Abstract
This study aimed to evaluate the evolution of iron overload, assessed by serum ferritin (SF), in transfusion-dependent lower risk patients with myelodysplastic syndrome (MDS), as well as to describe the occurrence of organ complications, and to analyze its relationship with iron chelation therapy. This observational retrospective study was conducted from March 2010 to March 2011 in 47 Spanish hospitals. A total of 263 patients with lower risk MDS (International Prognostic Scoring System [IPSS] low/intermediate-1 risk or Spanish Prognostic Index [SPI] 0–1 risk), transfusion-dependent, and who had received ≥10 packed red blood cells (PRBC) were included. At MDS diagnosis, patients received a mean of 2.8 ± 3.9 PRBC/month, and 8.7 % of patients showed SF ≥1000 μg/L. Over the course of the disease, patients received a mean of 83.4 ± 83.3 PRBC, and 36.1 % of patients presented SF ≥2500 μg/L. Cardiac, hepatic, endocrine, or arthropathy complications appeared/worsened in 20.2, 11.4, 9.9, and 3.8 % of patients, respectively. According to investigator, iron overload was a main cause of hepatic (70.0 %) and endocrine (26.9 %) complications. A total of 96 (36.5 %) patients received iron chelation therapy for ≥6 months, being deferasirox the most frequent first chelation treatment (71.9 %). Chelation-treated patients showed longer overall survival (p < 0.001), leukemia-free survival (p = 0.007), and cardiac event-free survival (p = 0.017) than non-chelated patients. In multivariable analyses, age (p = 0.011), IPSS (p < 0.001), and chelation treatment (p = 0.015) were predictors for overall survival; IPSS (p = 0.014) and transfusion frequency (p = 0.001) for leukemia-free survival; and chelation treatment (p = 0.040) and Sorror comorbidity index (p = 0.039) for cardiac event-free survival. In conclusion, these results confirm the potential survival benefit of iron chelation therapy and provide additional evidence on the deleterious effect of iron overload in lower risk MDS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The myelodysplastic syndromes (MDS) are clonal stem cell disorders characterized by ineffective hematopoiesis and by a significant risk of progression to acute myeloid leukemia. Anemia is the most frequent peripheral cytopenia described in MDS patients, with up to 85 % of patients manifesting anemia at diagnosis [1], and most MDS patients develop transfusion dependence.
Because of transfusion dependence, MDS patients are at high risk of transfusional iron overload evidenced by high serum ferritin (SF) levels, which has been associated with inferior overall survival (OS) and leukemia-free survival (LFS) in multiple retrospective studies in lower risk MDS [2–7]. Iron chelation has emerged as a therapeutic option to prevent the potential complications associated with regularly transfused MDS patients. Multiple analyses suggest that an appropriate iron chelation therapy is associated with improved OS and LFS in this group of patients [8–12].
Additionally, there is increasing evidence relating iron overload with cardiac and hepatic dysfunction in MDS patients [13–18]. In this regard, multiple retrospective analyses suggest that mortality is higher in MDS iron overloaded patients, with cardiac dysfunction being the primary cause of nonleukemic death [2, 4, 19]. The impact of iron overload on patients with thalassemia and the consequent benefits of iron chelation therapy have been widely validated. Nevertheless, the benefits of iron chelation therapy on organ damage, OS, and LFS in MDS patients are still a clinical debate [15]. Thus, specific studies are needed in order to provide sound evidence to support treatment guidelines in MDS patients.
The goals of this study were to evaluate the evolution of iron overload, assessed by SF, in transfusion-dependent lower risk MDS patients, as well as to describe the occurrence of clinical complications, and to analyze its relationship with chelation therapy.
Patients and methods
Study design
This was an observational, retrospective, and multicenter study conducted over 12 months (March 2010 to March 2011) in 47 Hematology Departments in Spanish hospitals. This study was conducted in accordance with the Guidelines for Ethical Review of Epidemiological Studies, Spanish Society of Epidemiology, the principles of the Helsinki Declaration, and its subsequent amendments. The study was approved by the Ethics Committee from Hospital Clínico Universitario San Carlos (Madrid, Spain). Written informed consent was obtained from patients prior to their inclusion in the study.
