial species is shown in Table 3. Coagulase-negative staphylococci (12/21) were the most frequent Gram-positive bacterial species. Escherichia coli dominated the Gram-negative pathogen spectrum (3/6). Twelve of 21 isolated Gram-positive and three of six Gram-negative pathogens, respectively, were FQ resistant.
Altogether, pulmonary infiltrates were detected 16 times. Ten pulmonary infiltrates were classified as possible (n = 9) and probable (n = 1) IFD. Six pulmonary infiltrates were classified as caused by other, possibly bacterial or viral, infections. Finally, IFD were diagnosed in ten of 47 patients with one proven, one probable, and eight possible IFD, respectively.
Acute GvHD grade III/IV according to the revised Glucksberg criteria occurred in 17 % (8/47) of patients. The median duration of hospitalization was 36 days with a median of 26 days after alloHCT.
Discussion
To our knowledge, this is the first report on patients undergoing alloHCT without fluconazole and FQ prophylaxis. The investigated regimen to perform alloHCT without either prophylaxis did not lead to an increase in non-relapse mortality. The day 100 non-relapse mortality (NRM) of 9 % is within the expected range for this patient population. Therefore, it should be questioned critically if either of the so-far standard prophylaxis is actually necessary.
Within both, the German and the European guideline, a FQ prophylaxis is recommended for patients undergoing alloHCT [4, 5]. However, recommendations refer to studies, in which only small numbers of alloHCT recipients have been included into large series of patients with cancer and hematologic malignancies undergoing conventional chemotherapy [11]. Moreover, studies on FQ prophylaxis in neutropenic patients did not demonstrate uniformly a significant reduction of mortality [11–14]. Prospective randomized studies comparing FQ prophylaxis versus placebo are missing completely in the setting of alloHCT [14]. There are only few small retrospective studies investigating the efficacy of FQ prophylaxis in patients undergoing alloHCT in the existing literature [15, 16]. Simondsen et al. [16] showed no significant difference in day 100 mortality (8.3 versus 10.4 %) in their analysis comparing alloHCT with or without FQ prophylaxis. Similarly, Kanda et al. [15] demonstrated that there is no significant impact on early mortality after discontinuation of FQ prophylaxis for patients with alloHCT.
BSI have been demonstrated to be associated with an increased mortality in adult alloHCT recipients [1–3]. The incidence of BSI varies between 13–60 % among patients after alloHCT [1, 17, 18]. Liu et al. [2] demonstrated that BSI occurred in 24.8 % of alloHCT recipients and found a predominance of Gram-negative bacteria (54 %) despite the use of FQ prophylaxis. In our analysis, BSI occurred in 36 % of the patients. In contrast to Liu and colleagues, we saw a predominance of approximately 80 % Gram-positive bacteria. However, only 43 % of these Gram-positive bacteria would have been susceptible to FQ. Recently, two further studies showed a predominance of Gram-positive BSI [1, 8]. Consistent with these data, coagulase-negative staphylococci were the most frequent isolated Gram-positive pathogens in our analysis (57 % of Gram-positive bacteria). Despite omitting FQ prophylaxis, the proportion of Gram-negative isolates among BSI was low in our cohort (approximately 30 %). The corresponding data in the literature with FQ prophylaxis are 37 and 40 %, respectively [1, 8]. The relatively low frequency of Gram-negative BSI might be due to the use of colistin for selective gut decontamination.
In our analysis, three out of six isolated Gram-negative bacteria were FQ resistant. This is in line with the recently reported increasing emergence of FQ-resistant bacteria in patients undergoing alloHCT [1, 6]. Bock et al. [19] demonstrated for their center a rate of FQ-resistant E. coli of >60 % in alloHCT patients in comparison with only 20 % in the general hospital population. Due to their advanced hematological disease, the majority of patients of our cohort had experienced one or more episodes of antibiotic therapy, particularly by FQs, before their allogeneic transplantation. These previous exposure to antibiotics might have facilitated the emergence of FQ-resistant bacteria. The rising frequency of FQ-resistant bacteria is a further rationale for omitting prophylaxis by FQ in patients undergoing alloHCT.
