Abstract
Background
There are controversies about the ability of neutrophil to lymphocyte ratio to predict the recurrence and survival in patients with locally advanced rectal cancer (LARC) treated with neoadjuvant chemoradiation. The objective of this study is to investigate the prognostic potential of combined lymphocyte count (LC) and neutrophil count (NC) in LARC patients treated with chemoradiotherapy (CRT) followed by curative surgery.
Methods
Patients with LARC who underwent surgical resection between January 2010 and December 2017 were reviewed retrospectively. We divided the patients into three groups: high LC and low NC, low LC and high NC, and the remaining patients. The cut-off values of LC and NC were determined by receiver operating characteristic curve analysis and log-rank test statistics. We compared the disease-free survival (DFS) rate between the groups.
Results
A total of 176 consecutive patients were included in this study. The 5 year DFS rate was significantly different among the three groups in pathologic node (pN)+ patients (73.2% vs. 61.9% vs. 14.2%; P = 0.025). Cox multivariate analysis for pN+ patients demonstrated that combination of low LC and high NC (hazard ratio, 3.630; 95% confidence interval [CI], 1.306–10.093; P = 0.013) was significantly correlated with decreased DFS.
Conclusions
This study showed that the combination of LC and NC is a powerful predictive factor for disease recurrence in pN+ LARC patients who underwent CRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard treatment of locally advanced rectal cancer is neoadjuvant chemoradiation therapy followed by total mesorectal excision to decrease the local recurrence. Many studies have been performed to identify prognostic factors that can predict recurrence in rectal cancer patients after radical resection. Pathological lymph node status has been considered the most powerful prognostic factor in rectal cancer after chemoradiation therapy. In particular, pathological complete response is associated with better outcome after radical resection [1]. Multiple biologic markers have been suggested as prognostic markers [2,3,4]. However, none have been validated as prognostic tools, and their application in clinical practice is limited [5,6,7].
Systemic inflammation plays a key role in tumor progression and metastasis [8]. DNA variation explains the quantitative variation of specific inflammatory immune cell types. However, the extent to which variation in inflammatory immune cell subtypes is heritable is unknown. A large number of studies have used the neutrophil to lymphocyte ratio (NLR) in patients with LARC treated with neoadjuvant chemoradiation [9,10,11,12,13,14,15]. However, there are controversies about the ability of NLR to predict recurrence in patients with LARC. While some studies demonstrated that the NLR was a significant prognostic factor for disease-free survival (DFS) [9,10,11], others suggested that the NLR did not correlate with DFS [12,13,14,15]. One possible reason for these contradictory results is the heterogeneity of patients.
Some reports have suggested that lymphocyte count (LC) or neutrophil count (NC) alone can predict recurrence in patients with LARC treated with neoadjuvant chemoradiation [16,17,18,19]. Because of the controversies about the ability of NLR to predict recurrence, we hypothesized that the combination of neutrophil and lymphoct count (NLC) can predict recurrence in patients with LARC treated with neoadjuvant chemoradiation. As far as I know, this combination method has never been reported.
The objectives of this study are to investigate the prognostic potential of NLC and develop the prognostic model in LARC patients after preoperative chemoradiotherapy (CRT).
Material and methods
Patients with LARC who underwent surgical resection at Ajou University Hospital between January 2010 and December 2017, were retrospectively reviewed. We utilized Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement [20]. We selected this period because MRI has been routinely performed to evaluate depth of invasion and regional nodal status of rectal tumors from 2010. This period overlaps that of a prior study [16]. A total of 105 patients from the previous study were included in this current study. This study included all patients with radiologic evidence of node-positive disease and most patients with T3/4 disease. Patients were excluded if distant metastasis was diagnosed by initial operation (n = 4) or if curative resection was not performed after preoperative CRT (n = 1) (Fig. 1). Data on age at diagnosis, sex, tumor location, operation type, tumor (T) stage, node (N) stage, histological grade, carcinoembryonic antigen (CEA) level, and hematologic profile were gathered without missing data. Staging was performed according to the tumor-node-metastasis (TNM) classification of the American Joint Committee on Cancer (7th edition). The study was approved by the hospital’s institutional review board (MED-MDB-20-534).
The distance from the anal verge to the lower margin of the tumor was measured by rigid proctoscopy before surgery. We defined lower rectal cancer as lesions located within 5 cm of the anal verge, whereas middle rectal cancer was defined as tumors located between 5 and 10 cm of the anal verge.
Hemoglobin, platelet count, LC, NC, NLR, CEA and BMI were analyzed to develop a prognostic model. Baseline blood samples were obtained within 2 weeks prior to CRT, and preoperative blood samples were obtained within 2 weeks prior to operation.
