Abstract
Purpose
To retrospectively evaluate the effectiveness of computed tomography-guided percutaneous microwave ablation (MWA) and cementoplasty in patients with painful bone metastases at high risk of fracture.
Materials and Methods
Thirty-five patients with 37 metastatic bone lesions underwent computed tomography-guided MWA combined with cementoplasty (polymethylmethacrylate injection). Vertebrae, femur, and acetabulum were the intervention sites and the primary end point was pain relief. Pain severity was estimated by visual analog scale (VAS) before treatment; 1 week post-treatment; and 1, 6, and 12 months post-treatment. Functional outcome was assessed by improved patient walking ability. Radiological evaluation was performed at baseline and 3 and 12 months post-procedure.
Results
In all patients, pain reduction occurred from the first week after treatment. The mean reduction in the VAS score was 84, 90, 90 % at week 1, month 1, and month 6, respectively. Improved walking ability occurred in 100 and 98 % of cases at the 1- and 6-month functional outcome evaluations, respectively. At the 1-year evaluation, 25 patients were alive, and 10 patients (28 %) had died because of widespread disease. The mean reduction in the VAS score and improvement in surviving patients’ walking ability were 90 and 100 %, respectively. No patients showed evidence of local tumor recurrence or progression and pathological fracture in the treated sites.
Conclusion
Our results suggest that MWA combined with osteoplasty is safe and effective when treating painful bone metastases at high risk of fracture. The number of surviving patients at the 1-year evaluation confirms the need for an effective and long-lasting treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Painful bone metastases are a common cause of morbidity in patients with metastatic cancer and they indicate widespread disease. Therefore, the treatment goals are to palliate pain and prevent complications by reducing the length of hospital stay and improving performance status. Standard treatments include localized (radiation and surgery) and systemic therapies (chemotherapy, hormones, bisphosphonates, and analgesics) [1]. Radiation therapy is another therapeutic strategy, but 20–30 % of patients do not respond [2–6]. Because of the short life expectancy and poor quality of life in these patients, a minimally invasive approach is desirable and new therapeutic strategies that offer a curative option have recently become available. These techniques are based on image guidance to direct devices into metastatic tissue to ablate lesions without causing damage to healthy tissue, along with other procedures and agents such as alcohol, [7, 8] interstitial laser [9, 10], methylmethacrylate [11–14], cryoablation [15–17], radiofrequency [18, 19], and microwaves [20].
Our aim was to evaluate the technical effectiveness of percutaneous microwave ablation (MWA) combined with cementoplasty to treat painful bone metastases refractory to conventional approaches and at high risk of fracture.
Materials and Methods
Informed consent was obtained from all individual participants included in the study. Thirty-five patients with 37 skeletal metastatic lesions (16 men and 19 women; mean age, 64 ± 11 years) underwent computed tomography (CT)-guided percutaneous MWA and cementoplasty of symptomatic bone metastases. All patients had previously undergone standard treatments: 10 received radiotherapy, 8 received radiotherapy in combination with chemotherapy, and 17 received chemotherapy alone.
