Abstract
We report here anatomic variants which were found during a retrospective study of a male patient, 54 years old, evaluated in computed tomography: heptafurcation of the celiac trunk (CT) and bilateral double renal arteries. The seven branches of the heptafurcated CT were the (1) left and (2) right inferior phrenic arteries, the (3) splenic and (4) left gastric artery, the (5) common hepatic artery, further sending off the (a) proper, continued as left, hepatic artery and (b) the gastroduodenal artery, (6) a replaced right hepatic artery and (7) the dorsal pancreatic artery. To our knowledge, heptafurcation of the CT was not reported previously. The arterial variants have great importance during various surgical and interventional procedures and should be documented prior to respective procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The celiac trunk (CT), or celiac artery, is the first ventral, thus unpaired, branch of the abdominal aorta, emerging at the level of the 12th thoracic vertebra, when the aorta passes through its diaphragmatic hiatus. The CT usually trifurcates to form the tripus Halleri, which was described by Haller in 1756 [12]. In this common variant, the CT gives off, but not at the same point, the left gastric artery (LGA), the common hepatic artery (CHA) and the splenic, or lienal, artery (SA) [26]. The CHA further sends off the proper hepatic artery (PHA) and the gastroduodenal artery (GDA). Various classifications (four to eight types) of the CT branching patterns were proposed across time in literature [9]. Lipshutz (1917), quoted in [9], identified four types of CT variation by observing only the main branches of the CT, the CHA, LGA and SA [17]. Adachi (1928), also quoted in [9], included the superior mesenteric artery (SMA) in his analysis and got six types of CT variation resulting from variant trunks: hepatogastrosplenic, hepatosplenic, hepatosplenomesenteric, celiacomesenteric, hepatomesenteric and gastrosplenic [1]. Adachi’s six CT types were further evaluated and detailed by Shoumura et al. (1991) [35]. A study of 5002 patients found normal anatomy of CT in 89.1% of these, and indicated 15 possibilities of the celiac axis variation [37]. A 16-type/subtype classification of the branching pattern of the CT was also proposed (Table 1). Evidence of CT absence was also brought [2, 3, 14, 20, 23, 25, 40, 41]. Marco-Clement et al. (2016) suggested that different other classifications could be simplified by referring the anatomic variation of the CT to one of the following types defined by the emergence of its main branches: type I or complete, type II or incomplete, type III—absent CT, and type IV—celiacomesenteric trunk [19].
There are two types of aberrant hepatic arteries, accessory and replaced. The accessory hepatic arteries are supplemental arteries which are added to a normal one. Replaced arteries substitute a normal one [33]. A replaced hepatic artery (rHA) originates so from an artery other than the proper hepatic artery [16]. A common variation is that of a replaced right hepatic artery (rRHA), which originates the superior mesenteric artery (SMA).
Different branching patterns of the CT were sampled with volume-rendered three-dimensional images in a pictorial review in which was discussed that the a priori knowledge of the anatomic possibilities is mandatory in planning surgical and interventional procedures [26], being thus useful for transplantation and general surgeons, as well as vascular radiologists [7].
Anatomic variation
The anatomic variants reported here were found during a retrospective study of computer tomography scans, in a 54-year-old male patient. In that patient, an iodine radiocontrast agent was injected in the left brachial vein (100 ml, with 6 ml/s flow), followed by 40 ml saline medium mixed with 10 ml iodine contrast. A 16-slice scanner was used: 1.2 mm collimation and reconstructions of 3 mm thickness with no overlap for primary diagnosis; and 1.5 mm thickness with 50% overlap for multiplanar, MIP and 3D volume rendering technique (3D-VRT). The arterial variant was documented using the OsiriX Lite software and its 3D Volume Rendering application.
Although arterial anatomic variations were suggested on MPRs, only on three-dimensional volume renderizations they were adequately documented anatomically. Anatomical variants of the CT as well as of the renal arterial system were found (Figs. 1, 2, 3, 4, 5).
