Abstract
Tumor immune evasion is a hallmark of cancer. The programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) pathway has been suggested to play an important role in T cell tolerance and tumor immune escape. In this study, we aimed to evaluate the correlation between the expression of PD-1/PD-L1 and the post-treatment outcome in patients with nasopharyngeal carcinoma (NPC). Formalin-fixed, paraffin-embedded tissue biopsies from 139 patients with histological diagnosis of NPC treated with conventional chemoradiotherapy were studied. By using immunohistochemistry staining, expressions of PD-1 on tumor-infiltrating lymphocyte and PD-L1 on tumor tissue were detected. The staining results were evaluated with H-score. The correlation between PD-1/PD-L1 expression and clinical characteristics and post-treatment outcome were analyzed. PD-1+ immune cell were present in 52 of these 139 tumors (37.4 %). PD-L1 expression was detected in 132 patients (95.0 %), which located on tumor tissue. High expression of PD-L1 (median H-score >35) in tumor tissue significantly correlated with a poor prognosis of disease-free survival (P = 0.009). Co-expression of PD-1 and PD-L1 in NPC at diagnosis correlated with the poorest prognosis of disease-free survival (P = 0.038). PD-1/PD-L1 co-expression reflected the selective suppression of cytotoxic lymphocytes in the tumor microenvironment and predicted recurrence and metastasis of NPC after conventional therapies. Blocking this pathway in patients with co-expression of PD-1/PD-L1 provides a potential therapy target for NPC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is distinguished from other head and neck epithelial cancers, which showed specific epidemiological and pathological features [1]. The incidence of NPC is 80,000 worldwide with 50,000 deaths every year [2]. In southern China, the incidence is one of the highest all over the world (15–50 per 100,000) [3]. Radiotherapy alone or chemoradiotherapy is effective in curing the cancer [4]. Currently, with the advances in tumor imaging and radiotherapy technique, the 5-year local control rate reaches up to 95 % and the 5-year disease-free survival rises to 77 % for early stage NPC [4, 5]. However, distant metastasis remains the obstacle for improving survival [5].
In southern China, the majority (>90 %) histopathologic subtype of NPC is poor or undifferentiated (WHO grade II or III) squamous carcinoma, which is characterized by prevailing Epstein–Barr virus infection and the presence of a substantial immune infiltrate in the primary tumor consisting mainly of T cells [6–8]. Activated immune cells such as cytotoxic tumor-infiltrating lymphocytes (TILs) are important for eliminating residual cancer cells and monitoring recurrence. It has been reported that local infiltration of T lymphocyte was a prognostic factor in patients with NPC [9]. However, many immunosuppressive mechanisms in microenvironments can inactivate these T cells, leading to increasing the risk of recurrence after conventional treatment. Program death-1 (PD-1) is one of the immunosuppressive receptor expressing on activated T cells [10–12].
PD-1 is kind of suppressive receptor expressed by activated T cells, which mediated immunosuppression and tumor immune escape [11]. PD-1 functions through binding with its ligands, PD-L1 and PD-L2, which were expressed on tumor cells, stromal cells, or tumor-associated macrophage (TAM) [13, 14]. PD-1 is a marker for T lymphocytes malfunctioning in response to viral infection [15–18]. It has been reported that PD-1 expression in intratumoral lymphocytes is associated with the malfunctioning of TIL in melanoma, liver cancer, and lymphoma [19–21]. Besides, PD-L1 expression was also found in several kinds of virus-associated malignancies [22]. Increase of PD-1 expression in intratumoral T cells predicts poor prognosis in renal cell carcinoma, classical Hodgkin’s lymphoma and NPC [19, 20, 23]. However, elevation of PD-1 expression is associated with favorable prognosis in follicular lymphoma and HPV-associated head and neck cancer [24, 25]. In our previous study, the detail mechanism of PD-L1 up regulation in EBV-infected NPC had been demonstrated. High PD-L1 expression in NPC is related to lower disease control [26].