The presence of pain refractory to conventional approaches was documented in all cases and all were receiving analgesic therapy [opioids or a combination of opioids and nonsteroidal anti-inflammatory drugs (NSAIDs)]. Before the ablation treatment, pain severity was evaluated using a validated pain assessment tool, the visual analog scale (VAS), which rates pain on a continuous scale from 0–10 to indicate the intensity of the pain [21]. The use of analgesics was also recorded. Inclusion criteria were VAS score ≥4; lesions not responding to chemotherapy and/or radiation therapy at least 3 weeks before the ablation session; chemotherapy-associated complications that required discontinuation of this treatment; lesions adjacent to structures sensitive to irradiation; life expectancy greater than 2 months; and ineligibility for surgical treatment. All patients received pre-procedural contrast-enhanced CT scans to assess the location, size, and radiological aspects of the lesions. In 8 patients, there was a solitary lesion, while the remaining 27 patients had two or more lesions. The topographic distribution of the skeletal metastasis and histology of the primary malignancies are summarized in Table 1. In patients with two or more bone metastatic lesions, only those who were symptomatic were treated. Treated lesion diameter ranged from 2.0–12.0 cm (4.0 ± 2.0 cm). Lesions located in bones subjected to load (vertebrae, femoral head and neck, and acetabulum), lesions which disrupted cortical bone with tumor tissue arising from the bone, and very large osteolytic lesions were considered at high risk of fracture. In our series, all treated lesions were osteolytic, with bone destruction accompanied or not by a soft tissue mass, and at high risk of fracture. Physical examination was performed by the oncologist collaborating with the radiologist performing the ablation treatment. Analgesic use and pain symptoms (assessed by VAS score) were monitored at 1 week, 3 and 6 months after treatment, and yearly, thereafter. Drug therapy (NSAIDs and opioids) was discontinued 1 week after treatment and resumed in cases of persistence or exacerbation of painful symptoms. Functional outcome was evaluated using a qualitative scale for the assessment of patients’ walking ability that rates this ability as worse, unchanged, or improved. Radiological follow-up consisted of contrast-enhanced CT scans acquired 3 and 12 months after the procedure. Radiological imaging 3 months after treatment was performed to identify residual disease or local tumor progression.
Treatment Technique
Percutaneous MWA was performed using a 2.45 GHz microwave generator (AMICA-GEN, HS Hospital Service, Aprilia, Italy) delivering energy via a 14- or 11-gauge, mini-choked, water-cooled, interstitial antenna (AMICA-GEN). Tumors smaller than 3.5 cm maximum diameter were treated with a single 14-gauge antenna that completely destroyed the tumor. Tumors with maximum diameter larger than 3.5 cm were treated using two antennae operated simultaneously. In some patients, the bone cortex was pierced with a 10-gauge bone marrow biopsy needle (Stryker® Instruments, Kalamazoo, MI, USA), serving as a coaxial introducer for the antenna, to comfortably reach the osteolytic lesion. To protect heat-sensitive anatomical structures such as the spinal cord or nerve roots, one or more thermocouples were positioned before beginning the ablative treatment. Once the antenna was inserted into the tumor, the introducer was retracted to avoid interfering with microwave emissions by the active tip of the probe to avoid overheating the cannula during ablation, and energy delivery was then begun. We used a 20-cm-long microwave antenna to allow for sufficient retraction of the cannula from the ablation area. Once ablation was complete, the antenna was withdrawn and the introducer was left in situ and subsequently used for the osteoplastic procedure. In cases of cortical bone erosion or interruption by the tumor tissue, an 11-gauge antenna was directly inserted into the target lesions without using a coaxial introducer.
Microwave Ablation Procedure
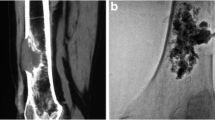
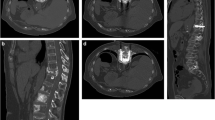
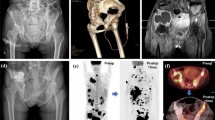
In all patients, MWA was performed under CT-guidance, with 5-mm collimation at 80–140 mA (CT system: SOMATOM Sensation, Siemens, AG, Forchheim, Germany). Dual guidance with CT and fluoroscopy was used to allow: precise needle placement, increased operator comfort, reduced rate of complications, and to avoid leakage during cement injection. A board-certified interventional radiologist performed all treatments. Throughout the procedure, patients were under conscious sedation with continuous intravenous infusion of fentanyl citrate (0.1 mg/2 mL diluted 1:10 with saline solution) and received local anesthesia comprising subcutaneous injection of 2 % lidocaine hydrochloride. The probe choice was at the discretion of the interventional radiologist and was based on several factors, including, but not limited to tumor size, morphology, location, adjacent structures, and access route. In 35 ablation sessions, a single antenna was used in 94 % of sessions (n = 33) and two antennae were used in 6 % (n = 2). Ablation treatment was combined with cementoplasty, achieved by PMMA injection (mean volume, 7.5 mL; range 3–13 mL) (Fig. 1). Plain CT was performed after the procedure and patients were immediately transferred to the recovery room for observation. All patients were discharged 24 h after treatment in stable condition and without complications.