Three-dimensional volume rendering of the abdominal aorta branches, right antero-inferior view. AA abdominal aorta, CHA common hepatic artery, CT celiac trunk, D duodenum, DPA dorsal pancreatic artery, GDA gastroduodenal artery, IMA inferior mesenteric artery, LGA left gastric artery, LIPA left inferior phrenic artery, LRAs left renal arteries, P/LHA proper hepatic artery, continued as left hepatic artery, RIPA right inferior phrenic artery, RRAs right renal arteries, rRHA replaced right hepatic artery (*with an initial retroportal segment), SA splenic artery, SMA superior mesenteric artery
Three-dimensional volume rendering of the celiac trunk and superior mesenteric artery, anterior-left view. CHA common hepatic artery, CT celiac trunk, DPA dorsal pancreatic artery, GDA gastroduodenal artery, LGA left gastric artery, LIPA left inferior phrenic artery, P/LHA proper hepatic artery, continued as left hepatic artery, RIPA right inferior phrenic artery, rRHA replaced right hepatic artery, SA splenic artery, SMA superior mesenteric artery
Three-dimensional volume rendering of the seven branches of the celiac trunk, anterior view (with exclusion of the celiac trunk). CHA common hepatic artery, DPA dorsal pancreatic artery, GDA gastroduodenal artery, LGA left gastric artery, LIPA left inferior phrenic artery, P/LHA proper hepatic artery, continued as left hepatic artery, RIPA right inferior phrenic artery, rRHA replaced right hepatic artery, SA splenic artery
Coronal MPR (a) and inferior-to-superior sequence of axial MPRs depicting the origin of the celiac trunk branches. AA abdominal aorta, CHA common hepatic artery, CT celiac trunk, GDA gastroduodenal artery, LGA left gastric artery, LIPA left inferior phrenic artery, PV portal vein, RIPA right inferior phrenic artery, rRHA replaced right hepatic artery, SA splenic artery, SMA superior mesenteric artery
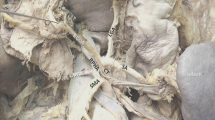
The CT appeared as a long, thick and hook-shaped branch leaving the abdominal aorta above the SMA (Figs. 1, 2). On each side of the proximal half of the CT were inserted the thin inferior phrenic arteries, left (LIPA) and right (RIPA) (Figs. 1, 4, 6), which were ascending on the sides of the aorta at the level of the diaphragmatic pillars. Then, immediately before its termination, the CT was sending an ascending branch, the LGA (Figs. 1, 2, 3, 4, 6). The distal end of the CT was quadrifurcated (Figs. 1, 2, 4, 5, 6). The left branch was the SA. The right branch of the CT was the CHA further divided into a proper, continued as left, hepatic artery (P/LHA), and the GDA. The P/LHA continued antero-laterally to the portal vein towards the hepatic hilum (Fig. 4). The right inferior branch leaving the CT was an rRHA which crossed posteriorly the retropancreatic segment of the portal vein (Figs. 1, 4b), then it changed its direction posterior to the GDA (Figs. 1, 4d) and ascended on the postero-lateral side of the portal vein (Fig. 4e) towards the liver. The 7th branch leaving the CT was the dorsal pancreatic artery (DPA) (Figs. 1, 2, 3, 4, 5, 6).
Drawing of the celiac trunk (CT) heptafurcation. CHA common hepatic artery, DPA dorsal pancreatic artery, GDA gastroduodenal artery, LGA left gastric artery, LIPA left inferior phrenic artery, P/LHA proper hepatic artery, continued as left hepatic artery, RIPA right inferior phrenic artery, rRHA replaced right hepatic artery, SA splenic artery
On each side two distinctive and distanced renal arteries, superior and inferior, were leaving the abdominal aorta to enter the respective renal hilum at its superior and, respectively, inferior margins. The superior renal arteries, left and right, emerged at almost the same level from the abdominal aorta. The left inferior renal artery was inserted into the abdominal aorta at a superior level, as referred to the corresponding right one (Fig. 1). From the aortic segment between the origins of the inferior renal arteries, left and right, emerged the inferior mesenteric artery (IMA) (Fig. 1).
Discussion
Extremely rare anatomical variations of arteries can be identified in the current practice [31]; thus an accurate knowledge of these is relevant. As the interventional techniques developed, the accurate depiction and definition of the CT and its branches are important in planning surgical and interventional procedures, especially in patients with malignancies [26].
Tandler (1904), quoted in [10, 39], discussed that the anterior branches of the abdominal aorta arise from a series of primitive arteries subjected to a craniocaudal shift (the “caudal wandering” of Evans (1912) and Mall (1981), quoted in [24]) along the aorta; due to further processes of absorption and diversion the final anatomical pattern becomes variable. During their morphogenesis, these primitive segmental arteries constitute the longitudinal anterior anastomosis of Tandler ahead of the aorta that will be correlated in adult with the coeliomesenteric and intermesenteric anastomoses [32]. However, the embryologic explanation of Tandler is not enough to explain the shift of the phrenic arteries and DPA to the CT and the resulting heptafurcated CT.
