Abstract
A recent meta-analysis, which included non-placebo open-labeled trials, showed that fluoroquinolone prophylaxis reduces mortality in neutropenic patients, whereas two recent large trials failed to show a similar benefit. Therefore, we performed a meta-analysis of randomized, blinded, placebo-controlled trials of fluoroquinolone prophylaxis in neutropenic patients. We searched several databases for relevant trials in any language. We used random effects models for pooling dichotomous data and assessed the between-study inconsistency with I 2. Two investigators independently assessed the eligibility and quality of the included trials. A total of 2,721 patients were randomized in eight eligible trials. Compared to the placebo, there was a statistically non-significant but consistent decrease in mortality with fluoroquinolone prophylaxis (4.5% vs. 3.9%, relative risk (RR) 0.76, 95% confidence interval (CI) 0.54, 1.08, p = 0.13, I 2 = 0%). Significant inconsistency, however, accompanied the pooled analysis of febrile episode (39% vs. 31%, RR 0.76, 95% CI 0.55, 1.03, p = 0.08, I 2 = 96.5%). To an extent, this inconsistency was explained in the subgroup analyses by the type of patient population studied and the type of fluoroquinolone used (p for interaction ≤0.01 for both). The RR of febrile episodes for two trials of outpatients with solid tumors, including lymphomas, was RR 0.34 (95% CI 0.14, 0.80) and 0.60 (95% CI 0.33, 1.10) for two trials using levofloxacin prophylaxis. The RR in one of the two trials that used levofloxacin significantly favored the intervention, 0.76 (95% CI 0.70, 0.83). Fluoroquinolone prophylaxis reduces the risk of febrile episodes in neutropenic outpatients with solid tumors, including lymphomas, and is associated with a statistically non-significant, yet clinically important, decrease in mortality in all neutropenic patients. Prophylaxis with levofloxacin may reduce febrile episodes in neutropenic hematology patients and stem cell transplant recipients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infectious complications remain as one of the most important challenges posed to physicians who manage neutropenic patients. An absolute neutrophil count (ANC) of <1,000 cells/mm3 is associated with an increased susceptibility to infection and profound (ANC≤100 cells/mm3) or protracted neutropenia carry an even greater risk [1–4]. The incidence of febrile episodes in neutropenic patients undergoing cancer chemotherapy and hematopoietic stem cell transplantation ranges from <10% to 85% [5–9]. Febrile episodes involving bacteremia, which have been documented in up to 34% of these patients [10], result in lengthened hospital stays and an increased case–fatality rate. Thus, efficacious antibiotic prophylaxis should have expected benefits.
Because several fluoroquinolones have broad antimicrobial coverage, bactericidal activity, high tissue concentrations, and adequate tolerability and safety profiles, they have been used extensively for prophylaxis in neutropenic patients in some countries [11, 12]. Concerns for the emergence of fluoroquinolone-resistant organisms among treated patients and in the community and the lack of impact on mortality in published trials have challenged this practice [13]. Although most randomized trials that investigated fluoroquinolone prophylaxis in neutropenic patients have not shown a significant survival benefit, a relatively small number of deaths among the trial populations resulted in imprecise estimates. When pooled, however, these trials, which included 1,244 patients, demonstrated a reduction in mortality with fluoroquinolone prophylaxis (relative risk (RR) 0.52, 95% confidence interval (CI) 0.35, 0.77) [14]. However, the meta-analysis included non-placebo open-labeled trials. Out of the many weaknesses of a non-placebo comparison, the introduction of co-interventions can potentially lead to a systematic bias, the so-called performance bias [15]. The possibility of the introduction of co-interventions during the conduct of such trials, even for hard endpoints like mortality, cannot always be ruled out. This could weaken the inferences from the pooled results of a meta-analysis. For example, in a previously published meta-analysis [14], 12 out of a total of 18 trials (that used fluoroquinolone prophylaxis) examined various fluoroquinolones in comparison with no-intervention, while six had a placebo as a comparison. The results of eight of the 12 trials favored fluoroquinolone use for a reduction in febrile episodes, while only one out of six placebo-controlled trials showed a significant reduction in febrile episodes. Two of eight trials (four did not report mortality data) examining various fluoroquinolones in comparison with no-intervention showed a reduction in the all-cause mortality, while none of the six placebo-controlled trials showed a significant benefit. Two subsequent large randomized trials have been published since and included 2,325 patients. With levofloxacin prophylaxis, a significant survival benefit in neutropenic patients was not found in either of the trials [5, 16]. Most recently, the previous meta-analysis was updated with data from the two large trials [17]. The pooled results favored a reduction in mortality with the use of fluoroquinolone prophylaxis in neutropenic patients. Nevertheless, it had the same limitations of the previous meta-analysis [14]. To further examine the ongoing controversy in the published data that characterizes the use of fluoroquinolone prophylaxis, we performed a meta-analysis of randomized, blinded, placebo-controlled trials of fluoroquinolone prophylaxis in neutropenic patients.