A Axial CT scan showing extensive osteolysis of the right femoral neck with large tumor invasion of the cortical profile (arrow) in a 48-year-old man (patient 33 in Table 1). B Axial CT scan acquired after positioning the MW antenna before starting the ablation procedure. C Fluoroscopic image acquired during the combined MWA-cementoplasty: a MW antenna (white arrow) and a bone marrow biopsy needle (black arrow) were positioned within the tumor lesion and in the cortical bone, respectively, for the thermal ablation. D Fluoroscopic image showing the injection of PMMA into the bone lesion. E Axial and F coronal CT scans of the right femur demonstrating cement filling the ablation cavity. CT computed tomography, MW microwave, MWA microwave ablation, PMMA polymethylmethacrylate
Statistical Analysis
Continuous variables are shown as mean ± standard deviation (SD). Differences between the average VAS score at baseline and at month 1, 3, 6, and 12 post-procedure were evaluated using the two-tailed Student’s t test for paired data, as appropriate. p < 0.05 was considered statistically significant. All statistical analysis was performed using an OpenStat software.
Results
Technical success, defined as the ability to achieve complete ablation at the interface between the soft tissue and bone followed by osteoplasty with PMMA injection, was obtained in 100 % of cases. In all cases, a post-procedural plain CT scan was performed immediately after the procedure to identify complications such as hemorrhage. PMMA leakage occurred in 7 (20 %) of 37 patients.
Contrast-enhanced CT scans were performed 3 months after treatment to identify local tumor progression and compared with the post-procedural CT. On the day of the procedure, the mean VAS score was 6.8 ± 1.4 (range 4.0–9.0). Only 4 (11 %) of 35 patients reported an increase in pain during the first 24 h post-treatment. Clinical evaluation showed that pain symptoms were reduced as early as 1 week post-treatment with a mean VAS score of 1.1 ± 1.6 and a mean reduction of 84 % (6.8 ± 1.4 vs. 1.1 ± 1.6; p < 0.000). One month after treatment and 3 weeks after stopping patients’ usual pain therapies, the mean VAS score was 0.7 ± 1.4. Twenty-nine of 35 patients (83 %) were pain-free, while the remaining 6 (17 %) reported an average reduction of 45 % in the VAS score. These patients resumed pain treatment with NSAIDs because their symptoms were mild. Six months after the procedure, the mean VAS score was 0.6 ± 1.6, with a mean reduction of 90 %. Twenty-three of 30 patients (77 %) were symptom-free and did not resume any therapy. Six of 30 patients (20 %) were symptomatic, although the mean reduction in VAS score was 53 %. Only 1 patient (3 %) had recurrence of symptoms; this patient underwent cordotomy and died 1 month after this procedure because of disease progression in the brain. One year after MWA and cementoplasty, 25 patients were still alive and 10 patients (28 %) had died because of widespread disease. The mean VAS score in surviving patients was 0.2 ± 0.6 and 22 of 25 patients (88 %) were symptom-free; 3 patients (12 %) were symptomatic and had a mean VAS score of 2.0 (Fig. 2). During follow-up, no patients had pathological fractures even though the lesions were subjected to load and radiological local tumor progression was detected in only 1 patient. Evaluation of walking ability showed that 1 month after treatment, this was improved in all cases. Six months after treatment, walking ability was improved in 34 patients (98 %) and worse in 1 (2 %). At the 1-year follow-up, walking ability was improved in all 25 surviving patients compared with baseline.