Patient population and study procedures
Inclusion criteria comprised patients aged over 18 years; with MDS according to the French-American-British cooperative group and the World Health Organization criteria, and an International Prognostic Scoring System (IPSS) low/intermediate-1 risk or Spanish Prognostic Index (SPI) 0–1 risk [5, 20, 21]; and red blood cell transfusion-dependent patients who had received ≥10 concentrates of packed red blood cells (PRBC) during at least 12 months previous to study inclusion.
The following variables were collected from all patients: (1) demographics (age and gender), (2) clinical data at MDS diagnosis (date of diagnosis, symptoms, comorbidities, and concomitant diseases), (3) hematological parameters at diagnosis (hemoglobin level, absolute neutrophil and platelet counts, and proportion of blast cells in bone marrow), (4) cytogenetics (conventional karyotype and fluorescence in situ hybridization [FISH], if available), (5) morphological classification (by IPSS and SPI), (6) treatment received for MDS (lenalidomide, azacitidine, and others), (7) data about PRBC transfusion requirements (date of first PRBC transfusion, and number of PRBC units administered from diagnosis and during the last 12 months), (8) SF levels (at diagnosis, at the start of iron chelation therapy, and at last assessment), (9) Sorror comorbidity index [22], (10) data about iron chelation therapy (date of first and last administration of an iron chelating agent, drug/s, dose/s, duration of chelation with every drug used, and reason for discontinuation or change in prescription), and (11) organ complications during the course of the disease (cardiac, hepatic, endocrine, and arthropathy). For cardiac complications, a patient was considered to experience a cardiac complication if he/she has suffered a complication related to cardiac insufficiency, arrhythmia, or both. The cause of complications was assessed as per investigator’s criteria. The investigator obtained retrospective patients’ information and survival status from medical charts and recorded them in case report forms.
Statistical analysis
Data were summarized using descriptive statistics. Continuous variables were described using central tendency and dispersion measurements (mean ± 1 standard deviation) and were compared using t tests or nonparametric methods (Mann-Whitney U tests or Kolmogorov-Smirnov Z tests, as appropriate). Frequency distributions of discrete variables were compared using chi-squared tests or Fisher’s exact tests, as appropriate. OS, LFS, and event-free survival were calculated by the Kaplan-Meier method; results were presented as survival curves and median survival times with 95 % confidence intervals. Patients receiving iron chelation therapy for ≥6 months were considered as chelated for survival analyses. Cardiac event-free survival was analyzed taking into account the first cardiac event per patient. The log-rank or Breslow test was used to compare survival curves, as appropriate. Multivariable proportional hazard regression models were used to evaluate prognostic factors for OS, LFS, and cardiac event-free survival. Independent variables (covariates) included for possible selection were age at diagnosis, gender, IPSS score at diagnosis, frequency of transfusions, iron overload (SF ≥2500 μg/L, as all patients were transfusion-dependent and had received ≥10 PRBC), chelation treatment, lenalidomide/azacitidine treatment, and Sorror comorbidity index. A forward stepwise variable selection procedure was used to determine the final multivariable model, which finally included the following covariates: age, gender, chelation treatment, iron overload, lenalidomide/azacitidine treatment, IPSS score, frequency of transfusions, and Sorror comorbidity index.
Missing data were not considered in the analyses, and a two-sided significance level of 0.05 was considered as statistically significant. Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS Inc, Chicago, USA).
Results
Patients’ characteristics
A total of 263 patients were evaluated in this study. Patient characteristics at diagnosis are described in Table 1. The median observation time (interquartile range) of patients included in the study analysis was 41 (19–80) months.
Overall iron metabolism
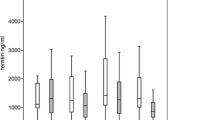
Data on iron metabolism is shown in Table 2. Patients had a mean transferrin saturation index (TSI) of 57.4 ± 25.0, with 100 (38.0 %) patients reporting TSI ≥50 %. In addition, SF levels at diagnosis were 515.6 ± 470.8 μg/L; 23 (8.7 %) patients had SF levels ≥1000 μg/L. Over the course of the disease, all patients received a mean of 83.4 ± 83.3 PRBC, and a total of 95 (36.1 %) patients showed SF levels ≥2500 μg/L.