Recently, in the phase I clinical of anti-PD-1 and anti-PD-L1 antibodies, patients with melanoma, non-small-cell lung cancer, and renal cell carcinoma have achieved clinical response, and the relationship between PD-L1 expression on tumor cells and objective response has been demonstrated by immunohistochemistry (IHC) [27, 28]. However, NPC was not included in the phase I study. The role of targeting programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) axis in NPC has not yet been fully evaluated. Besides, in previous study about PD-1/PD-L1, only single marker, PD-1 or PD-L1 was analyzed. Here, we aimed to explore the impact of co-expression of PD-1 and PD-L1 on prognosis in patients with NPC.
Patients and methods
General information
A total of 139 adult patients diagnosed of NPC at Sun Yat-sen University Cancer Center (Guangzhou, China), who had formalin-fixed paraffin-embedded tissue (FFPE) from the original diagnostic biopsy, were identified. The basic clinical data of these patients were collected, including gender, age, tumor stage, treatment regimen, and follow-up records.
Characteristics of these patients are summarized in Table 1. Among the 139 patients enrolled, 113 males and 26 females, with the median age 45 years (range from 18 to 81 years). All the patients were treated with conventional chemoradiotherapy. The median follow-up time was 50.3 months. Locoregional relapse or distant metastasis had occurred in 60 patients, and a total of 30 patients had died during follow-up. All tumors were classified as undifferentiated non-keratinizing phenotype.
The protocol was approved by the Institutional Review Board of Sun Yat-Sen University Cancer Center (Guangzhou, China), and the research was conducted in accordance with the Declaration of Helsinki and good clinical practice. Written informed consent had been provided before samples were collected.
Immunohistochemistry staining
FFPE sections (3 μm thick) were collected for staining PD-1 and PD-L1. Dewaxed paraffin sections were rehydrated by alcohol series, treated with 3 % H2O2for 10 min at room temperature and pressure cooked for 10 min to retrieve antigen using citrate-based buffer (Vector antigen unmasking solution 3300). PD-1 (Abcam, ab52587), and PD-L1 (E1L3 N™,Cell Signaling Technology, Danvers, MA) were stained using the Bond Max Microsystems.
The expression of PD-1 and PD-L1 was performed on two different slides. Tonsils tissue was taken as a positive control. The IHC results were score manually by two pathologists who counted 20 sequential high-power fields judged to be representative of the tumor, while remaining blinded to clinical information. The average and greatest number of PD-1+ on tumor-infiltrating lymphocytes and PD-L1+ in the tumors per high-power field (0.54 mm field diameter) were recorded. The staining results were recorded with H-score (giving a range of 0–300) [10]. According to the expression level of both PD-1 and PD-L1, four groups of patients were divided.
Statistical analysis
Patients were divided categorically according to the expression of each biomarker into cohorts on the basis of expression level (high and low). Optimal cut point for expression level of each marker was determined by using the X-Tile statistical package (Yale University, New Haven, CT) basing on the outcome [29]. Kaplan–Meier curves defined by these cut points were generated, and statistical significance of differences arising from differential expression of each marker were determined by using the log-rank test.
Outcomes were measured from the date of diagnosis to occurrence of event or date of last follow-up. Disease-free survival (DFS) was measured from the date of diagnosis to the time of recurrence, metastasis, or the date of last follow-up.
Student’s t-test was used to evaluate the association of high and low expression of PD-1 and PD-L1 with age. Chi-square test was used to assess the expression of PD-1 and PD-L1 correlated with clinical parameters such as gender and tumor staging. Survival analysis was depicted by Kaplan–Meier method. Univariate analysis and multivariate analysis were performed with log-rank test and Cox regression analysis, respectively. A p value < 0.05 used to denote statistical significant, and all reported p values were two sided. These statistical analyses were performed with SPSS 16.0.
Results
Prognostic significance of PD-1 or PD-L1 expression
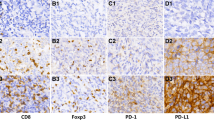
PD-1 expressed on TIL was only observed in 37.4 % of patients. PD-L1 expression was detected in 95 % of patients (Fig. 1). Univariate analysis between PD-1/PD-L1 expression, clinical characteristics and prognosis was performed for all patients. It was found that the expression level of PD-L1 was the only prognostic factor for DFS (Table 2). We showed that in patients with high expression of PD-L1 (H-score >35) was correlated with more poor DFS (39.6 vs. 65.2 months, P = 0.009), which had been reported in previous study [26]. Here, the impact of PD-1 expression on prognosis was not obvious (P = 0.57).