FQ prophylaxis is intended to diminish infection-related mortality by reducing the incidence of febrile neutropenia and bacterial infections. In this analysis, 62 % of patients developed febrile neutropenia. However, the high frequency of febrile neutropenia did not translate into an elevated NRM. Only six patients developed pulmonary infiltrates which were suspicious for bacterial infection. Thus, even without broad-spectrum antibiotic prophylaxis, bacterial pulmonary infection is a rare event, at least in this cohort.
Fungal diseases are frequent complications after alloHCT. To prevent fungal infections, an antifungal prophylaxis by fluconazole is widely used according to current guidelines [4, 7, 20]. However, invasive mold infections are the most relevant IFD in alloHCT. Of note, fluconazole is not effective against these infections. Nevertheless, the current German and ECIL 3–2009 guidelines still recommend fluconazole prophylaxis at least until day 75 after transplantation [4, 7]. These recommendations refer to two placebo-controlled studies from the early 1990s on patients being transplanted with bone marrow after myeloablative conditioning. Since these studies do not reflect the current practice of alloHCT, the decision to omit fluconazole prophylaxis was made locally at our center in 2010. Altogether, this approach did not result in an elevated NRM or excess of IFD. Only ten of 47 patients experienced the diagnosis of an IFD. And finally, only one of these ten patients was diagnosed for candidemia with a fluconazole-susceptible candida albicans. Solely, this patient, who survived the IFD, would have benefited possibly from a fluconazole prophylaxis. Despite the absence of fluconazole prophylaxis, the incidence of candidemia in the study cohort is within the range of published data for candidemia under fluconazole prophylaxis [1, 3, 21]. The remaining nine patients diagnosed for IFD were both, clinically and by radiologic imaging diagnostics, highly suspicious for invasive aspergillosis. These infections would not have been prevented by fluconazole prophylaxis at all. We therefore suggest re-evaluating the common policy of fluconazole prophylaxis in alloHCT.
The use of topical amphotericin B suspension as mouth rinse and topical prophylaxis against esophageal candidiasis might be a reason for the relatively low rate of IFD especially of candidemia. Topical amphotericin B suspension might have prevented IFD by reducing the burden of candida in the upper gastrointestinal tract. Furthermore, minimal absorption of topical amphotericin B had been demonstrated after oral rinsing [22]. Nonetheless, it is questionable whether the resulting very low plasma level of amphotericin B can act systemically as an antifungal agent. However, to our knowledge, topical amphotericin B suspension has not yet been assessed in the setting of alloHCT.
A further reason for the low rate of pulmonary mold infections is the consistent accommodation of all patients in rooms with high-efficiency particulate air filtration from the beginning of conditioning until neutrophil recovery. However, this type of accommodation should be the standard of care according to JACIE guidelines [23].
With the ECIL 3–2009 guideline [7, 24], fluconazole still is recommended with A1-rating for patients in the initial neutropenic phase. Additionally, voriconazole is recommended with a provisional A1-rating. The recommendation is based on the results of a randomized study comparing voriconazole versus fluconazole. The study showed a trend to fewer IFD for voriconazole prophylaxis. However, there was no statistically significant benefit for overall survival for voriconazole prophylaxis [25]. Thus, it is questionable whether fluconazole should be replaced by voriconazole for antifungal prophylaxis in alloHCT. The TRANSNET study has shown [26] that patients undergoing HCT are at different risk for IFD. The GITMO recommendations for primary antifungal prophylaxis [27] and the consensus guidelines for antifungal prophylaxis [20], both published in 2014, suggest a risk-adapted approach: high-risk patients should receive a mold-active antifungal prophylaxis. Basically, in our cohort, a similar approach had been already used. Only patients with a pre-existing long-lasting neutropenia at high risk for IFD received a primary and patients with previous IFD a secondary antifungal prophylaxis with a mold-active drug. According to our data, there is no need for a primary fluconazole prophylaxis for all other patients.