Iron metabolism according to chelation therapy
A total of 146 (55.5 %) patients received iron chelation therapy during the course of the disease. Fifty (19.0 %) patients discontinued it within 6 months of starting treatment, while 96 (36.5 %) patients received it for ≥6 months (PRBC transfusions at starting treatment, 31.7 ± 28.5; SF levels at starting treament, 1865.5 ± 1040.8 μg/L). Chelated patients were younger at MDS diagnosis than nonchelated patients (67.2 ± 11.5 vs 74.6 ± 9.0 years; p < 0.001) and showed lower Sorror comorbidity index (0.9 ± 1.2 vs 1.7 ± 1.9; p = 0.002); there were no significant differences with regard to patients’ gender.
Among patients under chelation therapy, most patients received deferasirox as the first iron chelation therapy (69 subjects, 71.9 %), followed by deferoxamine (21 subjects, 21.9 %), and deferiprone (4 subjects, 4.2 %).
As deferasirox was the agent most frequently used in our study population, detailed analyses of changes in iron balance throughout the course of the disease and causes for treatment discontinuation were performed in patients receiving deferasirox. Specifically, data were evaluated according to whether a decrease or an increase in SF levels was observed when deferasirox was used as a first iron chelation agent (Table 3). Patients with increased SF levels showed slightly lower deferasirox doses (16.8 ± 5.6 vs 18.3 ± 3.6 mg/kg/day), higher transfusion frequencies (2.7 ± 2.2 vs 2.2 ± 1.0 PRBC/months), and shorter treatment durations (20.8 ± 10.7 vs 24.6 ± 11.3 months).
Twenty-one (30.4 %) patients discontinued deferasirox. The main causes for deferasirox discontinuation were adverse events (eight patients), reaching normal SF levels (four patients), and lack of efficacy (two patients).
Overall survival
OS was longer for patients receiving chelation treatment (not reached vs 153 [78.0–228.0] months; p < 0.001) (Fig. 1a). When data were analyzed in patients who presented SF levels lower than 2500 μg/L, OS was also longer for chelated patients than nonchelated patients (median survival was not reached in both groups; p = 0.008). In patients who maintained SF levels ≥2500 μg/L, OS was also longer for chelated patients (177.0 [139.6–214.4] vs 98.0 [70.1–125.9] months; p = 0.011).
In a multivariable analysis, the independent variables predicting OS were age (p = 0.011), IPSS (p < 0.001), and chelation treatment (p = 0.015) (Table 4).
Leukemia-free survival
Only a small proportion of the study patients developed acute myeloid leukemia (18 subjects, 6.8 %), the majority of whom (14 subjects, 77.8 %) were from the nonchelated patient group. Patients receiving iron chelation therapy had longer LFS than those nonchelated (median survival was not reached in both groups; p = 0.007) (Fig. 1b).
In a multivariable analysis, the independent variables predicting LFS were IPSS (p = 0.014) and transfusion frequency (p = 0.001) (Table 4).
Organ complications and event-free survival
The information on organ complications is summarized in Table 5. Interestingly, the number of PRBC transfused (53.6 ± 61.2) and the SF levels (1945.4 ± 1527.6 μg/L) at the onset of cardiac complications were lower than those observed at the onset of hepatic complications (58.7 ± 73.7 and 2387.2 ± 1722.2 μg/L, respectively). According to the opinion of investigators who collected the data, iron overload was the main factor related to hepatic complications (21 subjects, 70.0 %), followed by drugs (7 subjects, 23.3 %), viral infections (3 subjects, 10.0 %), alcohol abuse (1 subject, 3.3 %), and others (6 subjects, 20.0 %); more than one cause was reported in 9 (30.0 %) subjects. For arthropathy, arthrosis was the main cause of complications (7 subjects, 70.0 %). For endocrine complications, diabetes (8 subjects, 30.8 %) and iron overload (7 subjects, 26.9 %) were described as the main causes of complications. In the case of cardiac complications, the cause was unknown in most cases (heart failure, 43 subjects, 93.5 %; heart arrhythmia, 23 subjects, 95.8 %). Iron overload was not reported as a cause for cardiac complication in any of the described cases.