Methods
Eligibility criteria
Eligible trials met the following criteria: (1) be a randomized controlled trial; (2) compare the use of prophylactic fluoroquinolone as a single agent with a placebo; (3) include adults (defined as those at least 16 years of age) who experienced episodes of neutropenia due to cancer chemotherapy and/or hematopoietic stem cell transplantation; and (4) measure the incidence of all-cause mortality and febrile episodes. Randomized controlled trials published only as abstracts were excluded.
Identification of the relevant literature
We performed a comprehensive search of several databases (in October 2005), including MEDLINE, EMBASE, the Cochrane Library (including its database of trials, CENTRAL, and its two databases of systematic reviews, CSDR and DARE), Web of Science, International Pharmaceutical Abstracts Online, and BIOSIS. The search strategies used a combination of controlled vocabulary supplemented by keywords to define the concept areas: quinolones, neutropenia, infection prophylaxis. Subject hierarchies (explosions) were used where that feature was available (MEDLINE, EMBASE, International Pharmaceutical Abstracts). An experienced reference librarian (P.J.E.) performed the database search. Box 1 shows the search string. In addition, we searched the reference sections of eligible trials and reviews. The search was not limited to English-language publications; the authors translated two articles, one published in French and the other in Spanish (I.M.T. and C.T., respectively), into English prior to data collection.
Data collection
Using a structured form, we abstracted patient characteristics (demographic variables, type of cancer; solid tumor or hematological malignancy), inpatient or outpatient status and patients undergoing stem cell transplantation, intervention characteristics (fluoroquinolone used, dose, duration, and time of prophylaxis), and outcomes (all-cause mortality and febrile episodes). We contacted authors of the primary studies requesting missing information. One reviewer (H.I.) abstracted the data. Two reviewers (H.I. and I.T.) independently assessed the methodologic quality of the included studies using the validity criteria proposed by Jüni et al. [15]. Disagreements were resolved by consensus.
Statistical analysis
We used a random effects model, the DerSimonian and Laird method [18], as the primary model for pooling dichotomous data across trials (for more generalizable results) and estimated a pooled relative risk (RR) and its associated 95% confidence interval (CI). We estimated I 2, the proportion of the total variability in results between studies that is due to true heterogeneity rather than to sampling error (chance). A value of >50% represents substantial inconsistency [19]. All analyses were performed with RevMan Analyses Version 4.2.7 (© 2004 the Cochrane Collaboration).
Inconsistency was explored by performing pre-specified subgroup analyses testing for a treatment–subgroup interaction [20]. The studied subgroups included the study population; inpatient (which, inevitably, included hematology patients and patients with various malignancies receiving stem cell transplantation) vs. outpatient (solid tumors, including lymphomas) and the type of quinolone used; levofloxacin vs. other fluoroquinolones (levofloxacin has broader antimicrobial coverage compared to others [21]).
Results
Yield of search strategy and eligible trials
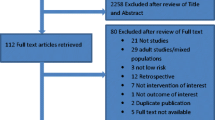
Seventeen randomized trials were evaluated for eligibility and eight were included in this analysis [5, 16, 22–27]. Figure 1 shows the reasons for exclusion. There was 100% agreement on the eligibility of the trials between the two reviewers.
