Abstract
Purpose
The systemic inflammatory response is attracting increasing attention as a predictive biomarker for oncological outcome in patients with colorectal cancer. This study is aimed at verifying if the lymphocyte–C-reactive protein (CRP) ratio (LCR) could be used as a predictor of oncological outcome in patients with rectal cancer (RC) receiving preoperative chemoradiotherapy (CRT).
Methods
We analyzed data for 86 patients with RC who received preoperative CRT followed by total mesorectal excision at our institution. A ratio of 6000 was used as the cut-off value for LCR for further analysis.
Results
The post-CRT LCR was significantly lower than the pre-CRT LCR in patients with RC. Although post-CRT LCR status was not significantly correlated with overall survival (OS), low pre-CRT LCR was significantly associated with shorter recurrence-free survival (RFS: p = 0.02) and OS (p = 0.017) in this population and was an independent prognostic factor for both RFS and OS (hazard ratio (HR) 3.19, 95% confidence interval (CI) 1.33–7.66, p = 0.009; HR 2.83, 95%CI 1.14–7.01, p = 0.025, respectively). Furthermore, low pre-CRT LCR was a stronger indicator of early recurrence (p = 0.001) and poor prognosis (p = 0.025) in RC patients without pathological lymph node metastasis compared with patients with pathological lymph node metastasis, and prognostic potential of pre-CRT LCR was clearly revealed especially RC patients receiving long-course CRT.
Conclusions
Assessment of pretreatment LCR status might aid decision-making regarding postoperative treatment strategies in patients with RC receiving CRT followed by potentially curative resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in Western countries.1 Rectal cancer (RC) has a high frequency of local recurrence, and preoperative chemoradiotherapy (CRT) followed by total mesorectal excision (TME) is currently the recommended standard treatment strategy in patients with advanced RC.2 Indeed, preoperative irradiation together with advanced surgical techniques has led to around a 10% decrease in the local recurrence rate of RC.3,4,5,6 However, recurrence of distant metastasis remains the major factor associated with a poor prognosis in RC patients.3, 6,7,8,9 Precise biomarkers are thus urgently needed to identify high-risk patients who might benefit from close follow-up and more intensive adjuvant chemotherapy, in order to improve the prognosis of RC patients receiving preoperative CRT.
We recently developed the lymphocyte–C-reactive protein (CRP) ratio (LCR) as a new predictive biomarker for oncological outcome in CRC patients.10 We comprehensively and systematically assessed the prognostic value of various combinations of inflammatory factors in preoperative blood samples and revealed the predictive value of preoperative LCR for recurrence and survival in CRC patients, using an optimal cut-off value of 6000. However, the clinical feasibility of pretreatment and preoperative LCR in RC patients receiving preoperative CRT followed by curative surgery remains unknown. In the current study, we directly compared pre- and post-CRT LCR statuses in RC patients receiving preoperative CRT and investigated the clinical predictive value of LCR using the previously determined cut-off value of 6000.10
Materials and Methods
Patients
We enrolled 86 patients with RC who received preoperative CRT followed by TME at our institution between 2001 and 2015. The criteria for preoperative CRT were as follows: (a) patients with tumors in the lower two-thirds of the rectum; (b) stage > T2 or T1 (tumor invading to submucosa) with clinical N1 (to improve resectability and likelihood of successful sphincter-saving procedure); (c) age 20–80 years; (d) Eastern Cooperative Oncology Group performance status of 0 or 1; (e) no invasion of external sphincter muscle or anal elevator muscle; and (f) no evidence of deep venous thrombosis, as described previously.11 Pretreatment clinical stage was assessed by digital examination, transrectal ultrasonography, computed tomography, and magnetic resonance imaging. All patients were classified according to the International Union Against Cancer TNM Classification (7th edition). Of the 86 patients, 16 had stage II, and 70 had stage III disease before undergoing CRT. Written informed consent was obtained from each patient, and the study was approved by the institutional review boards of our institution.