Patients receiving chelation treatment had longer cardiac event-free survival in comparison with nonchelated patients (137.0 [108.5–165.5] vs 96.0 [84.1–107.9] months; p = 0.017) (Fig. 1c); in a multivariable analysis, the independent variables predicting cardiac event-free survival were chelation treatment (p = 0.040) and Sorror comorbidity index (p = 0.039) (Table 4). Differences between chelated and nonchelated patients were not observed in terms of hepatic, endocrine, or arthropathy event-free survival (Table 5).
Discussion
This observational retrospective study shows that the administration of iron chelation therapy for ≥6 months results in longer patients’ survival and cardiac event-free survival. Almost 64 % of patients in our study reached SF levels higher than 1000 μg/L and 36 % higher than 2500 μg/L, while 36.5 % received chelation therapy for ≥6 months. Both the number of PRBC transfused and SF levels were over those recommended by current guidelines on MDS management [23–25]. Nonetheless, our data show an improvement on these parameters in comparison with previous assessments in Spain, where SF levels in the last follow-up reached 2480 ± 1648 μg/L [26]. Our findings are consistent with previous analyses evaluating the benefit of iron chelation therapy on patients’ survival [9, 11, 27, 28]. Adequate chelation therapy administered for ≥6 months has shown to improve OS [29, 30], and it has also been identified as a factor predicting for OS [8, 29], along with other variables such as age [4, 31], IPSS [4, 8, 31], transfusion requirements [3, 4, 8, 31], and the development of secondary iron overload [3, 4]. The increased risk of progression to acute myeloid leukemia under conditions of oxidative stress, characteristic of iron overload, has been validated by indirect data from preclinical studies showing the effect of iron modifying cell growth and differentiation [32]. Indeed, the analysis of risk transformation to acute myeloid leukemia has suggested that iron overload and transfusion dependence have a significant impact on this endpoint [33]. Although our survival analysis showed longer LFS in chelated patients, iron chelation therapy was not identified as an independent variable predicting for LFS. There is no agreement with regard to the effect of chelation therapy on the risk of progressing to acute myeloid leukemia, though Lyons et al. reported a trend to longer time in patients receiving iron chelation therapy [30]. Thus, further research is still needed to clarify its effect on LFS.
In addition to the positive effect of chelation therapy on patients’ survival, our results also support the benefit of chelation cherapy on cardiac event-free survival. The assessment of prevalence and impact of comorbidities in over 1700 patients from a US medical database revealed that 51 % of patients with newly diagnosed MDS suffered from comorbidities, and those with comorbidities had an increased risk of death [34]. Specifically, hepatic and cardiac dysfunctions associated to iron damage in transfusion-dependent low-risk MDS patients have been extensively reported to carry along a higher mortality rate [2–4]. In light of the high incidence of cardiac and hepatic comorbidities in MDS patients, efforts have been made to include these complications in specific indexes to predict the risk of nonleukemic death in MDS patients [35]. Furthermore, Hoffbrand et al. [36] recommended considering liver and cardiac iron concentrations as additional valuable parameters at the moment of deciding whether or not to start chelation treatment in low-risk PRBC transfusion-dependent MDS patients.
Our study’s assessment of organ complications during the course of the disease is a novel approach. In this regard, iron overload was reported as the main factor related to hepatic complications. Nonetheless, we did not find significant differences in hepatic event-free survival for chelated and nonchelated patients. One of the limitations of our study was the low number of hepatic events reported (n = 30), as this may have prevented statistically significant differences between groups to appear. In contrast, although researchers in our study did not relate cardiac complications to iron overload, the results showed a significant difference between those treatment groups for cardiac event-free survival (137 months in chelated vs 96 months in nonchelated patients; p = 0.017). Interestingly, the number of PRBC transfused and SF levels at the onset of cardiac complications (54 PRBC and 1945 μg/L) were lower than those observed at the onset of hepatic complications (59 PRBC and 2387 μg/L). Differences are even more striking when compared with evidence from thalassemic patients where the onset of cardiac complications is associated to PRBC values ranging from 75 to 100 and SF levels above 2500 μg/L [37].