Characteristics of the included trials
Table 1 describes the characteristics of the eight eligible trials enrolling 2,721 adult patients spanning the period 1987–2005. Three trials included only patients with acute leukemia [22, 24, 26]. Two trials included patients with solid tumors, including lymphomas [16, 25]. Three trials included patients with both solid and hematologic malignancies; solid-tumor patients were one-third or less of the trial population in two studies [23, 27] and 90% of the patients with solid tumors underwent autologous stem cell transplantation in the third trial [5]. All studies that included patients with hematologic malignancies or stem cell transplantation were conducted in the inpatient setting [5, 22–24, 26, 27]. In contrast, all studies of solid-tumor patients, including lymphoma, were conducted in the outpatient setting [16, 25]. The sample sizes ranged from 18 to 1,565 patients.
Fluoroquinolone prophylaxis was started at the time of initiation of chemotherapy in five studies [22, 24–27] or upon the resolution of acute emesis [24]. In two studies, it was started within 48–96 h of the initiation of chemotherapy or stem cell infusion [5, 23] and in one study it varied depending on the expected neutropenia due to a specific regimen (day 5, day 8, or day 15 post-chemotherapy) [16]. Prophylaxis duration varied among studies. The fluoroquinolones used in these studies included levofloxacin [5, 16], norfloxacin [22], ciprofloxacin [23, 24], ofloxacin [25], enoxacin [26], and pefloxacin [27]. The duration of neutropenia was described in four studies and ranged from 2 to 55 days [5, 22, 23, 25]. It was defined as an ANC<500/mm3 in all but one study, in which <1,000/mm3 was considered as neutropenia [5]. Compliance was reported to be good or excellent in five out of eight trials [5, 16, 22, 26, 27].
Quality assessment of the included trials
Table 2 displays the quality assessment of the included trials. Trials were judged according to the key validity components that address biases in selection, performance, detection, and sample attrition. All trials were double-blind in nature. Outcome was ascertained without the knowledge of the assigned treatment. Allocation concealment and the method of randomization was adequate in five trials [5, 16, 22, 24, 27], but one or the other was not reported for the other three trials [23, 25, 26]. We either did not get a response for two primary studies [23, 25] or the author (because the old data was not accessible) was not able to provide information to our queries [26]. The patients in seven of eight trials were analyzed according to the intention-to-treat principle [5, 16, 22, 24–27]. A significant proportion of patients was lost to follow up in one trial in which participants in both prophylaxis and control arms experienced equal numbers of events. Thus, although included, it did not contribute to the meta-analysis [23]. In four trials, the follow up was complete [16, 22, 24, 26]. Small numbers of patients were lost to follow up in the remaining three trials (3–7%) [5, 25, 27]. The kappa statistic for interobserver agreement on various quality domains ranged from 0.74 to 0.85. Conceptually, κ (a statistic used to measure non-random agreement between observers, investigators, or measurements) accounts for the observed agreement by chance and informs the clinician of the extent of possible agreement over and above chance. A value of κ from 0.81 to 0.99 means almost perfect agreement, and a value from 0.61 to 0.80 means substantial agreement.
Quantitative results of the meta-analysis
Analysis of all-cause mortality data
Figure 2 displays the mortality meta-analysis. Compared to the placebo, fluoroquinolone prophylaxis led to a statistically non-significant decrease in all-cause mortality (4.5% vs. 3.9%, RR 0.76, 95% CI 0.54, 1.08, p = 0.13). There was no inconsistency in the results between studies (I 2 = 0%). Table 3 summarizes the results of the subgroup analyses. All analyses suggested a statistically non-significant decrease in mortality with prophylaxis.
