Abstract
Programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) are new targets in cancer immunotherapy. PD-1 protein is an immune checkpoint expressed in many tumors. Epstein–Barr virus (EBV) is present in malignant Hodgkin/Reed–Sternberg (HRS) cells in approximately 40–50 % of Hodgkin lymphoma (HL). The aim of this study is to evaluate the clinical and prognostic importance of PD-1 and/or PD-L1 in HL and also to determine the association between EBV-encoded RNA (EBER) and PD-1/PD-L1. Formalin-fixed, paraffin-embedded tissue samples from 87 cases with HL were analyzed in this study. Immunohistochemical staining was performed to detect the PD-1 and PD-L1 expressions. Chromogenic in situ hybridization for EBER was performed using fluorescein-labeled oligonucleotide probes. PD-1 and PD-L1 expressions were found in 20 % of the cases. The EBER positivity was found in 40 cases (45 %). It has been found that co-expression of PD-1 and PD-L1 was associated with shorter survival although PD-1 or PD-L1 expressions were not found to be related with survival. Overall survival (OS) and disease-free survival (DFS) in cases without PD-1 and PD-L1 expressions were 135 and 107 months, respectively. OS and DFS in cases with co-expression for PD-1 and PD-L1 were 24 and 20 months, respectively, and these differences were found to be statistically significant for both OS and DFS (p = 0.002 and p = 0.003, respectively). Cox regression analysis showed that co-expression of PD-1 and PD-L1 was found to be an independent risk factor for prognosis (OR 6.9, 95 % CI 1.9–24.3). Targeting PD-1 and/or PD-L1 is meaningful due to the 20 % expression of each in HL, and we did not find an important association between PD-1 and PD-L1 and EBER expression in HL. Very poor outcome in cases with co-expression of PD-1/PD-L1 suggests new avenues to detect the new prognostic markers and also therapeutic approaches in HL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Programmed death-1 (PD-1) protein is one of the most important immune checkpoints and its receptor is expressed by activated T cells. PD-1 mediates immunosuppression and is expressed on T cells after activation [1]. PD-1 is present on exhausted T cells, B cells, NK cells, dendritic cells, and activated monocytes [2]. PD-1 has two ligands known as programmed death-ligand 1 (PD-L1) (B7-H1) and PD-L2 (B7-DC) [3–5]. The physiological role of PD-1 is to guarantee T cell homeostasis and to provide balance between T cell activation and proliferation. Binding of the ligand PD-L1 to PD-1 expressed on the surface of activated T cells delivers an inhibitory signal and reduces cytokine production and proliferation. So, PD-L1/PD-1 interactions confer tumor escape from immune control [6]. This signaling is important for both tumor biology and treatment including the interactions between tumor, tumor microenvironment, and immune system and to target associated pathways in malignant tumors. PD-1/PD-L1 axis has been implicated in prognosis in several solid tumors, and results of many studies suggest that PD-1 pathway is a rationale target for therapeutic inhibition [7]. Tumor PD-L1 expression is a hot topic as a possible predictive biomarker for patient benefit from PD-1 pathway. It has been shown many times that blockade of PD-1 or PD-L1 by monoclonal antibodies may lead to significant antitumor effects in various types of malignant tumors [8, 9]. On the other hand, it has been shown an association between Epstein–Barr virus (EBV)-associated neoplasias and PD-1 and/or PD-L1 expression in some lymphomas [10, 11]. Here, we presented the study about the expression patterns of PD-1 and PD-L1 in Hodgkin lymphoma (HL) and their association with EBV status and also prognostic value of PD-1/PD-L1 pathway in this disease.