As MDS is primarily a hematologic malignancy of the elderly [38], with 86 % of cases diagnosed in individuals older than 60 years [25], comorbidities may compromise elderly patients to a greater degree, and they become more susceptible to the deleterious effect of iron overload. This can be particularly relevant for cardiac complications and might partly explain the differential thresholds in PRBC and SF levels observed for cardiac versus hepatic events in our population. In addition, our study pointed out comorbidities—Sorror comorbidity index—as predictive for cardiac event-free survival, along with chelation treatment; although chelated patients showed less comorbidities, the benefit of chelation treatment remained irrespective of comorbidity occurrence. Furthermore, cardiac complications in MDS patients may be compounded by several factors (e.g., hypertension and coronary artery disease) and may easily hide behind the usual causes of death in the elderly [39].
One caveat of this study is common to the potential biases and confounding effects of all retrospective analyses. Thus, these findings—especially those on cardiac complications and cardiac event-free survival—must be confirmed in prospective trials.
Nevertheless, the study results provide further evidence on the deleterious effect of iron overload and support the potential survival benefit of iron chelation therapy in lower risk MDS patients. Indeed, chelation-treated patients showed longer OS, LFS, and cardiac event-free survival. Therefore, we believe that this study provides physicians with meaningful information to take into account when managing MDS patients.
References
Steensma DP, Bennett JM (2006) The myelodysplastic syndromes: diagnosis and treatment. Mayo Clin Proc 81(1):104–130. doi:10.4065/81.1.104
Takatoku M, Uchiyama T, Okamoto S, Kanakura Y, Sawada K, Tomonaga M, Nakao S, Nakahata T, Harada M, Murate T, Ozawa K (2007) Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol 78(6):487–494. doi:10.1111/j.1600-0609.2007.00842.x
Malcovati L, Della Porta G, Cazzola M (2006) Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica 91(12):1588–1590
Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, Passamonti F, Arcaini L, Maffioli M, Bernasconi P, Lazzarino M, Cazzola M (2005) Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol 23(30):7594–7603. doi:10.1200/JCO.2005.01.7038
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89(6):2079–2088
Bennett JM, Catovsky D, Daniel MÄ, Flandrin G, Galton D, Gralnick HT, Sultan C (1982) Proposals for the classification of the myelodysplasic syndromes. Br J Haematol 51(2):189–199
Sanz GF, Sanz MA, Vallespi T, Canizo MC, Torrabadella M, Garcia S, Irriguible D, San Miguel JF (1989) Two regression models and a scoring system for predicting survival and planning treatment in myelodysplastic syndromes: a multivariate analysis of prognostic factors in 370 patients. Blood 74(1):395–408
Rose C, Brechignac S, Vassilief D, Pascal L, Stamatoullas A, Guerci A, Larbaa D, Dreyfus F, Beyne-Rauzy O, Chaury MP, Roy L, Cheze S, Morel P, Fenaux P (2010) Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myelodysplasies). Leuk Res 34(7):864–870. doi:10.1016/j.leukres.2009.12.004
Neukirchen J, Fox F, Kundgen A, Nachtkamp K, Strupp C, Haas R, Germing U, Gattermann N (2012) Improved survival in MDS patients receiving iron chelation therapy—a matched pair analysis of 188 patients from the Dusseldorf MDS registry. Leuk Res 36(8):1067–1070. doi:10.1016/j.leukres.2012.04.006
Komrokji RS, Al Ali NH, Padron E, Lancet JE, List AF (2011) Impact of iron chelation therapy on overall survival and AML transformation in lower risk MDS Patients treated at the Moffitt Cancer Center. Blood 118(21):1196–1197
Leitch HA (2007) Improving clinical outcome in patients with myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk Res 31(Suppl 3):S7–9. doi:10.1016/S0145-2126(07)70460-5