5-Fluorouracil-Based CRT Regimen
The 86 patients were treated randomly with short-course (n = 35, 20 Gy in 4 fractions) or long-course (n = 51, 45 Gy in 25 fractions) radiotherapy, using a four-field box technique.12 All patients also received concurrent 5-fluorouracil (5-FU)–based chemotherapy, including 5-FU/leucovorin and tegafur/uracil, with S-1 as a sensitizer drug.13 The mean interval between the completion of CRT and surgery was 2–3 weeks for patients receiving the short course and 6–8 weeks for the long course. Surgical approaches included laparotomy and laparoscopic surgery, with standard curative resection based on TME. All patients received 5-FU-based adjuvant chemotherapy for 6 months to 1 year after surgery. Patients were followed up every 12–16 weeks for at least 1 year, using our standard protocol including tumor marker studies, computed tomography, endoscopic examinations, ultrasonography, and chest radiography. Bone scans were performed if bone metastasis was indicated. Data collected from inpatient and outpatient records included age and sex and tumor-specific data including histology, T classification, lymphatic and venous invasion, and lymph node metastasis (LNM).
Laboratory Measurement of LCR
Blood samples were collected from the enrolled patients within 1 week prior to CRT (pre-CRT) and within 1 week prior to surgery after CRT (post-CRT). CRP levels and total lymphocytes were analyzed in routine blood tests, and LCR was calculated according to the formula: total lymphocytes (number/μl)/CRP (mg/dl) (as described previously).10 Based on the previous study,10 we used 6000 as the cut-off value for LCR to clarify the clinical impact of LCR in RC patients receiving preoperative CRT.
Tumor Regression After CRT and Pathological Staging Using Surgical Specimens
All surgical specimens were analyzed histopathologically, and pathologic TNM classification and staging were determined according to the American Joint Committee on Cancer. The histopathologic degree of tumor regression was based on the Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Colorectum and was classified into four categories: grade 0, no necrosis or regressive changes; grade 1a, > two-thirds vital residual tumor cells (VRTCs) and grade 1b, approximately one-third VRTCs; grade 2, < one-third VRTCs; and grade 3, no VRTCs.14 Non-responders were defined as patients with histopathological tumor regression grades 0–1b, and responders as those with grades 2–3, as described previously.11,15, 16
Statistical Methods
Statistical analysis was performed using MedCalc version 16.8.4 (Broekstreet 52, 9030; Mariakerke, Belgium). Differences between groups were estimated by χ 2 tests. Differences between pre- and post-CRT LCR levels in the same group of patients were compared using Wilcoxon’s test for paired samples. Regarding time-to-event analyses, survival estimates were calculated by Kaplan–Meier analysis and groups were compared with log-rank tests. Overall survival (OS) was measured from the date of the patient’s surgery until the date of death from any cause (i.e., cancer-unrelated deaths were not censored) or the date of last known follow-up for patients who were still alive. Recurrence-free survival (RFS) was measured from the date of curative surgery to the date of disease recurrence or until the last contact with the patient. Cox proportional hazards models were used to estimate hazard ratios (HR) for recurrence and death. Assumptions of proportionality were confirmed for the Cox proportional hazards analyses by generating Kaplan–Meier survival curves (e.g., high vs. low LCR groups) and ensuring that the two curves did not intersect each other. Variables with p < 0.05 in univariate analysis were selected for multivariate analysis using the Cox proportional hazards regression model. In addition to target LCR status, the following confounding factors that reportedly affected prognosis in RC were also considered for univariate and multivariate analyses: sex, age at diagnosis, pathological differentiation (well–moderate or poor), T stage (T1/2 or T3/4), venous invasion (present or absent), lymphatic vessel invasion (present or absent), LNM (present or absent), pathological regression grade for CRT, operative procedure (APR or others), and surgical approach (laparoscopic surgery or open surgery). All p values were two-sided and p < 0.05 was considered significant.
Results
Comparison Between Peri-treatment LCR Statuses and Correlations with Clinical Variables in Patients with RC
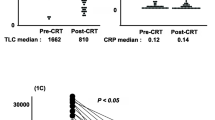
We initially assessed the differences between pre- and post-CRT LCR levels in our cohort. Post-CRT LCR was significantly reduced compared with pre-CRT LCR (p < 0.0001) (Fig. 1a). Regarding the correlations between pre- and post-LCR statuses and peri-CRT clinical variables, no clinical variables were significantly correlated with post-CRT LCR status. However, decreased pre-CRT LCR or post-CRT LCR was significantly correlated with male sex (p = 0.017, 0.03, respectively, compared with female, Table 1). Other examined factors, including advanced T category, presence of venous and lymphatic vessel invasion, LNM, advanced TNM stage classification, and treatment course of preoperative radiation, were not correlated with pre-CRT LCR status in RC patients.