Anatomic variants of the CT are consistently reported and patterned in the literature [17, 26, 35]. Although the heptafurcation of the CT was not reported previously, its possible hexafurcation was found twice, as documented in “Bergman’s Encyclopaedia of Human Anatomic Variation” [33]. Chitra (2010) documented the branching patterns of CT in 50 cadavers and found abnormal trifurcation, bifurcation, quadrifurcation, pentafurcation and hexafurcation of it [8]. The pattern of hexafurcation in that study included the three usual branches of the trunk (LGA, SA and CHA) and, additionally, the inferior phrenic, middle colic and duodenal branches [8]. Paraskevas and Raikos (2011) found the additional three branches of the CT being the LIPA, an accessory left suprarenal artery and an accessory jejunal artery [28]. It appears that the combinations of branches of an excessively branched CT are individual-specific and should not be referred to a certain pattern.
Vandamme and Bonte (1985) proposed a simple classification of the CT branches, as main (SA, CHA and LGA) and collateral branches [39]. They found that the most common collateral of the CT was the inferior phrenic artery [39]. They also discussed that if a CHA divides into its left and right branches medially of the portal vein, the right branch runs behind the portal vein, as also does a rRHA arising from the SMA [39]. Also the rRHA we found arising as one of the seven branches of the heptafurcated CT had an initial retroportal course.
Numerous possibilities of variation of hepatic arteries are actually reported [31]. Replaced right hepatic arteries, were found arising from the superior mesenteric artery in 106 of 1000 cases [13]. However, in that study, a direct origin from the CT of a replaced right hepatic artery, such as we found here, was not documented. A CT origin of a replaced right hepatic artery was documented by Kishi et al. (2010), as quoted in Bergman’s Encyclopaedia of Human Anatomic Variation [33]. Kishi et al. (2010) found in 3/361 cases a replaced right hepatic artery leaving the CT [16], without indicating any of these situations to correspond to a CT heptafurcation, such as we report here.
The dorsal pancreatic artery, or Haller’s Pancreatica Suprema, is a derivative of the longitudinal anterior anastomosis of Tandler and it can originate from the hepatic, splenic or superior mesenteric arteries, or from the hepatosplenic junction [10]. A variant dorsal pancreatic artery leaving the CT can determine its quadrifurcation, or its hexafurcation [33] and, as we found, its heptafurcation. The origin of the dorsal pancreatic artery from an accessory right hepatic artery emerging the superior mesenteric artery was also reported [33]. Nelson et al. (1988) indicated as possible collateral branches of the CT the inferior phrenic arteries, the DPA, the middle colic artery and an esophageal artery [24].
Although anatomic variations of the renal arteries are common, they are of utmost importance during surgery, especially for donor nephrectomies [30]. A hilar renal artery enters the renal hilum, while a polar one directly penetrates one of the renal poles. Sampaio and Passos (1992) evaluated 133 pairs of kidneys and found various combinations of hilar and polar renal arteries: one hilar artery, one hilar + one superior polar arteries, two hilar arteries, three hilar arteries, or just polar arteries, superior or inferior [34]. We found here bilaterally two hilar arteries, a type previously found by Sampaio and Passos (1992) in 7.9% of 266 kidneys. However, anatomical variation of the number of the hilar renal arteries was found ranging from one to six [4, 11, 15, 18, 21, 22, 29, 36, 38]. For double hilar renal arteries Khamanarong et al. (2004) reported a prevalence of 7.5% [15] but a recent study found this variant in 18.6% in a cadaveric study but in 12.3% in a computed tomographic study [6]. Also different patterns of the course of double renal arteries were described, parallel, divergent, convergent and crossed [5], the first type corresponding to the renal arteries variation in the present case. Therefore, the anatomic variation of renal arteries is rather a common feature, as also Özkan et al. (2006) observed [27], and not an exceptional one.
In conclusion, beyond their great importance during various surgical procedures and the mandatory preoperative documentation of the individuals’ specific arterial variants, it should not be ignored that finding variants of a certain vascular bed/axis should not exclude supplemental variations in the same individual, but in different vascular beds.