Analysis of febrile episodes data
Figure 3 displays the febrile episodes meta-analysis. Compared to the placebo, fluoroquinolone prophylaxis led to a non-significant reduction in the rate of febrile episodes (39% vs. 31%, RR 0.76, 95% CI 0.55, 1.03, p = 0.08). There was, however, large inconsistency in the results between studies (I 2 = 95.8%). We explored the sources of inconsistency by performing a priori hypothesized subgroup analyses (Table 4).
The pooled RR for the two trials in outpatients and the six trials conducted in the inpatient setting was 0.34 (95% CI 0.14, 0.80) and 0.90 (95% CI 0.70, 1.16), respectively (p for interaction <0.001).
The pooled RR for the two trials that used levofloxacin and the six trials that used fluoroquinolones other than levofloxacin was 0.60 (95% CI 0.33, 1.10) and 0.89 (95% CI 0.65, 1.22), respectively (p for interaction 0.01). The RR estimate of 0.76 (95% CI 0.70, 0.83) from the single inpatient trial that used levofloxacin was possibly different to the estimate from the other inpatient trials (p for interaction 0.08).
Discussion
Our meta-analysis suggests a statistically non-significant but a clinically important trend towards a decrease in mortality in neutropenic patients with fluoroquinolone prophylaxis, RR 0.76 (95% CI 0.54, 1.08, p = 0.13), whereas substantial inconsistency (I 2 = 95.8%) across studies precluded a meaningful interpretation of the pooled febrile episodes data.
The validity of the meta-analysis regarding febrile episodes is hindered by the lack of a consistent definition of fever and operant dependency of its measurement. The results of between-studies subgroup analyses appear to explain inconsistency to a certain extent on the basis of the type of patient receiving the treatment and the type of fluoroquinolone used.
Some investigators have proposed risk-based therapy in febrile neutropenia [28]. We also found that outpatients with solid tumors, including lymphomas, a low-risk population with 8–31% risk of febrile episodes in placebo-controlled subjects, are more responsive to prophylaxis. Conversely, hematology inpatients and stem cell transplant recipients, a high-risk population with 83–100% risk of febrile episodes in placebo-controlled subjects, are less responsive to prophylaxis. One of many plausible explanations might be that factors other than bacterial infections are a significant cause of fever in this population. All except one inpatient trial included in this review permitted the use of antiviral and/or antifungal prophylaxis. We also found significantly different estimates favoring levofloxacin use when trials that used fluoroquinolones other than levofloxacin were compared to trials that used levofloxacin (p for interaction 0.01). This subgroup analysis was associated with residual inconsistency, which, likely, was due to a difference in the risk profile amongst participants of these trials. Similarly, yet not statistically significant, the estimate of RR favored the trial [5] that used levofloxacin in patients with hematologic malignancies and stem cell transplant recipients was compared to trials of other fluoroquinolones conducted in the same patient population (p for interaction 0.08). Although these are between-study subgroup analyses, the results are supported by the fact that levofloxacin has a broader antimicrobial coverage than the earlier used fluoroquinolones [21]. There was only one trial that used levofloxacin in patients with hematologic malignancies and one in solid tumors, including lymphoma, and both of these recent large trials demonstrated a significant decrease in the risk of febrile episodes with levofloxacin [5, 16].
The most recent Infectious Disease Society of America (IDSA) guidelines do not recommend the routine use of antimicrobial prophylaxis in neutropenic patients [13]. Their conclusions stem from two basic concerns: (1) emerging drug-resistant pathogens due to extensive antibiotic use and (2) prophylaxis has not been shown to consistently reduce mortality rates, despite adequate evidence that supports its efficacy in reducing febrile episodes.