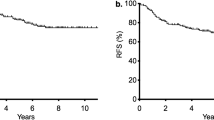
Differences between pre- and post-CRT LCR statuses and their prognostic impacts of in patients with RC undergoing preoperative CRT. a Post-CRT LCR levels were significantly reduced compared with pre-CRT LCR levels (p < 0.0001, Wilcoxon’s test). b, c Kaplan–Meier survival curves for RFS (b) and OS (c) in RC patients based on pre-CRT LCR levels. Patients with low pre-CRT LCR status according to a cut-off value of 6000 had significantly poorer prognoses than those with a high pre-CRT LCR status in terms of both RFS and OS (log-rank test, p = 0.02, 0.017, respectively). d, e Kaplan–Meier survival curves for RFS (d) and OS (e) in RC patients. Although decreased post-CRT LCR was significantly correlated with poor RFS (p = 0.045, log-rank test, d), there was no significant correlation between post-CRT LCR levels based on a cut-off value of 6000 and OS (p = 0.07, log-rank test, e). All statistical tests were two-sided
Low Pre-CRT LCR Status Was Significantly Correlated with Shorter RFS and OS in Patients with RC
We performed time-to-event analyses to evaluate the prognostic values of pre- and post-CRT LCR levels. According to Kaplan–Meier survival curves subdivided by LCR scores (cut-off value 6000), patients with low pre-CRT LCR had significantly shorter RFS (p = 0.02, log-rank test; Fig. 1b) and OS (p = 0.017; Fig. 1c) than patients with higher pre-CRT LCR. Although decreased post-CRT LCR was significantly correlated with poor RFS (p = 0.045; Fig. 1d), there was no significant correlation with OS (p = 0.07; Fig. 1e).
We determined the potential of pre-CRT LCR status as a predictive biomarker of recurrence and prognosis in RC patients receiving CRT using multivariate Cox regression analysis. This analysis showed that decreased LCR status, the presence of pathological LNM (ypN(+)), and operative procedure (APR) were independent prognostic factors for RFS in RC patients with preoperative CRT (HR 3.19, 95% confidence interval (CI) 1.33–7.66, p = 0.009; HR 6.26, 95%CI 1.91–20.6, p = 0.003; HR 6.5, 95%CI 2.45–17.3, p = 0.002, respectively, Table 2). Furthermore, decreased LCR status, the presence of pathological LNM (ypN(+)), and operative procedure (APR) were also independent prognostic factors for OS in RC patients (HR 2.83, 95%CI 1.14–7.01, p = 0.025; HR 6.01, 95%CI 2.03–17.8, p = 0.001; HR 5.32, 95%CI 1.46–19.5, p = 0.011, respectively, Table 3).
Pre-CRT LCR Status Could Identify a Population at High Risk of Oncological Outcomes Among RC Patients Without Pathological LNM Receiving CRT
Multivariate analysis identified the presence of pathological LNM (ypN+) as an independent prognostic factor for both DFS and OS in RC patients with CRT. We therefore assessed the prognostic impact of pre-CRT LCR status with respect to pathological LNM to identify patients requiring aggressive chemotherapy after curative resection, especially among low-risk ypN− RC patients. Among patients with pathological TNM stages I–II (ypN−), low pre-CRT LCR was significantly associated with shorter RFS and OS compared with high pre-CRT LCR (p = 0.001, 0.025, respectively, log-rank test; Fig. 2a, b). However, although low post-CRT LCR status was significantly associated with poorer OS (p = 0.018, log-rank test), there was no significant correlation between post-CRT LCR status and RFS in these patients (Fig. 2c, d). Furthermore, multivariate analyses identified low pre-CRT LCR as an independent risk factor for poor prognosis, especially in terms of RFS in RC patients without pathological LNM (RFS, HR 9.19, 95%CI 1.78–47.6, p = 0.008; OS, HR 2.83, 95%CI 0.62–12.9, p = 0.18, Tables 4 and 5).