Preoperative radiotherapy was administered to the pelvis 5 days per week for 5 weeks with a daily fraction of 1.8 Gy. A boost of 5.4 Gy was applied to the tumor bed. During the radiotherapy, concomitant chemotherapy was performed. Chemotherapy regimen was selected from 5-fluorouracil, capecitabine, or FOLFOX. Surgery was scheduled 6–8 weeks after completion of CRT.
Patients were scheduled to visit an outpatient clinic every 3–6 months for the first 2 years after surgery, every 6 months for the next 3 years, and yearly thereafter. Physical examination and serum CEA test were performed at each visit. Chest X-ray, chest and abdominopelvic computed tomography scan, and colonoscopy were performed annually, and on suspicion of recurrence. Positron emission tomography was performed on suspicion of recurrence. Recurrence was detected by a combination of imaging studies and CEA measurements and confirmed by pathologic examination. The patients were followed up until death or the cut-off date (December 31, 2019). Median follow-up time was 48.0 months (range 4–115 months). DFS was calculated for all patients from the date of surgery until recurrence and death.
Statistics
Chi-square test or Fisher’s exact test was used to compare differences in clinicopathological features. Cut-off values for platelet count, LC, NC and NLR were determined using receiver operating characteristic (ROC) curve analysis and log-rank test statistics. Cut-off values for hemoglobin, CEA and BMI were determined according to normal clinical values. Patients were divided into two groups according to each cut-off value. The study endpoint was DFS, which was defined as the time from the date of irradiation initiation to the date of disease recurrence or to the date of last follow-up. DFS was compared using the Kaplan–Meier survival analysis with a log-rank test between groups. Among the inflammatory immune cells, we selected LC, which was significantly associated with DFS on multivariate analysis for all the patients and NC, which was significantly associated with DFS on multivariate analysis for pN+ patients. We divided patients into three groups according to LC and NC: Group 1 was patients with higher LC and lower NC; Group 3 was patients with lower LC and higher NC; and Group 2 included patients with higher LC and NC, or lower LC and NC. Linear by linear association was used to analyze the relationship between recurrence pattern and the combination group. Univariate and multivariate analyses were performed to determine prognostic predictors for disease recurrence including combination groups according to pathologic LN status. Data were analyzed using SPSS software for Windows (Version 18.0; SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological characteristics
A total of 176 consecutive patients who underwent curative resection for LARC from 2010 to 2017 were included in this study. Table 1 lists the characteristics of the patients. The 176 patients comprised 126 men and 50 women with a median age of 59 years (range 26–79 years). The optimal cut-off values for initial LC, NC, NLR and platelet count were 1.8, 6.5, 3.6, and 274, respectively. Among the 176 patients, 68 (38.6%) had low LC and 100 (56.8%) had low NC.
Univariate and multivariate analyses for DFS and sub-analysis by pathologic lymph node status
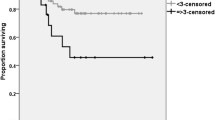
Of the 176 patients, 45 recurrences were reported, with a median time to recurrence of 14 months (range 4–59 months). The 5 year DFS rate was significantly lower in LARC patients with low LC compared with those with high LC (60.0% vs. 78.3%, P = 0.043). Patients with high NC had a significantly shorter 5 year DFS compared with those with low NC (64.0% vs. 77.3%, P = 0.036) (Fig. 2). The 5 year DFS rate was lower in patients with pT3–4, pN+ , initial CEA level > 5, and post-CRT CEA level > 5. However, the 5 year DFS rate did not show a significant difference between patients with low and high NLR (73.1% vs. 61.3%, P = 0.123) (Table 2). Multivariate analysis demonstrated that high initial LC (hazard ratio [HR], 2.037; 95% confidence interval [CI], 1.129–3.675; P = 0.018), pN+ (HR, 2.638; 95% CI, 1.414–4.925; P = 0.002), and pT3–4 (HR, 2.477; 95% CI, 1.181–5.192; P = 0.016) were significant predictors for 5 year DFS (Table 2). Multivariate analysis for pN− patients demonstrated that initial CEA (HR, 3.915; 95% CI, 1.409–10.879; P = 0.009) was a significant predictor for 5 year DFS. Multivariate analysis for pN+ patients demonstrated that female sex (HR, 2.432; 95% CI, 1.084–5.452; P = 0.031), and initial NC (HR, 2.658; 95% CI, 1.167–6.053; P = 0.020) were significant predictors for 5 year DFS (Table 3).
Correlation between clinicopathological characteristics and combination of LC and NC according to pathologic lymph node status
There was no significant difference between clinicopathological characteristics and combination in pN− rectal cancer patients. There was no significant difference except in platelet count (P = 0.015) among the three groups in pN+ rectal cancer (Table 4).