The routine use of fluoroquinolone prophylaxis has been challenged because of the potential for the selection and dissemination of fluoroquinolone-resistant organisms. The rapid emergence of these organisms is a legitimate concern and has been reported in multiple centers that use fluoroquinolone prophylaxis [11, 29, 30]. A recent meta-analysis, however, failed to show a significant increase in colonization or the number of infections caused by quinolone-resistant bacteria in patients treated with quinolones [31]. Widespread dissemination of resistant organisms in hematology units has also not been a major problem. Several studies suggest that resistance to fluoroquinolones in Escherichia coli is of multiclonal origin and that the horizontal spread of a single clone of fluoroquinolone-resistant E. coli appears to be an uncommon event [32, 33]. Another concern is the possible lack of prophylaxis efficacy over time due to the widespread use of fluoroquinolones and subsequent patient colonization with resistant organisms. Epidemiologic studies have not convincingly supported the assumption of a theoretical lack of efficacy of fluoroquinolones with continued use due to expected bacterial resistance [34]. In addition, fluoroquinolones do have a better safety and tolerability profile compared to other antibiotics. The two largest studies [5, 16] to date reported a minimal increase in adverse events with fluoroquinolone use that were limited to gastrointestinal effects and a rash.
In comparison with a previous meta-analysis and its updated results [14], our meta-analysis has several different features. First, we limited our meta-analysis to placebo-controlled blinded trials. Second, the previous meta-analysis included a study that used a fluoroquinolone in combination with a macrolide antibiotic [35], but we limited our analyses to trials that used only fluoroquinolones as prophylaxis. The addition of antimicrobials with gram-positive coverage to fluoroquinolone prophylaxis has been shown to decrease the rate of febrile episodes as compared to fluoroquinolone monoprophylaxis in another published meta-analysis [36]. Third, the presence of significant inconsistency, as measured by the I 2 statistic, precludes a reliable interpretation of the single pooled estimate of febrile episodes meta-analysis. We, therefore, attempted to explore sources of inconsistency by performing pre-specified subgroup analyses.
It could be argued that the results of our meta-analysis did not add significantly to the evidence provided by the two recent large trials. This is not known a priori and does not support dismissing the pooled evidence when it concurs with single trials. First, a statistically non-significant, yet consistent, improvement in all-cause mortality from the pooled analysis adds important conclusions to the best available evidence in the literature, i.e., from placebo-controlled blinded trials. Second, the pooled estimate from a meta-analysis is more applicable to the general population than a single trial. Third, one could not ignore the contribution of the old small trials to the pooled results; the weight of small trials in the pooled analysis was approximately 25% for mortality and 63% for febrile episodes.
Finally, publication bias can affect the findings of a meta-analysis. In our meta-analysis, the small number of studies limits our ability to assess for publication bias (for instance, using a funnel plot, which, at times, can be misleading, as reported in the past [37, 38]) or to draw conclusions about publication bias. Nevertheless, our process of literature identification was comprehensive and was performed with the collaboration of an experienced librarian, so that our review should have identified the majority of published studies that examined the role of fluoroquinolone prophylaxis in neutropenic patients.
Conclusion
Cumulative data from placebo-controlled trials demonstrates a statistically non-significant, yet clinically important, decrease in mortality in neutropenic patients receiving fluoroquinolone prophylaxis and merits further investigation. The data indicate that prophylaxis with fluoroquinolone reduces the risk of febrile episodes in neutropenic outpatients with solid tumors, including lymphomas. In addition, levofloxacin may have a different effect than other fluoroquinolones, and its use may reduce febrile episodes in hematology patients and stem cell transplant recipients, at the risk of neutropenic infections. In selected adult populations, fluoroquinolone prophylaxis could be used with surveillance for the emergence of resistant pathogens.