Association of PD-1/PD-L1 co-expression with post-treatment outcome in NPC
As the activation of PD-1 pathway calls for the bindin g of PD-L1, here we analyzed the expression of PD-1 on TILs and PD-L1 on tumor cells in different combination basing on the expression level of the two markers. Patients were allocated into four groups: PD-1 negative and PD-L1 low expression (PD-1 = 0/PD-L1 ≤ 35), PD-1 negative and PD-L1 high expression (PD-1 = 0/PD-L1 > 35), PD-1 positive and PD-L1 low expression (PD-1 > 0/PD-L1 ≤ 35), and PD-1 positive and PD-L1 high expression (PD-1 > 0/PD-L1 > 35). There were no significant difference among the four groups on clinical parameters of age, tumor stage, lymph node metastasis, and clinical TNM staging (Table 3). Outcome of the four groups was significantly different for DFS (P = 0.038; Fig. 2), with the median DFS of 65.2 versus 38.2 versus 94.1 versus 28.1 months, respectively. The group of patients with PD-1 > 0/PD-L1 ≤ 35 had best prognosis, and the group with PD-1/PD-L1 co-expression (PD-1 > 0/PD-L1 > 35) showed the poorest DFS.
Time to first-line treatment failure (recurrence or metastasis) of patients on the basis of the expression level of PD-1 (negative vs. positive) and PD-L1 (high vs. low) in different combination. PD-1 = 0/PD-L1 ≤ 35: PD-1 negative and PD-L1 low expression; PD-1 = 0/PD-L1 > 35: PD-1 negative and PD-L1 high expression; PD-1 > 0/PD-L1 ≤ 35: PD-1 positive and PD-L1 low expression; PD-1 > 0/PD-L1 > 35: PD-1 positive and PD-L1 high expression
Discussion
NPC was one of Epstein–Barr virus (EBV)-associated malignancies with high metastatic potential compared to other head and neck cancers [1, 30]. To identify the patients who had the potential of immune escape and had great risk of relapse after primary treatment is extremely important. In this study, we analyzed the co-expression of PD-1 and PD-L1 together with clinical parameters in the same cohort of NPC patients. We found that high expression of PD-L1 on tumor cells predicted a poor clinical outcome in terms of DFS. Furthermore, co-expression of PD-1 and PD-L1 in patients with NPC might predict the highest recurrence or metastasis incidence. To our knowledge, this is first study to explore prognostic value of co-expression of the two immune escape markers in NPC patients.
PD-1 delivers inhibitory signals to T cells when binding with PD-L1 and PD-L2 [11, 31]. The inhibitory function of PD-1 has been shown to be relevant during autoimmunity, allergy, chronic virus infections, and anti-tumor immunity [12, 32]. It has been reported that PD-1 expression on immune cells was correlated with poor prognosis in renal cell carcinoma and Hodgkin’s lymphoma [19, 20]. Hsu et al. [23] also reported that increase of PD-1 expressing on CD8+ T cells predicted poor outcome in NPC. However, in some other studies, it has been demonstrated that PD-1 expression on T cells was associated with favorable outcome in follicular lymphomas and HPV-associated head and neck cancers [24, 25]. In our research, PD-1+ TILs were detected in 37.4 % patients. PD-1 expression was not a prognostic factor in our study, which was similar to previous reports of 28.4 % by immunofluorescence staining methods [23].
PD-L1 is an immune-modulatory cell-surface glycoprotein, which is primary expressed by antigen-presenting cells and tumor cells [11]. Binding of PD-L1 to its receptor PD-1 inhibits proliferation of activated T cells leading to apoptosis of T cell, which is so-called “T cell exhaustion” [14]. Chen et al. [33] reported that PD-L1 expression is a characteristic of virus- and immunodeficiency-associated malignancies, including NPC. It has been demonstrated that 89 % (16/18) of EBV-associated NPC cases had PD-L1 expression in malignant cells, and the results of which was consistent with our study [26]. Additionally, poor clinical prognosis in renal cell carcinoma, hepatocellular cancer, and breast cancer was found to correlate with the expression of PD-L1 in the tumor microenvironment [34–37]. In our analysis, PD-L1 expression was also an independent prognostic factor in NPC. Patients with high expression of PD-L1 were correlated with poor DFS.