Limitations of the present analysis are (1) the retrospective design and (2) the limited number of patients. Additionally, our data might be biased by the use of metronidazole and colistin for intestinal bacterial decontamination. The aim of this strategy was to reduce the risk of acute GvHD as suggested by Beelen et al. [28]. However, neither colistin nor metronidazole is a broad-spectrum antibiotic prophylaxis with systemic action. Thus, the prophylactic use of both drugs does not contradict the intention of our approach performing alloHCT without antibiotic prophylaxis.
This analysis demonstrates as a proof of concept that performing alloHCT without any FQ and fluconazole prophylaxis is feasible. All relevant outcome parameters such as day 100 NRM, overall survival, and the frequency of IFD, BSI, and acute GvHD are within the range published for patients transplanted with antimicrobial prophylaxis. Considering the increasing emergence of FQ and fluconazole resistance and the missing scientific evidence for a benefit by the prophylactic use of both drugs, these prophylaxes should be re-evaluated as common standards in alloHCT. Alternatively, a risk-adapted approach for an aspergillus-effective antifungal prophylaxis should be assessed in the future.
References
Mikulska M, Del Bono V, Raiola AM, Bruno B, Gualandi F, Occhini D, di Grazia C, Frassoni F, Bacigalupo A, Viscoli C (2009) Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant 15(1):47–53
Liu CY, Lai YC, Huang LJ, Yang YW, Chen TL, Hsiao LT, Liu JH, Gau JP, Chen PM, Tzeng CH, Chiou TJ (2011) Impact of bloodstream infections on outcome and the influence of prophylactic oral antibiotic regimens in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant 46(9):1231–1239
Ortega M, Rovira M, Almela M, Marco F, de la Bellacasa JP, Martinez JA, Carreras E, Mensa J (2005) Bacterial and fungal bloodstream isolates from 796 hematopoietic stem cell transplant recipients between 1991 and 2000. Ann Hematol 84(1):40–46
Kruger WH, Bohlius J, Cornely OA, Einsele H, Hebart H, Massenkeil G, Schuttrumpf S, Silling G, Ullmann AJ, Waldschmidt DT, Wolf HH (2005) Antimicrobial prophylaxis in allogeneic bone marrow transplantation. Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Haematology and Oncology. Ann Oncol 16(8):1381–1390
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ (2009) Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Preface Bone Marrow Transpl 44(8):453–455
Bonadio M, Morelli G, Mori S, Riccioni R, Papineschi F, Petrini M (2005) Fluoroquinolone resistance in hematopoietic stem cell transplant recipients with infectious complications. Biomed Pharmacother 59(9):511–516
Maertens J, Marchetti O, Herbrecht R, Cornely OA, Fluckiger U, Frere P, Gachot B, Heinz WJ, Lass-Florl C, Ribaud P, Thiebaut A, Cordonnier C, Third European Conference on Infections in L, (2011) European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3—2009 update. Bone Marrow Transplant 46(5):709–718
Busca A, Cavecchia I, Locatelli F, D'Ardia S, De Rosa FG, Marmont F, Ciccone G, Baldi I, Serra R, Gaido E, Falda M (2012) Blood stream infections after allogeneic stem cell transplantation: a single-center experience with the use of levofloxacin prophylaxis. Transpl Infect Dis 14(1):40–48
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK (2009) Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49(1):1–45
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for R, Treatment of Cancer/Invasive Fungal Infections Cooperative G, National Institute of A, Infectious Diseases Mycoses Study Group Consensus G, (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46(12):1813–1821
Gafter-Gvili A, Fraser A, Paul M, Leibovici L (2005) Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 142(12 Pt 1):979–995
Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, Allione B, D'Antonio D, Buelli M, Nosari AM, Cilloni D, Zuffa E, Cantaffa R, Specchia G, Amadori S, Fabbiano F, Deliliers GL, Lauria F, Foa R, Del Favero A, Gruppo Italiano Malattie Ematologiche dell'Adulto Infection P (2005) Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med 353(10):977–987