Patients and methods
Formalin-fixed, paraffin-embedded tissue samples from 87 cases with newly diagnosed HL were analyzed in this study. NCCN risk scoring system was used to determine the risk group of the patients. All the cases had been treated with ABVD regimen (doxorubicin, bleomycin, vinblastine, DTIC) as a first-line treatment. Salvage regimen was DHAP (dexamethasone, Ara-C, cisplatinum) regimen in the majority of cases in the first relapse. Immunohistochemical staining was performed on 5-μm sections from tissue using antibody to PD-1 (MRQ-22, Ventana) and PD-L1 (Monoclonal antibody CD274/PDL1 AM26531AF-N.Acris Germany). The visualization system used was the BenchMark XT (Ventana) with heat-induced epitope retrieval (CC1 solution, Ventana) and the iView DAB detection kit (Ventana). The visualization system used was the BenchMark XT with enzymatic digestion (ISH Protease 2, Ventana) and the iViewBlue detection kit (Ventana). Cases stained with anti-PD-1 were scored according to intensity of cytoplasmic and/or membranous positivity as follows: 0 (no staining), 1+ (weak or equivocal staining), 2+ (moderate staining), or 3+ (strong staining). Tumor cells, Hodgkin/Reed–Sternberg (HRS) cells, and peritumoral microenvironment were evaluated separately. Tumor positivity was considered when more than 5 % tumor population was stained. Microenvironment was considered positive when more than 20 % of all cell population was stained. HRS cells were evaluated as positive or negative, regardless of intensity and rate. PD-L1 was evaluated cytoplasmic and/or membranous positive and negative for HRS cells and microenvironment by immunohistochemistry. PD-L1 positivity in malignant cells and microenvironment was made according to the scoring system used in a previous article [9, 10]. Chromogenic in situ hybridization for EBV-encoded RNA (EBER) was performed using fluorescein-labeled oligonucleotide probes (INFORM EBER Probe, Ventana). EBER was considered as positive in case of dark-blue nuclear staining.
Statistical analyses
The variables between the groups were analyzed by using the chi-square test and McNemar’s test. Cohen’s kappa was used for agreement statistics. Strength of agreement was evaluated by kappa statistic (<0.00 is poor. 0.00–0.20 is slight, 0.21–0.40 is fair, 0.41–0.60 is moderate, 0.61–0.80 is substantial, 0.81–1.00 is almost perfect). The predictors of survival were analyzed by the Kaplan–Meier method and compared by the Mantel log-rank test. Cox proportional hazard regression model was applied to identify multivariate predictors (forward procedure, Wald method). The results are reported as mean ± SD, median, number (n), and percent (%). A p value <0.05 was considered as significant. Statistical analyses were performed using the statistical package SPSS v 22.0.
Findings
Male to female ratio was 46:41. Mean age was 35.3 ± 14.5 (range between 15 and 71). Sixty percent of the cases had early-stage disease while 40 % had advanced stage disease. According to the risk scores, 15 cases had early stage-favorable disease, 34 cases had early stage-unfavorable disease, and 38 cases had advanced stage disease. Table 1 shows the demographic features of the patients.
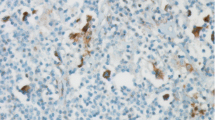
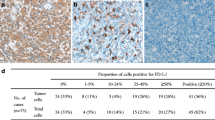
PD-1 expression was detected in 18 cases (20 %), and PD-L1 expression was detected in 18 cases (20 %). EBER-ISH was detected in 40 cases (45 %). Figures 1, 2, and 3 show the EBV, PD-1, and PD-L1 expressions, respectively. Table 2 shows the associations between EBV, PD-1, and PD-L1. We did not find an association between EBV and PD-1 (kappa coefficient = 0.08) and between EBV and PD-L1 (kappa coefficient = −0.01). There was a fair association between PD-1 and PD-L1 (kappa coefficient = 0.23).
Immunohistochemically, three cases had 2+ staining and 15 cases had 1+ staining with PD-1. We did not detect 3+ staining with PD-1. PD-L1 was found to be positive in 18 cases, and staining was detected in HRS cells and microenvironment by immunohistochemistry.