Lyons RM, Marek BJ, Sharma S, Paley C, Esposito J, Garbo L, DiBella N, Garcia-Manero G (2011) 24-month analysis of the impact of chelation on clinical outcomes in a 600 patient registry of lower-risk MDS patients. Blood 118(21):1207–1208
Porter JB (2001) Practical management of iron overload. Br J Haematol 115(2):239–252
Dreyfus F (2008) The deleterious effects of iron overload in patients with myelodysplastic syndromes. Blood Rev 22(Suppl 2):S29–34. doi:10.1016/S0268-960X(08)70006-7
Gattermann N (2012) Pathophysiological and clinical aspects of iron chelation therapy in MDS. Curr Pharm Des 18(22):3222–3234
Goldberg SL (2007) Novel treatment options for transfusional iron overload in patients with myelodysplastic syndromes. Leuk Res 31(Suppl 3):S16–22. doi:10.1016/S0145-2126(07)70462-9
Leitch HA (2011) Controversies surrounding iron chelation therapy for MDS. Blood Rev 25(1):17–31. doi:10.1016/j.blre.2010.09.003
Della Porta MG, Malcovati L, Travaglino E, Pascutto C, Maffioli M, Invernizzi R, Cazzola M (2007) A prognostic model for predicting the impact of comorbidities on survival of patients with myelodysplastic syndromes. Blood 110(11), abstract 24103
Della Porta G, Kuendgen A, Malcovati L, Zipperer E, Pascutto C, Travaglino E (2008) Myelodysplastic syndrome (MDS)-specific comorbidity index for predicting the impact of extra-hematological comorbidities on survival of patients with MDS. Blood 112(11):925–926a
WHO classification of tumours (2008) In: Swerdlow SH, Campo E, Harris NL et al (eds) WHO classification of tumours of hematopoietic and lymphoid tissues. IARC Press, Lyon, pp 109–138
Germing U, Aul C, Niemeyer CM, Haas R, Bennett JM (2008) Epidemiology, classification and prognosis of adults and children with myelodysplastic syndromes. Ann Hematol 87(9):691–699. doi:10.1007/s00277-008-0499-3
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B (2005) Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106(8):2912–2919. doi:10.1182/blood-2005-05-2004
Gattermann N (2007) Guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. Leuk Res 31(Suppl 3):S10–15. doi:10.1016/S0145-2126(07)70461-7
Gattermann N (2008) Overview of guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. Int J Hematol 88(1):24–29. doi:10.1007/s12185-008-0118-z
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD (1999) World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol 17(12):3835–3849
Remacha AF, Arrizabalaga B, Del Canizo C, Sanz G, Villegas A (2010) Iron overload and chelation therapy in patients with low-risk myelodysplastic syndromes with transfusion requirements. Ann Hematol 89(2):147–154. doi:10.1007/s00277-009-0794-7
Komrokyi R, Al Ali N, Padras E (2011) Impact of iron chelation therapy on overall survival and AML transformation in lower risk MDS patients treated at the Moffitt Cancer Centre. Blood 118:2776
Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, Laouri M (2010) Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol 28(17):2847–2852. doi:10.1200/JCO.2009.25.2395
Delforge M, Selleslag D, Beguin Y, Triffet A, Mineur P, Theunissen K, Graux C, Trullemans F, Boulet D, Van Eygen K, Noens L, Van Steenweghen S, Lemmens J, Pierre P, D’Hondt R, Ferrant A, Deeren D, Van De Velde A, Wynendaele W, Andre M, De Bock R, Efira A, Breems D, Deweweire A, Geldhof K, Pluymers W, Harrington A, MacDonald K, Abraham I, Ravoet C (2014) Adequate iron chelation therapy for at least six months improves survival in transfusion-dependent patients with lower risk myelodysplastic syndromes. Leuk Res 38(5):557–563. doi:10.1016/j.leukres.2014.02.003
Lyons RM, Marek BJ, Paley C, Esposito J, Garbo L, DiBella N, Garcia-Manero G (2014) Comparison of 24-month outcomes in chelated and non-chelated lower-risk patients with myelodysplastic syndromes in a prospective registry. Leuk Res 38(2):149–154. doi:10.1016/j.leukres.2013.11.004
Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, Giagounidis A, Hildebrandt B, Bernasconi P, Knipp S, Strupp C, Lazzarino M, Aul C, Cazzola M (2007) Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 25(23):3503–3510. doi:10.1200/JCO.2006.08.5696