References
Bodey GP, Buckley M, Sathe YS, Freireich EJ (1996) Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64:328–340
Dale DC, Guerry DT, Wewerka JR, Bull JM, Chusid MJ (1979) Chronic neutropenia. Medicine (Baltimore) 58:128–144
Lucas KG, Brown AE, Armstrong D, Chapman D, Heller G (1996) The identification of febrile, neutropenic children with neoplastic disease at low risk for bacteremia and complications of sepsis. Cancer 77:791–798
Schimpff SC (1986) Empiric antibiotic therapy for granulocytopenic cancer patients. Am J Med 80:13–20
Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, Allione B, D’Antonio D, Buelli M, Nosari AM, Cilloni D, Zuffa E, Cantaffa R, Specchia G, Amadori S, Fabbiano F, Deliliers GL, Lauria F, Foà R, Del Favero A (2005) Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med 353:977–987
Kamana M, Escalante C, Mullen CA, Frisbee-Hume S, Rolston KVI (2005) Bacterial infections in low-risk, febrile neutropenic patients: over a decade of experience at a comprehensive cancer center. Cancer 104:422–426
Meijer E, Dekker AW, Lokhorst HM, Petersen EJ, Nieuwenhuis HK, Verdonck LF (2004) Low incidence of infectious complications after nonmyeloablative compared with myeloablative allogeneic stem cell transplantation. Transpl Infect Dis 6:171–178
Fernández-Mosteirin N, Salvador-Osuna C, Gimeno Lozano JJ, Marco Lamata ML, Blasco Alberdi M, Rubio Felix D, Giralt Raichs M (2005) Incidence of febrile episodes in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation (in Spanish). An Med Interna 22:213–216
Lyman GH, Delgado DJ (2003) Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 98:2402–2409
Sigurdardottir K, Digranes A, Harthug S, Nesthus I, Tangen J-M, Dybdahl B, Meyer P, Hopen G, Løkeland T, Grøttum K, Vie W, Langeland N (2005) A multi-centre prospective study of febrile neutropenia in Norway: microbiological findings and antimicrobial susceptibility. Scand J Infect Dis 37:455–464
Kern WV, Klose K, Jellen-Ritter AS, Oethinger M, Bohnert J, Kern P, Reuter S, von Baum H, Marre R (2005) Fluoroquinolone resistance of Escherichia coli at a cancer center: epidemiologic evolution and effects of discontinuing prophylactic fluoroquinolone use in neutropenic patients with leukemia. Eur J Clin Microbiol Infect Dis 24:111–118
Carratalá J, Fernández-Sevilla A, Tubau F, Callis M, Gudiol F (1995) Emergence of quinolone-resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin. Clin Infect Dis 20:557–560; discussion 561–563
Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KVI, Shenep JL, Young LS (2002) 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34:730–751
Gafter-Gvili A, Fraser A, Paul M, Leibovici L (2005) Meta-analysis: Antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 142:979–995
Jüni P, Altman DG, Egger M (2001) Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 323:42–46
Cullen M, Steven N, Billingham L, Gaunt C, Hastings M, Simmonds P, Stuart N, Rea D, Bower M, Fernando I, Huddart R, Gollins S, Stanley A (2005) Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med 353:988–998
Leibovici L, Paul M, Cullen M, Bucaneve G, Gafter-Gvili A, Fraser A, Kern WV (2006) Antibiotic prophylaxis in neutropenic patients: new evidence, practical decisions. Cancer 107:1743–1751
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326:219
Fu KP, Lafredo SC, Foleno B, Isaacson DM, Barrett JF, Tobia AJ, Rosenthale ME (1992) In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob Agents Chemother 36:860–866
Karp JE, Merz WG, Hendricksen C, Laughon B, Redden T, Bamberger BJ, Bartlett JG, Saral R, Burke PJ (1987) Oral norfloxacin for prevention of gram-negative bacterial infections in patients with acute leukemia and granulocytopenia. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 106:1–7
Lew MA, Kehoe K, Ritz J, Antman KH, Nadler L, Takvorian T, Mayer R, Kalish L, Finberg R (1991) Prophylaxis of bacterial infections with ciprofloxacin in patients undergoing bone marrow transplantation. Transplantation 51:630–636