Prognostic impacts of pre- and post-CRT LCR statuses in patients with RC without pathological LNM (ypN−). a, b Kaplan–Meier survival curves for RFS (a) and OS (b) in RC patients without pathological LNM (ypN−) based on pre-CRT LCR levels using a cut-off value of 6000. Patients with low pre-CRT LCR showed poorer survival in terms of both RFS and OS (p = 0.001, 0.025, respectively, log-rank test) compared with patients with high pre-CRT LCR among patients with RC without pathological LNM (ypN−). c, d Kaplan–Meier survival curves for RFS (c) and OS (d) in patients with RC without pathological LNM (ypN−) based on post-CRT LCR levels using a cut-off value of 6000. Low post-CRT LCR status was significantly associated with poor OS (p = 0.018) but did not predict poor RFS in these patients (p = 0.18). e, f Kaplan–Meier survival curves for RFS (e) and OS (f) in patients with RC based on LNM and pre-CRT LCR statuses. Survival curve analysis for pre-CRT LCR combined with ypN status clearly revealed risk stratification for RFS (p < 0.0001) and OS (p < 0.0001, log-rank test) in patients with RC. All statistical tests were two-sided
In contrast, among patients with pathological stage III (ypN+), low post-CRT LCR status was significantly correlated with poor RFS (p = 0.017; log-rank test). However, other correlation showed that low LCR status was not significantly correlated with either RFS or OS (Supplementary Figure 1a-d). Interestingly, survival curve analysis of pre-CRT LCR combined with ypN status clearly revealed risk stratification for recurrence and survival in patients with RC (Fig. 2e, f). Notably, the survival curves for patients with ypN+, high pre-CRT LCR crossed those for patients with ypN−, low pre-CRT LCR for both RFS and OS.
Pre-CRT LCR Status Could Identify a Population at High Risk of Oncological Outcome Especially in RC Patients Receiving Long-Course CRT
We further evaluated dysregulation pattern and prognostic impact of LCR levels subdivided by short- and long-course CRT in RC patients. Consistent with the total cohort, post-CRT LCR was significantly reduced compared with pre-CRT LCR in both short- and long-course CRT groups (p = 0.009, 0.007, respectively, Supplementary Figure 2a, b). Next, we evaluated the prognostic impact of pre- and post-LCR status in RC patients subdivided by CRT regimens to investigate on impact of treatment times and LCR levels in these patients. Interestingly, survival curve analysis demonstrated no significant differences between high- and low LCR levels both pre- or post-CRT timing in RC patients receiving short-course CRT (pre-CRT: RFS: p = 0.82, OS: p = 0.55; post-CRT: RFS: p = 0.93, OS: p = 0.61, respectively, Supplementary Figure 3). In contrast, in RC patients receiving long-course CRT, patients with low pre-CRT or post-CRT LCR showed poorer RFS and OS compared with those with high pre-CRT or post-CRT LCR (pre-CRT: RFS: p = 0.0001, OS: p = 0.005; post-CRT: RFS: p = 0.021, OS: p = 0.007, respectively, Supplementary Figure 3). Furthermore, multivariate analysis revealed that decreased pre-CRT LCR status was an independent prognostic factor for both RFS and OS in RC patients receiving long-course CRT (HR 7.37, 95%CI 1.68–32.4, p = 0.008; HR 13.4, 95%CI 1.28–140, p = 0.03, respectively, Tables 6 and 7). Overall, these findings indicated that pre-CRT LCR with a cut-off value of 6000 could identify patients with RC receiving preoperative CRT who were at high risk of oncological outcomes.
Discussion
The systemic inflammatory responses caused by host–tumor interaction and host nutritional status are currently gathering attention as potential predictive biomarkers for recurrence and prognosis in patients with various malignancies, including CRC.17 Indeed, various systemic inflammation-based markers, such as CRP, neutrophil–lymphocyte ratio, and platelet–lymphocyte ratio, have recently demonstrated prognostic values in RC patients receiving preoperative CRT.18,19,20 Although emerging studies have indicated various molecules, including genetic and epigenetic molecules, as feasible “liquid biopsy” markers in CRC,21 markers that can be identified using simple blood tests with consistent cut-off values are likely to have greater actual clinical benefits. We recently developed the LCR as a new inflammation–nutrition marker in CRC and revealed that an optimal cut-off value of LCR allowed the identification of a subset of CRC patients at high risk of poor oncological outcomes, including recurrence and survival. However, the prognostic impact and clinical significance of LCR in RC patients receiving CRT remain unclear.
