Abstract
Purpose
Anatomic variants of the celiac trunk and superior mesenteric artery are common, thus knowledge of these variants is important for preoperative planning of abdominal surgery and interventional procedures.
Methods
We report a rare anatomic variant of replaced proper hepatic and gastroduodenal arteries discovered upon CT angiography and diagnostic angiogram.
Results
Emergent angiogram performed on a 61-year-old male who presented with signs and symptoms of upper gastrointestinal hemorrhage revealed a rare variant of an absent common hepatic artery and its branches with aberrant origins. The replaced proper hepatic artery originated from the superior mesenteric artery and the replaced gastroduodenal artery originated from a gastrosplenic trunk.
Conclusion
This case emphasizes the importance of evaluating preoperative imaging to identify vascular variants prior to undergoing abdominal surgery or interventional procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The celiac trunk is the first ventral branch of the abdominal aorta and provides arterial supply to most of the foregut. The normal branching pattern of the celiac trunk is a trifurcation of the splenic artery (SA), the left gastric artery (LGA), and the common hepatic artery (CHA). As the CHA courses towards the porta hepatis, it gives rise to the gastroduodenal artery (GDA) inferiorly, and becomes the proper hepatic artery (PHA) [4]. The PHA terminates by bifurcating into the right and left hepatic arteries to provide arterial blood supply to the liver.
Anatomical variants of the celiac trunk and hepatic arteries have been thoroughly described and classified in the literature and new classifications have been proposed and used to apply them in clinical scenarios [6, 7, 12]. Variation in hepatic arterial anatomy is common and is seen in approximately 50% of the population [3]. Understanding these variants are of great importance to surgeons and interventional radiologists to prevent vascular injury during procedures. Thus, high quality imaging is necessary for preoperative planning to evaluate for aberrant anatomy.
Case report
We report a case of a 61-year-old male with no known past medical history and daily NSAID use who presents with hematemesis and hematochezia. Vitals signs on admission demonstrate tachycardia to 111 bpm, normotensive blood pressure of 148/71 mmHg, and respiratory rate of 20. Laboratory results reveal anemia with a hemoglobin level of 6.7 g/dL. The patient was subsequently transfused 2 units of packed red blood cells and underwent emergent endoscopy. During endoscopy, a giant duodenal ulcer with active bleeding was found and treated with epinephrine injection. Cessation of bleeding from the ulcer base was achieved after epinephrine injection. The patient remained hemodynamically stable without evidence of bleeding for 36 h before having melena and a drop in hematocrit.
MDCT (multidetector computed tomographic) angiography of the chest, abdomen and pelvis was done using a General Electric Revolution EVO scanner. The scanning parameters were as follows: 120 kVp; Auto mA; 0.5 s gantry rotation time; 1.375 mm pitch; 2.5 mm slice thickness. The scanning area included the chest, abdomen and pelvis. CT angiography demonstrated a replaced PHA with SMA origin and a replaced GDA arising from a gastrosplenic trunk (Fig. 1). Interventional radiology was consulted and upon multidisciplinary discussion with gastroenterology and general surgery, it was decided that empiric embolization of the replaced GDA is the appropriate next course of treatment.
Anatomical anomalies of the gastroduodenal and proper hepatic arteries discovered on CT angiography. a Schematic drawing of the abdominal aorta (AO), gastrosplenic trunk, splenic artery (SA), left gastric artery (LGA), replaced gastroduodenal artery (rGDA), superior mesenteric artery (SMA), replaced proper hepatic artery (rPHA), left hepatic artery (LHA), and right hepatic artery (RHA). b 3D reconstruction of a MDCT angiogram of the abdomen and pelvis with IV contrast demonstrates an oblique view of the abdominal aorta (AO) demonstrating aberrant origins of the replaced proper hepatic artery (rPHA) arising from the superior mesenteric artery (SMA) and replaced gastroduodenal artery (rGDA) arising directly from the gastrosplenic trunk
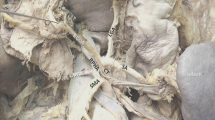
Emergent angiography was preformed and confirmed the absence of the CHA. Complete arterial supply to the liver via PHA originated from the SMA. The replaced GDA originated directly off the gastrosplenic trunk (Fig. 2).
Anatomic anomalies of the gastroduodenal and proper hepatic arteries visualized on angiography. a Digital subtraction angiogram of the gastrosplenic trunk demonstrates trifurcation of the replaced gastroduodenal artery (rGDA), splenic artery (SA), and left gastric artery (LGA). b Digital subtraction angiogram of the superior mesenteric artery (SMA) demonstrates a replaced proper hepatic artery (rPHA) giving rise the right hepatic artery (RHA) and left hepatic artery (LHA)
A micro-catheter was used to selectively catheterize the replaced GDA and an arteriogram demonstrated no active extravasation. Empiric coil embolization of the replaced GDA was subsequently performed. Post embolization angiography of the gastrosplenic trunk showed no antegrade flow into the replaced GDA.
Discussion
Anatomic variants of the celiac trunk and SMA occur due to anomalous embryogenesis of the primitive ventral blood vessels from the abdominal aorta [1, 4, 5, 9, 10, 15]. Michels set the benchmark for classifications of hepatic artery variants in 1966 [12]. Since his work there has been further investigation and modifications on the classification of these variants [7, 16, 18, 19].