Discussion
Bone metastases occur in approximately 20 % of cancer patients [22]. Complications from skeletal metastases include intractable pain, fracture, and decreased mobility, reducing mobility, and quality of life. In patients with cancer, pain originating from bone metastases can be difficult to treat because it is often intolerable or unresponsive to standard treatments such as radiation, surgery, and systemic therapies (chemotherapy, hormonal therapy, bisphosphonates, and analgesics) [1]. A limited number of reports using MWA to treat patients with painful metastases have demonstrated promising results [23, 24]. Our results suggest that combining MWA and cementoplasty is a safe and effective treatment for painful metastatic bone lesions. Comparing our results with other studies evaluating the efficacy of different ablation techniques (radiofrequency and cryoablation) combined with cementoplasty [25], MWA combined with cementoplasty appears to be more effective. Six months post-treatment, Masala et al. [25] reported a 79 % mean reduction in VAS score in patients treated with radiofrequency and osteoplasty and 71 % in patients treated with cryoablation and cementoplasty.
Osteolytic metastases can lead to pathological fracture and impending fracture should be treated before fracture occurs [26]. Cementoplasty is an interventional radiology technique that is most often performed alone but can also be performed with percutaneous thermal ablation techniques. Cementoplasty is performed with a palliative intent and does not stop tumor progression; it treats pain and allows fast consolidation of weight-bearing bones. Tumor tissue ablation may be complemented by cement injection with optimal results, as reported in previous studies [27–29]. The advantages of combined MWA-cementoplasty result from optimal cement distribution into the ablated tissue and bone stabilization in a single session. Cavitation after ablation of the osteolytic lesion promotes cement distribution, especially in infiltrative tumors that have extended to nearby tissues, by engulfing the extraosseous extension itself and enhancing the efficacy of the ablative technique. Our results demonstrate that combined MWA-cementoplasty is effective not only as a palliative treatment for intractable pain but, by bone stabilization, is also effective in preventing pathologic fractures caused by bone lesions subjected to load.
Regarding functional evaluation, Clarençon et al. [29] treated metastatic bone lesions with a maximum diameter of 1.1–10 cm with radiofrequency ablation with or without cementoplasty and reported functional improvement in 74 % of cases. In our study, 98 % of patients with larger lesions obtained an overall functional improvement 6 months post-procedure. Only 1 patient experienced worsened functional outcome. At the 1-year evaluation, 22 of 25 patients (88 %) were asymptomatic and all surviving patients obtained functional improvement compared with baseline. Our results show better functional outcomes than those obtained by Clarençon et al. [29] likely because of the different ablation techniques used (MWA vs. radiofrequency ablation) and the fact that cementoplasty was performed in all of our patients.
As limitations, our study was retrospective and non-randomized, and the sample size was small.
Conclusions
Our number of surviving patients at 1 year confirms the need for an effective and durable treatment for patients with painful bone metastases at high risk of fracture with the aim of avoiding further complications, such as pathological fractures. Given the promising results of MWA combined with cementoplasty demonstrated in the present study, larger investigations are indicated.
References
Janjan N. Bone metastases: approaches to management. Semin Oncol. 2001;28:28–34.
Poulsen HS, Nielsen OS, Klee M, Rorth M. Palliative irradiation of bone metastases. Cancer Treat Rev. 1989;16:41–8.
Massie MJ, Holland JC. The cancer patient with pain: psychiatric complications and their management. J Pain Symptom Manag. 1992;7:99–109.
Spiegel D, Sands S, Koopman C. Pain and depression in patients with cancer. Cancer. 1994;74:2570–8.
Jeremic B, Shibamoto Y, Acimovic L, et al. A randomized trial of three single-dose radiation therapy regimens in the treatment of metastatic bone pain. Int J Radiat Oncol Biol Phys. 1998;42:161–7.
Gaze MN, Kelly CG, Kerr GR, et al. Pain relief and quality of life following radiotherapy for bone metastases: a randomized trial of two fractionation schedules. Radiother Oncol. 1997;45:109–16.