In the current study, we systemically investigated the clinical feasibility of preoperative LCR using clinical data derived from a large cohort of RC patients receiving CRT. First, we found that post-CRT LCR was significantly reduced compared with pre-CRT LCR in patients with RC. Furthermore, although post-CRT LCR was not significantly correlated with OS, low pre-CRT LCR status, based on the previously determined cut-off value of 6000,10 was significantly correlated with both poor RFS and OS. Second, decreased pre-CRT LCR and the presence of pathological LNM emerged as independent predictors of poor RFS and OS in RC patients receiving preoperative CRT. Third, low pre-CRT LCR was also a convenient predictor of poor prognosis and early recurrence, especially in RC patients without pathological LNM, and was an independent risk factor for prognosis, especially RFS in ypN− RC patients, who are less likely to require postoperative chemotherapy than ypN+ RC patients. Finally, we further evaluated the prognostic potential of pre- or post-LCR in RC patients subdivided by CRT course and revealed that prognostic potential of pre-CRT LCR was clearly revealed especially in RC patients receiving conventional long-course CRT.
Serum CRP is a well-recognized systemic inflammatory response marker, and previous studies have revealed the potential of CRP as a prognostic biomarker in RC patients receiving preoperative CRT.19, 22 Toiyama and colleagues directly compared the prognostic potentials of several inflammatory markers, including neutrophil–lymphocyte ratio, platelet–lymphocyte ratio, and CRP, in 84 RC patients, and reported that elevated CRP level in pre-CRT serum was the only independent predictive factor for both DFS and OS. Kim and coworkers recently identified preoperative CRP as the strongest predictive factor for cancer-specific survival in 125 patients with RC who received TME after CRT. Peripheral lymphocytes also play a pivotal role in immunosurveillance in antitumor reactions and have been established as a blood-based marker reflecting nutritional status.23 Based on these background studies, circulating pretreatment lymphocytes and combined scoring systems including lymphocytes have been demonstrated as feasible prognostic markers of oncological outcomes in RC patients receiving CRT.24, 25 Kitayama et al. investigated the clinical burden of subsets of white blood counts before and after radiation treatment and demonstrated that a low lymphocyte percentage was significantly correlated with a poorer prognosis in terms of DFS and OS in RC patients.25 Absolute lymphocyte count at baseline was also recently shown to be an independent favorable prognostic marker in RC patients treated with preoperative CRT.24 Consistent with these results, the current study confirmed that pre-CRT LCR using a consistent cut-off value10 could identify a subpopulation of RC patients receiving preoperative CRT who were at high risk of recurrence and poor survival.
The current study also showed that low pre-CRT LCR was an independent prognostic factor for RFS in RC patients without pathological LNM. Neoadjuvant chemoradiotherapy followed by curative surgery including TME is currently recognized as the standard treatment for locally advanced RC.26 Although accumulating evidence has revealed that ypN status is one of the major prognostic factors in RC patients receiving preoperative CRT,27 the clinical benefit of postoperative adjuvant chemotherapy remains controversial, especially in RC patients without pathological LNM.28,29,30 Considering these results, a selection method for high-risk RC patients without pathological LNM after neoadjuvant CRT followed by TME, such as pre-CRT LCR, could identify those patients who need adjuvant chemotherapy to inhibit recurrence and improve their prognosis.
Currently, preoperative radiotherapy for rectal cancer is recognized as two main standards, including short-course radiation therapy (RT) and conventional long-course RT. Although pathological response in surgical specimens for preoperative RT was superior in long-course RT compared with that in short-course RT, previous studies revealed no difference between these two therapies in terms of survival and local recurrence.31,32,33 Considering the differences of treatment course and duration of interval from CRT to surgery, we further evaluated the prognostic potential of pre- or post-CRT LCR in RC patients subdivided by CRT course to make a more impactful statement after standardization of CRT course in our study. Interestingly, prognostic potential of pre-CRT LCR was clearly revealed especially in RC patients receiving conventional long-course CRT. Recently, we investigated a direct comparison between short- and long-course CRT in clinicopathological responses and oncological outcome in RC patients.34 In this study, the presence of lymph node metastasis was a predictor of poor DFS in short-course CRT, whereas poor pathological response was a predictor of recurrence in long-course CRT due to the radiation effect of long treatment course. Considering this evidence with our findings, identification of patients with high risk for poor oncological outcomes using pre-CRT LCR might be necessary to discriminate candidates from RC patients receiving preoperative CRT who might benefit from more intensive adjuvant therapy after curative surgery.