In previous studies of PD-1 pathway in NPC patients, only one marker, PD-1 or PD-L1 was involved. Here, we analyzed expression of PD-1 by TILs and PD-L1 by tumor cells and the correlation of these markers with patient prognosis in the same cohort of patients. Interestingly, when expression of PD-1 and PD-L1 was considered together, we found that low expression of PD-L1 seemed to be a dominant favorable prognostic factor in NPC, while expression level of PD-1 was an additional factor. Patients with high expression of PD-L1 plus positive of PD-1 had significantly high recurrence or metastasis rate than patients with PD-L1 low expression plus PD-1 positive expression. Patients with negative PD-1 with or without favorable PD-L1 expression showed an intermediate median disease-free survival. In fact, recent research had demonstrated that high PD-L1 expression was associated with EBV infection in a broad range of EBV-associated malignancies [31]. Green et al. [38] had also demonstrated that the expression of the EBV-encoded latent membrane protein (LMP)-1 promotes both AP1 signaling and JAK–STAT signaling to up regulate PD-L1. They also confirm this result in the majority of EBV-positive post-transplant lymphoproliferative disorder (PTLD) patients.
In conclusion, NPC patients with PD-1 and PD-L1 co-expression showed poor prognosis due to the activation of PD-1 pathway, leading to T cell exhaustion, which demonstrated the importance of PD-1/PD-L1 axis in regulating anti-tumor immunity. This group of patients might be an ideal candidate for further clinical trial of anti-PD-1 or anti-PD-L1 therapy. Our study is limited by its retrospective nature with a relatively small sample size. Further study with greater sample size is warranted.
References
Chan AT, Teo PM, Johnson PJ. Nasopharyngeal cancer. Cancer Treat Res. 2003;114:275–93.
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30:114–9.
Ma BB, Hui EP, Chan AT. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci. 2008;99:1311–8.
Xiao WW, Huang SM, Han F, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117:1874–83.
Shanmugaratnam K, Sobin LH. The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer. 1993;71:2689–97.
Khanna R, Busson P, Burrows SR, et al. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res. 1998;58:310–4.
Lin X, Gudgeon NH, Hui EP, et al. CD4 and CD8 T cell responses to tumour-associated Epstein-Barr virus antigens in nasopharyngeal carcinoma patients. Cancer Immunol Immunother. 2008;57:963–75.
Li J, Zeng XH, Mo HY, et al. Functional inactivation of EBV-specific T-lymphocytes in nasopharyngeal carcinoma: implications for tumor immunotherapy. PLoS One. 2007;2:e1122.
Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–7.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–34.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34.
Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7.
Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7.
Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4.
Urbani S, Amadei B, Tola D, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–403.
Muenst S, Hoeller S, Dirnhofer S, Tzankov A. Increased programmed death-1 + tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol. 2009;40:1715–22.
Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–61.
Zeng Z, Shi F, Zhou L, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One. 2011;6:e23621.
Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–73.
Hsu MC, Hsiao JR, Chang KC, et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23:1393–403.
Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–38.
Carreras J, Lopez-Guillermo A, Roncador G, et al. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27:1470–6.
Fang W, Zhang J, Hong S et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget. 2014;5(23):12189–202.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9.
Pathmanathan R, Prasad U, Sadler R, et al. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–8.
Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209:201–9.
Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22:265–8.
Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–73.
Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s.
Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–96.
Ghebeh H, Tulbah A, Mohammed S, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121:751–8.
Muenst S, Soysal SD, Gao F, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–76.
Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611–8.
Acknowledgments
This work was supported by grants from the the National High Technology Research and Development Program of China (863 Program No. 2012AA02A501 and 2012AA02A502), the Natural Science Foundation of Guangdong (Grant No. S2013010016564). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
All authors declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianwei Zhang and Wenfeng Fang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, J., Fang, W., Qin, T. et al. Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol 32, 86 (2015). https://doi.org/10.1007/s12032-015-0501-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0501-6