Gangi A, Kastler B, Klinkert A, et al. Injection of alcohol into bone metastases under CT guidance. J Comput Assist Tomogr. 1994;18:932–5.
Cotten A, Demondion X, Boutry N, et al. Therapeutic percutaneous injections in the treatment of malignant acetabular osteolyses. Radiographics. 1999;19:647–53.
Gangi A, Gasser B, De Unamuno S, et al. New trends in interstitial laser photocoagulation of bones. Semin Musculoskelet Radiol. 1997;1:331–8.
Gröenemeyer DH, Schirp S, Gevargez A. Image-guided percutaneous thermal ablation of bone tumors. Acad Radiol. 2002;9:467–77.
Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. Am J Neuroradiol. 2001;22:373–81.
Cotten A, Boutry N, Cortet B, et al. Percutaneous vertebroplasty: state of the art. Radiographics. 1998;18:311–23.
Cotten A, Deprez X, Migaud H, et al. Malignant acetabular osteolyses: percutaneous injection of acrylic bone cement. Radiology. 1995;197:307–10.
Marcy PY, Palussiere J, Descamps B, et al. Percutaneous cementoplasty for pelvic bone metastasis. Support Care Cancer. 2000;8:500–3.
Cooper I. Cryogenic surgery: a new method of destruction or extirpation of benign or malignant tissues. N Engl J Med. 1963;268:743–9.
Sewell P, Jackson M, Dhillon G. Percutaneous MRI guided cryosurgery of bone tumors. Radiology. 2002;225:514.
Callstrom MR, Atwell TD, Charboneau JW, et al. Painful metastases involving bone: percutaneous image-guided cryoablation-prospective trial interim analysis. Radiology. 2006;241:572–80.
Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300–6.
Posteraro AF, Dupuy DE, Mayo-Smith WW. Radiofrequency ablation of bony metastatic disease. Clin Radiol. 2004;59:803–11.
Pusceddu C, Sotgia B, Fele RM, Melis L. Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol. 2013;24(2):229–33.
Chapman CR, Case KI, Dubner R, et al. Pain measurement: an update. Pain. 1985;22:1–31.
Wasserman J, De la Lande B, Pecking A, Brasseur A. Le patient métastatique et son environnement. Douleur et métastases osseuses. Prog Urol. 2008;18(Suppl 7):S399–409.
Janjan N. Bone metastases: approaches to management. Semin Oncol. 2001;28:28–34.
Carrafiello G, Laganà D, Pellegrino C, et al. Percutaneous imaging-guided ablation therapies in the treatment of symptomatic bone metastases: preliminary experience. Radiol Med. 2009;114(4):608–25.
Carrafiello G, Laganà D, Mangini M, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008;6(Suppl 1):S65–9.
Schaefer O, Lohrmann C, Herling M, et al. Combined radiofrequency thermal ablation and percutaneous cementoplasty treatment of a pathologic fracture. J Vasc Interv Radiol. 2002;13:1047–50.
Toyota N, Naito A, Kakizawa H, et al. Radiofrequency ablation therapy combined with cementoplasty for painful bone metastases: initial experience. Cardiovasc Intervent Radiol. 2005;28:578–83.
Georgy B, Wong W. Plasma-mediated radiofrequency ablation assisted percutaneous cement injection for treating advanced malignant vertebral compression fractures. Am J Neuroradiol. 2007;28:700–5.
Clarençon F, Jean B, Pham HP, et al. Value of percutaneous radiofrequency ablation with or without percutaneous vertebroplasty for pain relief and functional recovery in painful bone metastases. Skeletal Radiol. 2013;42:25–36.
Conflict of interest
All of the authors declare that they have no conflicts of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pusceddu, C., Sotgia, B., Fele, R.M. et al. Combined Microwave Ablation and Cementoplasty in Patients with Painful Bone Metastases at High Risk of Fracture. Cardiovasc Intervent Radiol 39, 74–80 (2016). https://doi.org/10.1007/s00270-015-1151-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-015-1151-y