There are several potential limitations to this study. First, although we could demonstrate several novel findings about preoperative LCR in RC patients, this was a relatively small and retrospective nature of study. Furthermore, all of the patients enrolled in this study were from a single institution in Japan. Second, the lack of clear selection criteria for selection of short- or long-course CRT caused different patient characteristics between two groups. The difference in patient characteristics between the two groups was based on the customary use of long-course CRT for the purpose of tumor shrinkage to allow curative resection. To overcome these limitations, a larger multi-institutional prospective study, including standardization of selection criteria for treatment course and longer follow-up, is needed to validate our feasible results in this study and to strengthen as a predictive biomarker of pre-CRT LCR in RC patients.
Conclusion
This study highlighted the clinical feasibility of LCR as a predictive biomarker for oncological outcome in patients with RC receiving preoperative CRT. Quantification of pretreatment LCR status using an optimal cut-off value could help physicians to make decisions regarding postoperative treatment strategies in RC patients undergoing CRT followed by potentially curative resection.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi:https://doi.org/10.3322/caac.20138.
Ludmir EB, Palta M, Willett CG, Czito BG. Total neoadjuvant therapy for rectal cancer: An emerging option. Cancer. 2017;123(9):1497–506. doi:https://doi.org/10.1002/cncr.30600.
Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet (London, England). 2009;373(9666):811–20. doi:https://doi.org/10.1016/s0140-6736(09)60484-0.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23. doi:https://doi.org/10.1056/NEJMoa060829.
Cedermark B, Dahlberg M, Glimelius B, Pahlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980–7. doi:https://doi.org/10.1056/nejm199704033361402.
Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246(5):693–701. doi:https://doi.org/10.1097/01.sla.0000257358.56863.ce.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40. doi:https://doi.org/10.1056/NEJMoa040694.
MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet (London, England). 1993;341(8843):457–60.
Gollins S, Sebag-Montefiore D. Neoadjuvant Treatment Strategies for Locally Advanced Rectal Cancer. Clin Oncol (R Coll Radiol). 2016;28(2):146–51. doi:https://doi.org/10.1016/j.clon.2015.11.003.
Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ide S, Kitajima T et al. Lymphocyte-C-reactive Protein Ratio as Promising New Marker for Predicting Surgical and Oncological Outcomes in Colorectal Cancer. Annals of Surgery. 2019. doi:https://doi.org/10.1097/sla.0000000000003239.
Toiyama Y, Inoue Y, Kawamura M, Kawamoto A, Okugawa Y, Hiro J et al. Elevated platelet count as predictor of recurrence in rectal cancer patients undergoing preoperative chemoradiotherapy followed by surgery. International surgery. 2015;100(2):199–207. doi:https://doi.org/10.9738/intsurg-d-13-00178.1.
Cedermark B, Johansson H, Rutqvist LE, Wilking N. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer. 1995;75(9):2269–75.
Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JI, Yamamura T et al. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer research. 2001;61(3):1029–37.
Japanese Research Society for Cancer of the Colon and Rectum. Kanehara T. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. 2006.
Inoue Y, Saigusa S, Hiro J, Toiyama Y, Araki T, Tanaka K et al. Clinical significance of enlarged lateral pelvic lymph nodes before and after preoperative chemoradiotherapy for rectal cancer. Molecular and Clinical Oncology. 2016;4(6):994–1002. doi:https://doi.org/10.3892/mco.2016.855.
Okugawa Y, Toiyama Y, Oki S, Ide S, Yamamoto A, Ichikawa T et al. Feasibility of Assessing Prognostic Nutrition Index in Patients With Rectal Cancer Who Receive Preoperative Chemoradiotherapy. JPEN Journal of Parenteral and Enteral Nutrition. 2018;42(6):998–1007. doi:https://doi.org/10.1002/jpen.1041.
Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future oncology (London, England). 2010;6(1):149–63. doi:https://doi.org/10.2217/fon.09.136.
Nagasaki T, Akiyoshi T, Fujimoto Y, Konishi T, Nagayama S, Fukunaga Y et al. Prognostic Impact of Neutrophil-to-Lymphocyte Ratio in Patients with Advanced Low Rectal Cancer Treated with Preoperative Chemoradiotherapy. Digestive Surgery. 2015;32(6):496–503. doi:https://doi.org/10.1159/000441396.
Toiyama Y, Inoue Y, Saigusa S, Kawamura M, Kawamoto A, Okugawa Y et al. C-reactive protein as predictor of recurrence in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Anticancer Research. 2013;33(11):5065–74.