Table 3 shows the results of survival analyses according to clinical and prognostic factors. When we evaluated the clinical parameters, we found that both overall survival (OS) and disease-free survival (DFS) were longer in patients with early stage than in patients with advanced stage disease (p values were 0.024 and 0.001, respectively). Additionally, OS and DFS were found to be shorter in cases with extranodal involvement as compared without extranodal disease (p values were 0.004 and 0.001, respectively) and in cases with high-risk disease compared with low-risk disease (p values were 0.024 and 0.001, respectively).
Although median OS and DFS were shorter in cases with EBV(+) compared with EBV(−) (100 vs. 142 months for OS and 107 vs. 100 months for DFS), differences were not statistically significant for both (p = 0.064 and p = 0.129, respectively). Figure 4 shows OS in cases with or without EBV.
The median OS and DFS were found to be shorter in cases with PD-1 expression than in those without PD-1 expression (100 vs. 135 months for OS and 107 vs. 100 months for DFS); differences were not significant (p = 0.928 and p = 0.607, respectively). Similarly, the median OS and DFS were found to be shorter in cases with PD-L1 expression (79 months for both OS and DFS) than in those without PD-L1 expression (135 months for OS and 100 months for DFS); again, differences were not significant (p = 0.232 for OS and p = 0.305 for DFS). Although PD-1 or PD-L1 expressions were not associated with survival times, we found that co-expression of PD-1 and PD-L1 was associated with shorter survival. Median OS and DFS times in cases not expressing PD-1 and PD-L1 were 135 and 107 months, respectively. However, these times were 24 and 20 months in cases with co-expression, and these differences were found to be statistically significant for both OS and DFS (p = 0.002 and p = 0.003, respectively). Figure 5 shows OS curves according to co-expression of PD-1 and PD-L1. Cox regression analysis showed (Table 4) that co-expression of PD-1 and PD-L1 was found to be independent risk factors that related with prognosis. The odds ratio was found to be 6.9 (95 % CI 1.9–24.3, p = 0.002) for the cases having co-expression of PD-1 and PD-L1 (both PD-1 and PD-L1(+)).
Discussion
Programmed death-1 (PD-1) and its ligands, PD-L1 and PD-L2, deliver inhibitory signals, and this signaling regulates the balance between T cell activation, tolerance, and immuno-pathology. Specific and balanced responses to eradicate pathogens and tumors are very important in immune responses to foreign and self-antigens. For this reason, PD-1/PD-L pathway is very important in the pathogenesis of chronic infections and tumors [2]. PD-1 protein expressed by activated T cells while PD-L1 (B7-H1) and PD-L2 (B7-DC) both expressed by tumor cells and microenvironment [6]. It is very well known that macrophages in microenvironment of HL are very important prognostic parameters [12]. PD-L1 is not expressed by normal epithelial tissues, normal lymph nodes, and plasma cells, but it is aberrantly expressed on various human cancers, lymphoma cells, and myeloma cells [13–15]. PD-L1 may promote Ca progression by disabling the host antitumor response, and its expression on tumor cells is associated with poor prognosis in various malignant tumors including renal cell cancer, breast cancer, pancreas cancer, ovarian cancer, gastric cancer, esophageal cancer, head and neck cancer, and non-small cell lung cancer [16–22].
PD-L1 has an important role in lymphomagenesis. PD-L1 has been detected in various types of lymphomas and lymphoma cell lines. However, expression levels and frequencies are highly variable between less than 5 % and more than 90 % in different types and subtypes of the lymphomas in different studies. Antibodies used in different studies were clone MIH1; eBioscience, BioLegend, Aperio Scan Scope XT Workstation and EH12 for PD-L1 ad PD-1 [11, 14, 23–27]. PD-L1 expression has been analyzed by using flow cytometry, immunohistochemistry, and fluorescein in situ hybridization in cell lines and lymphoma specimens. We must consider that differences in the specificity and sensitivity of the commercial antibodies used for immunohistochemical staining of PD-1 and/or PD-L1 may explain the differences between studies and indirectly the prognostic value of the PD-1/PD-L1 system [6]. In our study, we used 5 and 20 % cutoff values for tumor and microenvironment.