Univariate and multivariate analyses for DFS according to pathologic lymph node status
There were significant differences in DFS among three groups in all patients (P = 0.014) and pN+ patients (P = 0.025). However, there were no significant differences among three groups in pN− patients (Fig. 3). In pN− patients, pT stage (P = 0.003), initial CEA level (P = 0.005), and post-CRT CEA level (P = 0.031) were significantly associated with DFS in univariate analysis. In pN+ patients, combination was significantly associated with DFS in univariate analysis (P = 0.025) (Table 5). Multivariate analysis for pN− patients demonstrated that pT stage (HR, 4.175; 95% CI, 1.499–11.629; P = 0.006) was a significant predictor in DFS. The combination (HR, 3.630; 95% CI, 1.306–10.093; P = 0.013) remained significant in multivariate analysis for pN+ patients (Table 6).
Discussion
We found that combination of low LC and high NC was strongly predictive for disease recurrence in pN+ LARC patients who underwent CRT. Multivariate analysis demonstrated that LC alone was an independent prognostic factor for DFS. Additionally, we performed a subgroup analysis according to pathologic lymph node status. Multivariate analysis for pN+ patients showed that NC was an independent prognostic factor for DFS. Based on these results, we speculated that combination of these two markers would be a much more powerful predictor for disease recurrence compared to NLR, particularly, in pN+ patients. Therefore, we compared DFS among the three groups according to LN and NC in pN− and pN+ patients, respectively. This analysis demonstrated that combination of LC and NC measured before CRT can more accurately predict disease recurrence of LARC in pN+ patients compared with NC alone. Notably, this combination was not applicable among pN− patients. We speculate that pN+ disease is more likely to be systemic compared with pN− disease. Therefore, systemic inflammatory cells have a more important role in pN+ disease. In examining all the patients, combination of LC and NC on multivariate analysis was confirmed to be a much stronger marker for predicting disease recurrence than was LN alone.
NLR has been proposed as an easily available marker to predict recurrence in rectal cancer. However, in our study, the NLR did not correlate with DFS, in support of several studies [12,13,14,15]. There is no consensus about the cut-off values for NLR and various values were used in previous studies. The cut-off values in previous reports varies from 2.0, 2.5 3.0, 3.5, 4.0, to 5.0. We failed to find the correlation with DFS even though we analyzed with various cut-off values.
Instead of NLR, we used the combination of LC and NC as a new potential prognostic systemic inflammatory marker. We combined two-grouped lymphocyte count and neutrophil count into two-, three-, and four-grouped combination and analyzed DFS according to the combination groups. Although two- and three-grouped combinations showed definite prognostic differences, we selected three-grouped combination because of clinical usefulness. The combination of these inflammatory markers resulted in a powerful predictor for DFS in pN+ rectal cancer. Our results indicate that this combination can more precisely represent systemic inflammatory immune status than can NLR. Interestingly, analysis of recurrence patterns showed the ratio of local recurrence to distant metastasis increases as the group increases. These recurrence patterns were shown in Fig. 4 and Table 7.
This study showed that pathologic N and T status are strongly associated with recurrence-free survival in LARC treated with preoperative CRT. Dinaux et al. suggested that persistent nodal involvement after neoadjuvant therapy is associated with increased risk of distant metastases and shorter DFS [21].
Although pathologic N and T stages were strongly associated with recurrence of LARC, these factors did not predict recurrence before CRT. In contrast, baseline LC and combination with NC were predictors of recurrence of LARC before CRT. Okugawa et al. suggested that a low pre-CRT lymphocyte-C-reactive protein ratio was a stronger indicator of early recurrence, particularly for rectal cancer without pathological lymph node metastasis [22].
Based on sub-analysis, we demonstrated that pathologic T stage was an independent prognostic factor for DFS in rectal cancer without lymph node metastasis. In rectal cancer with lymph node metastasis, combination of LC and NC was an independent prognostic factor for DFS.
This study has several limitations. This was a single-institution study with a retrospective design. In addition, prospective validation was lacking. However, these data were collected prospectively in an electronic database and updated regularly.
Preoperative LC and a combination with NC in patients undergoing curative surgery for colorectal cancer are simple and inexpensive means of identifying patients with a potentially poor prognosis. Early detection of recurrence could allow resection of metastatic lesions in rectal cancer patients, and the factors predicting recurrence are useful in treating LARC. However, the optimal values of LC and NC for predicting recurrence remain to be determined.
Conclusions
This study showed that combination of LC and NC was a powerful prognostic factor in pN+ LARC patients who underwent CRT. Further studies using a larger sample size are warranted to confirm the prognostic role of this combination marker in LARC patients. Prospective validation in future studies is needed to demonstrate the prognostic ability of this inflammatory marker.