Leitch HA (2011) Optimizing therapy for iron overload in the myelodysplastic syndromes: recent developments. Drugs 71(2):155–177. doi:10.2165/11585280-000000000-00000
Sanz G, Nomdedeu B, Such E, Bernal T, Belkaid M, Ardanaz T, Cervera J (2008) Independent impact of iron overload and transfusion dependency on survival and leukemic evolution in patients with myelodysplastic syndrome. Blood 112(11):238–239a
Wang R, Gross CP, Halene S, Ma X (2009) Comorbidities and survival in a large cohort of patients with newly diagnosed myelodysplastic syndromes. Leuk Res 33(12):1594–1598. doi:10.1016/j.leukres.2009.02.005
Della Porta G, Malcovati L (2009) Clinical relevance of extra-hematological comorbidity in the management of patients with myelodysplastic syndrome. Haematologica 94(5):602–606
Hoffbrand AV, Taher A, Cappellini MD (2012) How I treat transfusional iron overload. Blood 120(18):3657–3669. doi:10.1182/blood-2012-05-370098
Buja LM, Roberts WC (1971) Iron in the heart. Etiology and clinical significance. Am J Med 51(2):209–221
Hellstrom-Lindberg E (2005) Management of anemia associated with myelodysplastic syndrome. Semin Hematol 42(2 Suppl 1):S10–13
Gattermann N (2010) How to treat MDS without stem cell transplantation. Biol Blood Marrow Transplant 16(1 Suppl):S30–36. doi:10.1016/j.bbmt.2009.10.017
Acknowledgments
This study was funded by Novartis Farmacéutica, S.A, Spain. The authors would like to acknowledge the remaining investigators participating in the IRON-2 Study: Juan Antonio Muñoz Muñoz (Hospital Universitario Puerta del Mar, Spain), Rafael Franco Osorio (Hospital Punta de Europa, Spain), Luis Palomera Bernal (Hospital Clínico Universitario Lozano Blesa, Spain), Gemma Azaceta Reinares (Hospital Clínico Universitario Lozano Blesa, Spain), Jose Antonio Moreno Chulilla (Hospital Clínico Universitario Lozano Blesa, Spain), Ana Díaz Trapiella (Hospital de Cabueñes, Spain), Teresa Bernal del Castillo (Hospital Universitario Central de Asturias, Spain), María Antonia Durán Pastor (Hospital Universitario Son Dureta, Spain), Joan Bargay Lleonart (Hospital Sont Llatzer, Spain), Antonia Cladera Sierra (Hospital Sont Llatzer, Spain), Jose María Guerra Hernando (Hospital Sont Llatzer, Spain), Martín Mascaró Riera (Hospital Sont Llatzer, Spain), Angelina Lemes Castellano (Hospital Universitario de Gran Canaria Doctor Negrín, Spain), Bernardo Javier González González (Hospital Universitario de Canarias, Spain), María Cristina Fernández Jimenez (Complejo Hospitalario de Toledo, Spain), Consuelo del Cañizo (Hospital Universitario de Salamanca, Spain), Marcos Barbón Fernández (Hospital de León, Spain), Laura Vicente Folch (Consorci Sanitari Terrassa, Spain), Jaume Orriols Bernet (Fundació Althaia Manresa, Spain), Albert Altés (Fundació Althaia Manresa, Spain), Elena Cabezudo (Fundació Althaia Manresa, Spain), María Esther Plensa Alberca (Hospital de Mataró, Spain), Albert Soley i Garasa (Hospital de Mataró, Spain), Blanca Xicoy Cirici (Hospital Universitari Germans Trias i Pujol, Spain), Alfonso Soler Campos (Hospital Universitari Parc Taulí, Spain), Salut Brunet (Hospital de la Santa Creu i Sant Pau, Spain), Javier Bueno Aribayos (Hospital Vall d’Hebron, Spain), Esther Sancho (Hospital Vall d’Hebron, Spain), David Gallardo Giralt (Hospital Universitari de Girona Doctor Josep Trueta, Instituto Catalán de Oncologia de Girona, Spain), Armando Luaña Galán (Hospital Universitari Arnau de Vilanova, Spain), Andreu Llorente Cabrera (Hospital Universitari Joan XXIII, Spain), Joan Cid Vidal (Hospital Universitari Joan XXIII, Spain), Rafael Andreu Lapiedra (Hospital Universitario Doctor Peset, Spain), Elena Gómez Beltran (Hospital Universitario Doctor Peset, Spain), Carlos Fernández Lago (Complejo Hospitalario Universitario Juan Canalejo, Spain), Beatriz Pazos García (Complejo Hospitalario Universitario Juan Canalejo, Spain), María Angeles Bendaña