Imran H, Tleyjeh IM, Arndt CA, Baddour LM, Erwin PJ, Tsigrelis C, Kabbara N, Montori VM (2008) Fluoroquinolone prophylaxis in patients with neutropenia: a meta-analysis of randomized placebo-controlled trials. Eur J Clin Microbiol Infect Dis 27(1):53–63
Kimura S, Akahoshi Y, Nakano H, Ugai T, Wada H, Yamasaki R, Ishihara Y, Kawamura K, Sakamoto K, Ashizawa M, Sato M, Terasako-Saito K, Nakasone H, Kikuchi M, Yamazaki R, Kako S, Kanda J, Tanihara A, Nishida J, Kanda Y (2014) Antibiotic prophylaxis in hematopoietic stem cell transplantation. A meta-analysis of randomized controlled trials. J Infect 69(1):13–25
Kanda J, Ichinohe T, Saito T, Yamashita K, Kondo T, Ishikawa T, Ichiyama S, Uchiyama T (2010) Impact of discontinuing fluoroquinolone prophylaxis on early mortality after allogeneic marrow or peripheral blood SCT with myeloablative conditioning. Bone Marrow Transplant 45(8):1369–1371
Simondsen KA, Reed MP, Mably MS, Zhang Y, Longo WL (2013) Retrospective analysis of fluoroquinolone prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplantation. J Oncol Pharm Pract 19(4):291–297. doi:10.1177/1078155212465215
Almyroudis NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small TN, Kiehn TE, Pamer E, Papanicolaou GA (2005) Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis 7(1):11–17
Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR (2007) Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant 40(1):63–70
Bock AM, Cao Q, Ferrieri P, Young JA, Weisdorf DJ (2013) Bacteremia in blood or marrow transplantation patients: clinical risk factors for infection and emerging antibiotic resistance. Biol Blood Marrow Transplant 19(1):102–108
Fleming S, Yannakou CK, Haeusler GM, Clark J, Grigg A, Heath CH, Bajel A, van Hal SJ, Chen SC, Milliken ST, Morrissey CO, Tam CS, Szer J, Weinkove R, Slavin MA (2014) Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern Med J 44(12b):1283–1297
Mikulska M, Del Bono V, Bruzzi P, Raiola AM, Gualandi F, Van Lint MT, Bacigalupo A, Viscoli C (2012) Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection 40(3):271–278
Epstein JB, Truelove EL, Hanson-Huggins K, Mancl LA, Chen A, Press OW, Petersdorf SH, Fritsche TR, Epstein JD (2004) Topical polyene antifungals in hematopoietic cell transplant patients: tolerability and efficacy. Support Care Cancer 12(7):517–525
International standards for cellular therapy product collection, processing and administration (2012). (Fifth edition)
Castagna L, Bramanti S, Sarina B, Todisco E, Ibatici A, Santoro A (2012) ECIL 3–2009 update guidelines for antifungal management. Bone Marrow Transplant 47(6):866
Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, Gersten ID, Mendizabal AM, Leather HL, Confer DL, Maziarz RT, Stadtmauer EA, Bolanos-Meade J, Brown J, Dipersio JF, Boeckh M, Marr KA, Blood, Marrow Transplant Clinical Trials N (2010) Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 116(24):5111–5118
Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG (2010) Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50(8):1091–1100
Girmenia C, Barosi G, Piciocchi A, Arcese W, Aversa F, Bacigalupo A, Bandini G, Bosi A, Busca A, Castagnola E, Caselli D, Cesaro S, Ciceri F, Locasciulli A, Locatelli F, Mikulska M, Pagano L, Prete A, Raiola AM, Rambaldi A (2014) Primary prophylaxis of invasive fungal diseases in allogeneic stem cell transplantation: revised recommendations from a consensus process by Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transpl 20(8):1080–1088
Beelen DW, Elmaagacli A, Muller KD, Hirche H, Schaefer UW (1999) Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood 93(10):3267–3275
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all patients.
Rights and permissions
About this article
Cite this article
Heidenreich, D., Kreil, S., Nolte, F. et al. Allogeneic hematopoietic cell transplantation without fluconazole and fluoroquinolone prophylaxis. Ann Hematol 95, 287–293 (2016). https://doi.org/10.1007/s00277-015-2535-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2535-4