References
Adachi B (1928) Das arteriensystem der Japaner, vol 2. Kenkyusha Press, Kyoto, pp 18–71
Armstrong PJ, Franklin DP (2006) Superior mesenteric artery branch aneurysm with absence of the celiac trunk. Vascular 14:109–112. https://doi.org/10.2310/6670.2006.00015
Augustyniak E (1965) Rare case of the absence of the celiac trunk. Folia Morphol (Warsz) 24:447–448
Bayazit M, Gol MK, Zorlutuna Y, Tasdemir O, Bayazit K (1992) Bilateral triple renal arteries in a patient with iliac artery occlusion: a case report. Surg Radiol Anat 14:81–83
Bordei P, Sapte E, Iliescu D (2004) Double renal arteries originating from the aorta. Surg Radiol Anat 26:474–479. https://doi.org/10.1007/s00276-004-0272-9
Cases C, Garcia-Zoghby L, Manzorro P, Valderrama-Canales FJ, Munoz M, Vidal M, Simon C, Sanudo JR, McHanwell S, Arrazola J (2017) Anatomical variations of the renal arteries: cadaveric and radiologic study, review of the literature, and proposal of a new classification of clinical interest. Ann Anat 211:61–68. https://doi.org/10.1016/j.aanat.2017.01.012
Chen H, Yano R, Emura S, Shoumura S (2009) Anatomic variation of the celiac trunk with special reference to hepatic artery patterns. Ann Anat 191:399–407. https://doi.org/10.1016/j.aanat.2009.05.002
Chitra R (2010) Clinically relevant variations of the coeliac trunk. Singap Med J 51:216–219
Dilli Babu E, Khrab P (2013) Coeliac trunk variations: review with proposed new classification. Int J Anat Res 1:165–170
Douard R, Chevallier JM, Delmas V, Cugnenc PH (2006) Clinical interest of digestive arterial trunk anastomoses. Surg Radiol Anat 28:219–227. https://doi.org/10.1007/s00276-006-0098-8
Geraci C, Mule G, Geraci G, Mogavero M, Cerasola G (2010) Bilateral double renal arteries and bilateral double renal veins. A color-Doppler sonographic finding in a patient with arterial hypertension. Minerva Urol Nefrol 62:332–333
Haller VA (1756) Icones anatomicae in quibus aliquae partes corporis humani delineatae proponuntur et arteriarum potissimum historia continetur. Vandenhoeck, Gottingen, pp 27–38
Hiatt JR, Gabbay J, Busuttil RW (1994) Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 220:50–52
Iezzi R, Cotroneo AR, Giancristofaro D, Santoro M, Storto ML (2008) Multidetector-row CT angiographic imaging of the celiac trunk: anatomy and normal variants. Surg Radiol Anat 30:303–310. https://doi.org/10.1007/s00276-008-0324-7
Khamanarong K, Prachaney P, Utraravichien A, Tong-Un T, Sripaoraya K (2004) Anatomy of renal arterial supply. Clin Anat 17:334–336. https://doi.org/10.1002/ca.10236
Kishi Y, Imamura H, Sugawara Y, Sano K, Kaneko J, Kokudo N, Makuuchi M (2010) Evaluation of donor vasculobiliary anatomic variations in liver graft procurements. Surgery 147:30–39. https://doi.org/10.1016/j.surg.2009.06.017
Lipshutz B (1917) A composite study of the coeliac axis artery. Ann Surg 65:159–169
Macalister A (1883) Multiple renal arteries. J Anat Physiol 17:250–252
Marco-Clement I, Martinez-Barco A, Ahumada N, Simon C, Valderrama JM, Sanudo J, Arrazola J (2016) Anatomical variations of the celiac trunk: cadaveric and radiological study. Surg Radiol Anat 38:501–510. https://doi.org/10.1007/s00276-015-1542-4
Matusz P, Miclaus GD, Ples H, Tubbs RS, Loukas M (2012) Absence of the celiac trunk: case report using MDCT angiography. Surg Radiol Anat 34:959–963. https://doi.org/10.1007/s00276-012-0989-9
Miclaus GD, Matusz P (2012) Bilateral quadruple renal arteries. Clin Anat 25:973–976. https://doi.org/10.1002/ca.22083
Miclaus GD, Matusz P (2015) Bilateral triple renal arteries. Rom J Morphol Embryol 56:1507–1511
Morettin LB, Baldwin-Price HK, Schreiber MH (1965) Congenital absence of the celiac axis trunk. Am J Roentgenol Radium Ther Nucl Med 95:727–730