López (Hospital Clínico Universitario de Santiago Compostela, Spain), Aida Fernández Montero (Hospital Clínico Universitario de Santiago Compostela, Spain), Beatriz Antelo Rodríguez (Hospital Clínico Universitario de Santiago Compostela, Spain), Jose Luis Bello López (Hospital Clínico Universitario de Santiago Compostela, Spain), Manuel Mateo Pérez Encinas (Hospital Clínico Universitario de Santiago Compostela, Spain), María José Rabuñal Martínez (Hospital Clínico Universitario de Santiago Compostela, Spain), Mercedes Castro Mouzo (Complejo Hospitalario de Vigo, Hospital Xeral-Cíes, Spain), Jose María Lapeña Aznar (Hospital San Pedro, Spain), Celina Benavente Cuesta (Hospital Clínico Universitario San Carlos, Spain), Fernándo Ataulfo González Fernández (Hospital Clínico Universitario San Carlos, Spain), Valle Gómez García de Soria (Hospital Universitario de La Princesa, Spain), Santiago Osorio Prendes (Hospital General Universitario Gregorio Marañón, Spain), Alejandro del Castillo Rueda (Hospital General Universitario Gregorio Marañón, Spain), Teresa Pascual Garcia (Hospital Universitario Príncipe de Asturias, Spain), Helga Gullén García (Hospital Universitario Príncipe de Asturias, Spain), Marta Barrionuevo González (Hospital Universitario Príncipe de Asturias, Spain), Emilio Ojeda Gutierrez (Hospital Universitario Puerta del Hierro Majadahonda, Spain), María Dolores López García-Carreño (Hospital General Universitario Santa María del Rosell, Spain), Izaskun Ceberio (Hospital de Navarra, Spain), Saioa Zalba Marcos (Hospital de Navarra, Spain), Carmen Menchaca Echevarría (Hospital Txagorritxu, Spain), Arantza Mendizábal Abad (Hospital Txagorritxu, Spain), Ernesto Pérez Persona (Hospital Txagorritxu, Spain), Jose María Guinea de Castro (Hospital Txagorritxu, Spain), María Teresa Viniegra Ros (Hospital Txagorritxu, Spain), Teresa Uranga (Hospital Universitario Donostia, Spain), Maialen Sirvent Auzmendi (Hospital Universitario Donostia, Spain), Cristina Martínez Bilbao (Hospital Galdakao-Usansolo, Spain), Cristina Cortés (Hospital Galdakao-Usansolo, Spain), Garazi Letamendi Madariaga (Hospital Galdakao-Usansolo, Spain), Jesús Ojanguren (Hospital Galdakao-Usansolo, Spain), Jose Enrique de la Puerta Rueda (Hospital Galdakao-Usansolo, Spain), Koldo Atucha Aresti (Hospital Galdakao-Usansolo, Spain), Tomás Carrascosa Vallejo (Hospital Galdakao-Usansolo, Spain), Jose María Beltrán de Heredia (Hospital de Basurto, Spain), Itziar Olabarría Santurtun (Hospital de Basurto, Spain), Jose Antonio Marquez Navarro (Hospital de Basurto, Spain), María Victoria García Menoyo (Hospital de Basurto, Spain), Pilar Aragues (Hospital Universitario Cruces, Spain), and Sara Erkiaga (Hospital Universitario Cruces, Spain).
Ethical standards
This study was conducted in accordance with the Guidelines for Ethical Review of Epidemiological Studies, Spanish Society of Epidemiology, the principles of the Helsinki Declaration, and its subsequent amendments. The study was approved by the Ethics Committee from Hospital Clínico Universitario San Carlos (Madrid, Spain). Written informed consent was obtained from patients prior to their inclusion in the study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation, and with the Helsinki Declaration of 1975 and its later revisions.
Conflict of interest
The authors declare that Dr. Maria Diez Campelo has received research funding and honoraria from Novartis; and Dr. Guillermo Sanz has received research funding from Celgene and Novartis, and serves as a consultant to Celgene, Amgen, Novartis and Boehringer Ingelheim Pharma GmbH. The remaining authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Remacha, Á.F., Arrizabalaga, B., Villegas, A. et al. Evolution of iron overload in patients with low-risk myelodysplastic syndrome: iron chelation therapy and organ complications. Ann Hematol 94, 779–787 (2015). https://doi.org/10.1007/s00277-014-2274-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2274-y