Rafecas FJ et al (1989) Oral ciprofloxacin in the prophylaxis of bacterial infection in neutropenic patients. A randomized, double blind, comparative clinical study. Revista Espanola de Quimioterapia 2:174–177
Schroeder M, Schadeck-Gressel C, Selbach J, Westerhausen M (1992) Antibiotic prophylaxis with gyrase inhibitors during cytostatically induced granulocytopenias in patients with solid tumors: a double-blind prospective randomized study. Onkologie 15:476–479
Talbot GH, Cassileth PA, Paradiso L, Correa-Coronas R, Bond L (1993) Oral enoxacin for infection prevention in adults with acute nonlymphocytic leukemia. The Enoxacin Prophylaxis Study Group. Antimicrob Agents Chemother 37:474–482
Thomas X, Troncy J, Belhabri A, Thiebaut A, Bouheddou N, Michallet M, Fleurette J, Pivot C, Fiere D, Archimbaud E (2000) Effectiveness of combined vancomycin and pefloxacine in gastrointestinal decontamination for preventing infections after chemotherapy-induced bone marrow aplasia. A randomized double-blind study (in French). Presse Med 29:1745–1751
Rolston KVI (1998) Risk assessment and risk-based therapy in febrile neutropenic patients. Eur J Clin Microbiol Infect Dis 17:461–463
Somolinos N, Arranz R, Del Rey MC, Jimenez ML (1992) Superinfections by Escherichia coli resistant to fluoroquinolones in immunocompromised patients. J Antimicrob Chemother 30:730–731
Kern WV, Andriof E, Oethinger M, Kern P, Hacker J, Marre R (1994) Emergence of fluoroquinolone-resistant Escherichia coli at a cancer center. Antimicrob Agents Chemother 38:681–687
Gafter-Gvili A, Paul M, Fraser A, Leibovici L (2007) Effect of quinolone prophylaxis in afebrile neutropenic patients on microbial resistance: systematic review and meta-analysis. J Antimicrob Chemother 59:5–22
Tascini C, Menichetti F, Bozza S, Fedele M, Preziosi R, Allegrucci M, Del Favero A, Micozzi A, Martino P, Bistoni F (1999) Molecular typing of fluoroquinolone-resistant and fluoroquinolone-susceptible Escherichia coli isolated from blood of neutropenic cancer patients in a single center. Clin Microbiol Infect 5:457–461
Martino R, Subira M, Altes A, López R, Sureda A, Domingo-Albós A, Pericas R, Brunet S (1998) Effect of discontinuing prophylaxis with norfloxacin in patients with hematologic malignancies and severe neutropenia. A matched case-control study of the effect on infectious morbidity. Acta Haematol 99:206–211
Reuter S, Kern WV, Sigge A, Döhner H, Marre R, Kern P, von Baum H (2005) Impact of fluoroquinolone prophylaxis on reduced infection-related mortality among patients with neutropenia and hematologic malignancies. Clin Infect Dis 40:1087–1093
Tjan-Heijnen VCG, Postmus PE, Ardizzoni A, Manegold CH, Burghouts J, van Meerbeeck J, Gans S, Mollers M, Buchholz E, Biesma B, Legrand C, Debruyne C, Giaccone G (2001) Reduction of chemotherapy-induced febrile leucopenia by prophylactic use of ciprofloxacin and roxithromycin in small-cell lung cancer patients: an EORTC double-blind placebo-controlled phase III study. Ann Oncol 12:1359–1368
Cruciani M, Malena M, Bosco O, Nardi S, Serpelloni G, Mengoli C (2003) Reappraisal with meta-analysis of the addition of gram-positive prophylaxis to fluoroquinolone in neutropenic patients. J Clin Oncol 21:4127–4137
Tang JL, Liu JL (2000) Misleading funnel plot for detection of bias in meta-analysis. J Clin Epidemiol 53:477–484
Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I (2006) The case of the misleading funnel plot. BMJ 333:597–600
Acknowledgment
We would like to extend our special thanks to Dr. Michael Cullen, Cancer Centre, University Hospital Birmingham, Birmingham, UK, for providing us with the mortality data [16] and reviewing the paper.
Conflict of interest statement
None for all authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. Imran and I. M. Tleyjeh have contributed equally to the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Imran, H., Tleyjeh, I.M., Arndt, C.A.S. et al. Fluoroquinolone prophylaxis in patients with neutropenia: a meta-analysis of randomized placebo-controlled trials. Eur J Clin Microbiol Infect Dis 27, 53–63 (2008). https://doi.org/10.1007/s10096-007-0397-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0397-y