Various origins of the CHA have been reported in approximately 4% of individuals [8, 17]. These include a replaced CHA arising from the SMA in 0.86–3% of cases (Michels type IX, 4.5%) [12]. The GDA, however, is found as a constant branch of CHA in 92–95% of patients [9, 12]. A replaced GDA with a celiac origin has been described in the meta-analysis as the hepatosplenic trunk by Macro-Clement [11].
To emphasize the rarity of this variation, we reference only two studies on cadaveric dissections that demonstrate CHA absence and coexistence of a replaced PHA and replaced GDA [2, 13]. The absence of the CHA and coexistence of a replaced PHA and GDA was not included in Michels classification, however it has particular relevance for patients who are having their hepatic arteries interrogated or, as in the presented case, being evaluated for bleeding duodenal ulcer.
Documenting new and rare variants of the visceral arteries is important to maximize the database available to provide optimal care for patients during invasive procedures [14, 16]. It is important that interventional radiologists and surgeons are familiar with both common and rare celiac trunk and SMA branch variants, because failure to recognize aberrant vasculature can result in incomplete therapy or severe vascular and surgical complications.
Conclusion
The precise knowledge of the celiac trunk and arterial variants is very important for preoperative and interventional planning. It is essential to identify and understand variant anatomy prior to any intervention to avoid vascular complications and decrease morbidity of patients.
References
Adachi B (1928) Das Arteriensystem der Japaner, vol 2. Maruzen, pp 18–73
Alakkam A, Hill RV, Saggio G (2016) Superior mesenteric origin of the proper hepatic artery: embryological and clinical implications. Surg Radiol Anat 38(6):747–750. https://doi.org/10.1007/s00276-015-1599-0
Covey AM, Brody LA, Maluccio MA et al (2002) Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology 224(2):542–547. https://doi.org/10.1148/radiol.2242011283
Deriu G (1991) Vascular anatomy in abdominal surgery. Georg Thieme Verlag
Estrada R, Barraza G, Toledo V et al (2021) Variations of the celiac trunk in Mexican population by MDCT angiography. Eur J Anat 25(1):57–64
Goss CM (1960) Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. JB Lippincott Company
Hiatt JR, Gabbay J, Busuttil RW (1994) Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 220(1):50–52. https://doi.org/10.1097/00000658-199407000-00008
Lippert H, Pabst R (1985) Arterial variations in man. JF Bergmann Verlag, pp 30–53
Lipshutz B (1917) A composite study of the coeliac axis artery. Ann Surg 65(2):159–169. https://doi.org/10.1097/00000658-191702000-00006
Losanoff JE, Millis JM, Harland RC et al (2007) Hepato-spleno-mesenteric trunk. J Am Coll Surg 204(3):511. https://doi.org/10.1016/j.jamcollsurg.2006.07.045
Marco-Clement I, Martinez-Barco A, Ahumada N et al (2016) Anatomical variations of the celiac trunk: cadaveric and radiological study. Surg Radiol Anat 38(4):501–510. https://doi.org/10.1007/s00276-015-1542-4
Michels NA (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 112(3):337–347. https://doi.org/10.1016/0002-9610(66)90201-7
Natsis K, Piagkou M, Lazaridis N et al (2017) The coexistence of both replaced proper hepatic and gastroduodenal arteries due to the common hepatic artery absence. Surg Radiol Anat 39(11):1293–1296. https://doi.org/10.1007/s00276-017-1866-3
Rubio-Manzanares-Dorado M, Marín-Gómez LM, Aparicio-Sánchez D et al (2015) Implication of the presence of a variant hepatic artery during the Whipple procedure. Rev Esp Enferm Dig 107(7):417–422. https://doi.org/10.17235/reed.2015.3701/2015
Santos P, Barbosa A, Targino VA et al (2018) Anatomical variations of the celiac trunk: a systematic review. Arq Bras Cir Dig 31(4):e1403. https://doi.org/10.1590/0102-672020180001e1403
Suzuki T, Nakayasu A, Kawabe K et al (1971) Surgical significance of anatomic variations of the hepatic artery. Am J Surg 122(4):505–512. https://doi.org/10.1016/0002-9610(71)90476-4
Ugurel MS, Battal B, Bozlar U et al (2010) Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Br J Radiol 83(992):661–667. https://doi.org/10.1259/bjr/21236482
Vandamme JP, Bonte J, Van der Schueren G (1969) A revaluation of hepatic and cystic arteries. The importance of the aberrant hepatic branches. Acta Anat 73(2):192–209. https://doi.org/10.1159/000143296
Whitley A, Oliverius M, Kocián P et al (2020) Variations of the celiac trunk investigated by multidetector computed tomography: systematic review and meta-analysis with clinical correlations. Clin Anat 33(8):1249–1262. https://doi.org/10.1002/ca.23576
Funding
None.
Author information
Authors and Affiliations
Contributions
PR Manuscript writing, schematic drawing. SS Manuscript writing, image selection.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rana, P., Singh, S. Aberrant gastroduodenal and proper hepatic arteries. Surg Radiol Anat 43, 1421–1424 (2021). https://doi.org/10.1007/s00276-021-02774-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-021-02774-x