PD-L1 expression has been studied in HL in a relatively limited number of the studies. HRS cells variably express PD-L1/B7-H1, whereas tumor-infiltrating T cells express the co-inhibitory receptor: PD-1 [27, 28]. In a study covering 280 cases with classical HL and 3 cases with nodular lymphocyte-predominant HL on a tissue microarray platform, PD-1(+) cells have been observed in all cases of nodular lymphocyte-predominant HL but only in 1 % of classical HLs. With these findings, the authors proposed that the presence of PD-1(+) rosettes around neoplastic cells is typical but not exclusive for nodular lymphocyte-predominant HL [29]. However, in another study, PD-L1 (9p24.to) amplification has been found to be restricted to the HL subtype classical HL and increased PD-L1 expression has been found on tumor cells [27]. In another study, PD-L1 expression has been studied by using immunohistochemistry (IHC), and PD-L1 has been found in the majority of classical and mixed cellular HL subtypes in malignant cells and in tumor-infiltrating macrophages. However, PD-L1 has not been found in nodular lymphocyte-predominant HL in this study [10]. In our study, we found PD-1 expression in 18 cases (20 %) and PD-L1 in 18 cases (20 %). Except one case, all the cases showed more than 20 % staining for PD-1 or PD-L1. As mentioned before, various methods with variable cutoffs have been used in different studies. For this reason, we could not strictly compare our results with other studies. However, our results suggest that both PD-1 and PD-L1 are meaningful targets for HL.
Prognostic significance of PD-1 or PD-L1 expression in solid tumors has been evaluated in many types of solid tumors and limited number of cases with lymphoma. It has been shown that expression of PD-L1 in cancers including colon, gastric, hepatocellular, melanoma, non-small cell lung, ovarian, and renal cell cancers predicts poor outcome [7, 30]. When we looked at the available lymphoma data, we found that increased level of PD-L1 was associated with high proliferative index or poor prognosis. In these studies, it has been proposed that PD-L1 can promote cyclinD1 and CDK4 expression, and this leads to cell arrest in G1/S phase and maintains high growth rates in lymphoma cells. Additionally, it has been suggested that PD-L1 contributes to resistance to the drugs, and it has been shown that blockade of PD-L1 or PD-1 can reverse drug resistance and improves therapeutic efficacy. In diffuse large B cell lymphoma, increased level of PD-L1 has been found to be associated with high proliferative index and poor response to treatment and an association between extranodal involvement, poor PS, and inferior survival [14, 31]. In a HL study, increased amount of PD-1(+) tumor-infiltrating lymphocytes has been found to be a stage-independent negative prognostic factor for OS [27]. Another study about classical HL suggests a critical role for the PD-1/PD-L1 signaling axis with high numbers of tumor-infiltrating macrophages and an inferior clinical outcome through the suppression of antitumor immunity [10]. In our study, although DFS and OS were found to be shorter in cases showing PD-1 or PD-L1(+), we did not find an important difference for these cases. We found that the cases with PD-1 and PD-L1 co-expression have significantly shorter DFS and OS times as compared with cases not expressing PD axis. However, PD-1 and PD-L1 co-expression was found to be a significant independent risk factor (OR 6.9), and the confidence interval of the parameter was found to be wide (95 % CI 1.9–24.3) due to insufficient sample size (only seven patients and four were censored). Although the number of these cases is low, this point has been found remarkable. Considering this limitation for obtaining more reliable results, further studies are needed in large number of cases.