Gu X, Gao XS, Qin S, Li X, Qi X, Ma M et al. Elevated Platelet to Lymphocyte Ratio Is Associated with Poor Survival Outcomes in Patients with Colorectal Cancer. PloS one. 2016;11(9):e0163523. doi:https://doi.org/10.1371/journal.pone.0163523.
Okugawa Y, Grady WM, Goel A. Epigenetic Alterations in Colorectal Cancer: Emerging Biomarkers. Gastroenterology. 2015;149(5):1204–25.e12. doi:https://doi.org/10.1053/j.gastro.2015.07.011.
Kim WR, Han YD, Min BS. C-Reactive Protein Level Predicts Survival Outcomes in Rectal Cancer Patients Undergoing Total Mesorectal Excision After Preoperative Chemoradiation Therapy. Annals of surgical oncology. 2018;25(13):3898–905. doi:https://doi.org/10.1245/s10434-018-6828-4.
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–48. doi:https://doi.org/10.1016/j.immuni.2004.07.017.
Oh SY, Heo J, Noh OK, Chun M, Cho O, Oh YT. Absolute Lymphocyte Count in Preoperative Chemoradiotherapy for Rectal Cancer: Changes Over Time and Prognostic Significance. Technology in cancer research & treatment. 2018;17:1533033818780065. doi:https://doi.org/10.1177/1533033818780065.
Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC cancer. 2011;11:64. doi:https://doi.org/10.1186/1471-2407-11-64.
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. The New England journal of medicine. 2001;345(9):638–46. doi:https://doi.org/10.1056/NEJMoa010580.
Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Saltz LB et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer. 2008;113(1):57–64. doi:https://doi.org/10.1002/cncr.23516.
Chung MJ, Lee JH, Lee JH, Kim SH, Song JH, Jeong S et al. Adjuvant Chemotherapy in Rectal Cancer Patients Treated With Preoperative Chemoradiation and Total Mesorectal Excision: A Multicenter and Retrospective Propensity-Score Matching Study. International journal of radiation oncology, biology, physics. 2019;103(2):438–48. doi:https://doi.org/10.1016/j.ijrobp.2018.09.016.
Garlipp B, Ptok H, Benedix F, Otto R, Popp F, Ridwelski K et al. Adjuvant treatment for resected rectal cancer: impact of standard and intensified postoperative chemotherapy on disease-free survival in patients undergoing preoperative chemoradiation-a propensity score-matched analysis of an observational database. Langenbeck’s archives of surgery. 2016;401(8):1179–90. doi:https://doi.org/10.1007/s00423-016-1530-0.
Govindarajan A, Reidy D, Weiser MR, Paty PB, Temple LK, Guillem JG et al. Recurrence rates and prognostic factors in ypN0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Annals of surgical oncology. 2011;18(13):3666–72. doi:https://doi.org/10.1245/s10434-011-1788-y.
Ceelen W, Fierens K, Van Nieuwenhove Y, Pattyn P. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer: a systematic review and meta-analysis. Int J Cancer. 2009;124(12):2966–72. doi:https://doi.org/10.1002/ijc.24247.
De Caluwe L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. The Cochrane database of systematic reviews. 2013(2):CD006041. doi:https://doi.org/10.1002/14651858.CD006041.pub3.
Latkauskas T, Paskauskas S, Dambrauskas Z, Gudaityte J, Saladzinskas S, Tamelis A et al. Preoperative chemoradiation vs radiation alone for stage II and III resectable rectal cancer: a meta-analysis. Colorectal Dis. 2010;12(11):1075–83. doi:https://doi.org/10.1111/j.1463-1318.2009.02015.x.
Yamamoto A, Toiyama Y, Okugawa Y, Saigusa S, Ide S, Fujikawa H et al. Identification of Predictors of Recurrence in Patients with Lower Rectal Cancer Undergoing Neoadjuvant Chemotherapy: A Direct Comparison of Short-Course and Long-Course Chemoradiotherapy. Oncology. 2019;96(2):70–8. doi:https://doi.org/10.1159/000492617.
Acknowledgments
We thank Susan Furness, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written informed consent was obtained from each patient, and the study was approved by the institutional review boards of our institution.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okugawa, Y., Toiyama, Y., Fujikawa, H. et al. Prognostic Potential of Lymphocyte–C-Reactive Protein Ratio in Patients with Rectal Cancer Receiving Preoperative Chemoradiotherapy. J Gastrointest Surg 25, 492–502 (2021). https://doi.org/10.1007/s11605-019-04495-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04495-4