Nelson TM, Pollak R, Jonasson O, Abcarian H (1988) Anatomic variants of the celiac, superior mesenteric, and inferior mesenteric arteries and their clinical relevance. Clin Anat 1:75–91
Okada S, Ohta Y, Shimizu T, Nakamura M, Yaso K (1983) A rare anomalous case of absence of the celiac trunk–the left gastric, the splenic and the common hepatic arteries arose from the abdominal aorta independently. Okajimas Folia Anat Jpn 60:65–71
Ozbulbul NI (2011) CT angiography of the celiac trunk: anatomy, variants and pathologic findings. Diagn Interv Radiol 17:150–157. https://doi.org/10.4261/1305-3825.DIR.3283-10.1
Ozkan U, Oguzkurt L, Tercan F, Kizilkilic O, Koc Z, Koca N (2006) Renal artery origins and variations: angiographic evaluation of 855 consecutive patients. Diagn Interv Radiol 12:183–186
Paraskevas GK, Raikos A (2011) Multiple aberrant coeliac trunk ramifications. Singap Med J 52:e147-149
Pestemalci T, Mavi A, Yildiz YZ, Yildirim M, Gumusburun E (2009) Bilateral triple renal arteries. Saudi J Kidney Dis Transpl 20:468–470
Rusu MC (2006) Human bilateral doubled renal and testicular arteries with a left testicular arterial arch around the left renal vein. Rom J Morphol Embryol 47:197–200
Rusu MC, Jianu AM, Sztika D, Cuzino D, Loreto C (2011) Three extremely rare anatomic variants of the hepatic artery. Ann Vasc Surg 25:1138.e1–1138.e7. https://doi.org/10.1016/j.avsg.2011.03.011
Rusu MC, Vlad M, Voinea LM, Curca GC, Sisu AM (2008) Detailed anatomy of a left accessory aberrant colic artery. Surg Radiol Anat 30:595–599. https://doi.org/10.1007/s00276-008-0362-1
Sahni D, Aggarwal A, Gupta T, Kaur H, Gupta R, Chawla K, Saini N, Garg S, Singla A, Deep A, Singh HJ, Shekhawat D, Rapotra M (2016) Abdominal Aorta. In: Tubbs RS, Shoja MM, Loukas M (eds) Bergman’s comprehensive encyclopedia of human anatomic variation. John Wiley, Hoboken, pp 1016–1106
Sampaio FJ, Passos MA (1992) Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat 14:113–117
Shoumura S, Emura S, Utsumi M, Chen H, Hayakawa D, Yamahira T, Isono H (1991) Anatomical study on the branches of the celiac trunk (IV). Comparison of the findings with Adachi’s classification. Kaibogaku Zasshi 66:452–461
Simmons RL, Tallent MB, Kjellstrand CM, Najarian JS (1971) Kidney transplantation from living donors with bilateral double renal arteries. Surgery 69:201–207
Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB, Cho BH, So YH, Park JH (2010) Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA. Radiology 255:278–288. https://doi.org/10.1148/radiol.09090389
Sylvia S, Kakarlapudi SV, Vollala VR, Potu BK, Jetti R, Bolla SR, Rao M, Pamidi N (2009) Bilateral variant testicular arteries with double renal arteries. Cases J 2:114. https://doi.org/10.1186/1757-1626-2-114
Vandamme JP, Bonte J (1985) The branches of the celiac trunk. Acta Anat (Basel) 122:110–114
Yamaki K, Tanaka N, Matsushima T, Miyazaki K, Yoshizuka M (1995) A rare case of absence of the celiac trunk: the left gastric, the splenic, the common hepatic and the superior mesenteric arteries arising independently from the abdominal aorta. Ann Anat 177:97–100
Yi SQ, Terayama H, Naito M, Hirai S, Alimujang S, Yi N, Tanaka S, Itoh M (2008) Absence of the celiac trunk: case report and review of the literature. Clin Anat 21:283–286. https://doi.org/10.1002/ca.20627
Author information
Authors and Affiliations
Contributions
Data collection and management: MCR. Data analysis: MCR, BAM. Manuscript writing: BAM. Literature search: BAM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rusu, M.C., Manta, B.A. Novel anatomic variation: heptafurcation of the celiac trunk. Surg Radiol Anat 40, 457–463 (2018). https://doi.org/10.1007/s00276-018-1995-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-018-1995-3