This finding suggests the considerable immune escape in cases with PD-1 and PD-L1 expression. Also, Cox regression analysis showed prognostic significance of PD-1/PD-L1 system in addition to the age. On the other hand, PD-1 and PD-L1 expressions were evaluated by IHC in cases with nasopharyngeal cancer recently, and they found poorest prognosis for DFS in cases with co-expression of PD-1 and PD-L1, and they concluded that PD-1/PD-L1 co-expression reflects the selective suppression of cytotoxic lymphocytes in the tumor microenvironment [32].
Epstein–Barr virus (EBV) has been implicated in approximately 40 % of classical HLs [33]. There are some studies evaluating the association between EBV and PD-1, or its ligands and results are complex. EBV infection has been implicated in PD ligand expression, and it has been shown that EBV-encoded LMP1 can promote PD-L1 expression in tumor cells [34]. However, in another study covering 12 cases with EBV(+) HL, 11 cases showed strong membranous PD-L1 staining in malignant RS cells while 20 of 29 cases with EBV(−) HL showed PD-L1 positivity. In this study, it has been suggested that EBV status does not predict the presence or absence of PD-L1 expression in HL [10]. Additionally, EBV-encoded RNA ISH has been studied in primary mediastinal large B cell lymphoma, and no significant correlation has been found between EBV positivity and PD ligand [11]. We did not find an important association between EBV and PD-1 or PD-L1.
Monoclonal antibodies targeting PD-1 and PD-L1 have been synthesized and used successfully with durable responses in many solid tumors [35, 36]. Recently, there are many trials establishing the beneficial effects of the PD-1 blockers in lymphomas [7, 37]. The PD-1 inhibitors seem to reverse tumor escape from immune surveillance across several cancers, including melanoma; lung, bladder, and kidney cancers; and HL. These cancers are being targeted with PD-1 inhibitors used alone or in combination with other inhibitors that target specific pathways associated with tumor proliferation. Downregulation of PD-L1 combined with chemotherapy can suppress lymphoma growth, promote antitumor activity, and prolong the survival rate. Targeted therapy with PD-L1 may provide a new promising approach in the treatment of lymphomas [14]. In vitro studies showed that T cells from primary HL expressing PD-1 responded to PD-1 blockade [28, 29, 38]. Lastly, very good results with nivolumab and pembrolizumab in relapsed/refractory cases with HL have been published and presented [39, 40].
In conclusion, we detected PD-1 or PD-L1 expression in 20 % of the cases. Although each of PD-1 or PD-L1 expression found to be not affecting the clinical outcome, it showed that the co-expression of PD-1 and PD-L1 expressions associated with poor clinical outcome. These results suggest that the PD-1 or PD-L1 are important targets in HL, and monoclonal antibodies targeting this system will be very useful and effective in cases with HL as observed in many solid tumors.
References
Nishimura H, Agata Y, Kawasaki A, Sato M, Imamura S, Minato N, Yagita H, Nakano T, Honjo T (1996) Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol 8:773–780
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Dong H, Zhu G, Tamada K, Chen L (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5:1365–1369
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2:261–268
Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H (2001) B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 193:839–846
Atanackovic D, Luetkens T, Kroger N (2013) Coinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myeloma. Leukemia 1–8
Hamid O, Carvajal RD (2013) Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther 13:847–861
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MGM, Xu ML, Yu H, Fletcher CDM, Freeman GJ, Shipp MA, Rodig SJ (2013) PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 19:3462–3473
Twa DDW, Chan FC, Ben-Neriah S, Woolcock BW, Mottok A, Tan KL, Slack GW, Gunawardana J, Lim RS, McPherson AW, Kridel R, Telenius A, Scott DW, Savage KJ, Shah SP, Gascoyne RD, Steidl C (2014) Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood 123:2062–2065
Tan KL, Scott DW, Hong F, Kahl BS, Fisher RI, Bartlett NL, Advani RH, Buckstein R, Rimsza LM, Connors JM, Steidl C, Gordon LI, Horning SJ, Gascoyne RD (2012) Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood 120:3280–3287
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Li Y, Wang J, Li C, Ke XY (2012) Contribution of PD-L1 to oncogenesis of lymphoma and its RNAi-based targeting therapy. Leuk Lymphoma 53:2015–2023
Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B (2007) Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 110:296–304
Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED (2006) Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 66:3381–3385
Mohammed S, Ghebeh H, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S (2006) The B7-H1 (PD-L1)T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 8:190–198
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S (2007) Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 104:3360–3365
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N (2006) Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 108:19–24
Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11:2947–2953
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J (2009) Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 15:971–979
Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H, Li H, Luo X, Ye T, Sun Y, Chen H (2014) Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. OncoTargets Therapy 7:567–573
Rosenwald A, Wright G, Leroy K, Yu X, Gaulard RD, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Campo E, Montserrat E, Lopez-Guillermo A, Ott G, Muller-Hermelink HK, Connors JM, Braziel R, Grogan TM, Fisher RI, Miller TP, LeBlanc M, Chiorazzi M, Zhao H, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Staudt LM (2003) Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 198:851–862
Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM (2011) Programmed death ligand 1 is expressed by non-Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res 17:4232–4244
Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR et al (2003) Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 170:1257–1266
Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ (2006) Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol 30:802–810
Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, Kutok JL, Shipp MA (2010) Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116:3268–3277
Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, Kondo T, Ohmori K, Kurata M, Hayashi T, Uchiyama T (2008) PD-1–PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood 111:3220–3224
Muenst S, Hoeller S, Dirnhofer S, Tzankov A (2009) Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol 40:1715–1722
Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8:467–477
Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Gouill SL, Haioun C, Tarte K, Lamy T, Milpied N, Fest T (2014) High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. doi:10.1038/leu.2014.137
Zhang J, Fang W, Quin T, Yang Y, Hong S, Liang W, Ma Y, Zhao H, Huang Y, Xue C, Huang Y, Xue C, Huang P, Hu Y, Zhao Y, Zhang L (2015) Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol 32:86. doi:10.1007/sl2032-015-0501-6
Hohaus S, Santangelo R, Giachelia M, Vannata B, Massini G, Cuccaro A, Martini M, Cesarini V, Cenci T, D’Alo F, Voso MT, Fadda G, Leone G, Larocca LM (2011) The viral load of Epstein-Barr virus (EBV) DNA in peripheral blood predicts for biological and clinical characteristics in Hodgkin lymphoma. Clin Cancer Res 17:2885–2992
Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O’Donnell E, Neuberg D, Shipp MA (2012) Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res 18:1611–1618
Shih K, Arkenau HT, Infante JR (2014) Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 74:1993–2013
Naidoo J, Page DB, Wolchok JD (2014) Immune checkpoint blockade. Hematol Oncol Clin North Am 28:585–600
Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, Romaguera J, Hagemeister F, Fanale M, Samaniego F, Feng L, Baladandayuthapani V, Wang Z, Ma W, Gao Y, Wallace M, Vence LM, Radvanyi L, Muzzafar T, Rotem-Yehudar R, Davis RE, Neelapu SS (2014) Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 15:69–77
Chemnitz JM, Eggle D, Driesen J, Classen S, Riley JL, Debey-Pascher S, Beyer M, Popov A, Zander T, Schultze JL (2007) RNA fingerprints provide direct evidence for the inhibitory role of TGF beta and PD-1 on CD4+ T cells in Hodgkin. Blood 110:3226–3233
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372(4):311–9. doi:10.1056/NEJMoa1411087
Craig H, Moskowitz M, Ribrag V, Michot J-M, Martinelli G, Zinzani PL (2014) PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013) ASH 2014 Abs No: 290
Acknowledgments
This study has been supported by the Cukurova University Research Fund.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paydas, S., Bağır, E., Seydaoglu, G. et al. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol 94, 1545–1552 (2015). https://doi.org/10.1007/s00277-015-2403-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2403-2