References
Maas M, Nelemans PJ, Valentini V et al (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–844
Karagkounis G, Kalady MF (2017) Molecular biology: are we getting any closer to providing clinically useful information? Clin Colon Rectal Surg 30:415–422
Zhang Z, Wang S, Ji D et al (2018) Construction of a ceRNA network reveals potential lncRNA biomarkers in rectal adenocarcinoma. Oncol Rep 39:2101–2113
Rampazzo E, Del Bianco P, Bertorelle R et al (2018) The predictive and prognostic potential of plasma telomerase reverse transcriptase (TERT) RNA in rectal cancer patients. Br J Cancer 118:878–886
Conde-Muíño R, Cuadros M, Zambudio N, Segura-Jiménez I, Cano C, Palma P (2015) Predictive biomarkers to chemoradiation in locally advanced rectal cancer. Biomed Res Int 2015:921435
Seicean R, Funariu G, Seicean A (2004) Molecular prognostic factors in rectal cancer. Rom J Gastroenterol 13:223–231
Zeestraten EC, Kuppen PJ, van de Velde CJ, Marijnen CA (2012) Prediction in rectal cancer. Semin Radiat Oncol 22:175–183
Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V (2012) Multifaceted link between cancer and inflammation. Biosci Rep 32:1–15
Ke TM, Lin LC, Huang CC, Chien YW, Ting WC, Yang CC (2020) High neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predict poor survival in rectal cancer patients receiving neoadjuvant concurrent chemoradiotherapy. Medicine 99:e19877
Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC (2012) Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis 14:e701–e707
Nagasaki T, Akiyoshi T, Fujimoto Y et al (2015) Prognostic impact of neutrophil-to-lymphocyte ratio in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy. Dig Surg 32:496–503
Portale G, Cavallin F, Valdegamberi A, Frigo F, Fiscon V (2018) Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio are not prognostic biomarkers in rectal cancer patients with curative resection. J Gastrointest Surg 22:1611–1618
Shen J, Zhu Y, Wu W et al (2017) Prognostic role of neutrophil-to-lymphocyte ratio in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Med Sci Monit 23:315–324
Dudani S, Marginean H, Tang PA et al (2019) Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer 19:664
Lino-Silva LS, Salcedo-Hernández RA, Ruiz-García EB, García-Pérez L, Herrera-Gómez Á (2016) Pre-operative neutrophils/lymphocyte ratio in rectal cancer patients with preoperative chemoradiotherapy. Med Arch 70:256–260
Oh SY, Heo J, Noh OK, Chun M, Cho O, Oh YT (2018) Absolute lymphocyte count in preoperative chemoradiotherapy for rectal cancer: changes over time and prognostic significance. Technol Cancer Res Treat 17:1533033818780065
Liu H, Wang H, Wu J, Wang Y, Zhao L, Li G, Zhou M (2019) Lymphocyte nadir predicts tumor response and survival in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: immunologic relevance. Radiother Oncol 131:52–59
Diefenhardt M, Hofheinz RD, Martin D et al (2019) Leukocytosis and neutrophilia as independent prognostic immunological biomarkers for clinical outcome in the CAO/ARO/AIO-04 randomized phase 3 rectal cancer trial. Int J Cancer 145:2282–2291
Meng X, Shi D, Zheng H, Xu Y, Li Q, Cai S, Cai G (2017) High preoperative peripheral blood neutrophil predicts poor outcome in rectal cancer treated with neoadjunctive chemoradiation therapy. Int J Clin Exp Pathol 10:7718–7725
Collins GS, Reitsma JB, Altman DG (2015) Moons KG (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Br J Cancer 112:251–259
Dinaux AM, Leijssen L, Bordeianou LG, Kunitake H, Amri R, Berger DL (2018) Outcomes of persistent lymph node involvement after neoadjuvant therapy for stage III rectal cancer. Surgery 163:784–788
Okugawa Y, Toiyama Y, Fujikawa H et al (2020) Prognostic potential of lymphocyte-C-reactive protein ratio in patients with rectal cancer receiving preoperative chemoradiotherapy. J Gastrointest Surg 25:492–502
Author information
Authors and Affiliations
Contributions
SYO contributed to design of this paper, analysis of data, revising this paper and final approval. SY contributed to design of this paper, analysis of data, revising this paper and final approval. YO contributed to design of this paper, analysis of data, revising this paper and final approval.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoon, S., Oh, Y. & Oh, S.Y. Clinical Implications of Combined Lymphocyte and Neutrophil Count in Locally Advanced Rectal Cancer After Preoperative Chemoradiotherapy. World J Surg 45, 2591–2600 (2021). https://doi.org/10.1007/s00268-021-06126-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06126-z