Abstract
Background
The incidence of papillary thyroid microcarcinoma (PTMC) has increased over the past decade. The American Thyroid Association (ATA) suggests that these patients may undergo either thyroid lobectomy or active surveillance. It remains unclear whether there exists a subgroup of PTMC patients who may benefit from more aggressive treatment due to increased risk of recurrence.
Methods
We retrospectively reviewed 357 patients with PTMC who underwent surgery at a single institution from 2004 to 2016. Patients were classified according to 2015 ATA risk stratification for structural disease recurrence. Demographic, oncologic, and clinicopathologic data were compared between groups.
Results
Out of 357 patients, 246 were classified as low-risk PTMC, 93 were intermediate-risk, and 18 were high-risk. There were more male patients in the high-risk group (38.9%) than the intermediate- (31.2%) or low-risk groups (15.4%) (p < 0.001). Patients with low-risk microcarcinomas were more likely to have an incidental PTMC when compared to intermediate- or high-risk groups (98[39.8%], 15[16.1%], 1[5.6%], respectively, p < 0.001). Patients with high-risk PTMCs, compared to those with intermediate- and low-risk PTMCs, were more likely to have rising postoperative thyroglobulin levels after total thyroidectomy (6[40.0%], 4[5.1%], 9[5.7%], respectively, p = 0.001) and structural recurrence after lobectomy or total thyroidectomy (3[16.7%], 0[0%], 0[0%], respectively, p < 0.001). The median follow-up time was 17.5 (IQR 3–55) months.
Conclusions
Patients with high-risk PTMC have an increased risk of recurrence when compared to low- and intermediate-risk microcarcinomas, whereas intermediate-risk PTMC may behave similarly to low-risk tumors. ATA risk stratification may inform clinical decision making for patients with PTMC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most common endocrine malignancy worldwide, with a rising incidence in the past few decades, specifically for papillary thyroid carcinoma (PTC). This increase in PTC diagnoses is largely due to tumors measuring less than one centimeter, which are defined as papillary thyroid microcarcinomas (PTMCs) [1,2,3,4]. It has been suggested that increased use of cervical ultrasound and fine needle aspiration (FNA) has resulted in this rise in incidence; nevertheless, the prognosis is excellent due to the indolent nature of the disease [3, 5, 6]. As such, the traditional management of immediate thyroid surgery for PTMC without high-risk features is being reconsidered.
Although PTMC is generally associated with an excellent prognosis, 0.5% patients may die of PTMC [7]. Loco-regional recurrence rates and distant recurrence rates are reported to be around 2–6% and 1–2%, respectively [8]. While these recurrence rates are relatively low, there remains a subset of patients with PTMC who present with clinically significant disease and carry an increased risk of mortality [9,10,11]. Yu et al. noted that age greater than 45 years, male sex, minority race, extrathyroidal extension, positive lymph nodes, and distant metastases have been associated with a poor overall survival in PTMC patients [12].
The 2015 American Thyroid Association (ATA) thyroid cancer management guidelines provide a stratification system to determine the risk of structural recurrence in patients being treated for differentiated thyroid cancer. They developed both a continuum of risk and a three-tiered risk system in order to aid in classifying these patients [13]. The most recent ATA guidelines continue to advise a risk-directed approach in the management of PTMCs. Thyroid lobectomy or an active surveillance protocol, which involves serial imaging studies and thyroglobulin (Tg) measurements, are suggested treatment options for PTMC without clinically or radiographically evident metastases or local invasion [6, 13,14,15]. However, the guidelines do not provide specific recommendations with regard to patient selection or whether completion thyroidectomy is advised in patients who fall into higher-risk stratifications. Thus, we sought to compare recurrence rates between low-risk, intermediate-risk, and high-risk PTMC and evaluate the applicability of the 2015 ATA risk stratification system in these subcentimeter tumors.
Methods
The study was approved by the institutional review board at Weill Cornell Medicine. A retrospective review and analysis were performed on the medical records of all consecutive patients with PTMC who underwent surgery at a single academic tertiary referral center between 2004 and 2016. All adult patients (≥18 years old) diagnosed with PTMC on final pathology were included in this study. Study participants underwent a total thyroidectomy with or without prophylactic or therapeutic central neck and lateral neck dissection or thyroid lobectomy. Prophylactic central neck lymph node dissection was performed routinely at our institution for all biopsy-proven thyroid cancers until 2015. Lateral neck lymph node dissection was only performed in the presence of biopsy-confirmed lateral neck lymph node metastases. Patients who did not have any lymph nodes removed were classified as Nx.
Surgical pathology records were reviewed, and PTMC tumors were classified as low, intermediate, or high risk of recurrence according to the 2015 ATA risk stratification (Table 1). Briefly, intermediate-risk PTMC was defined as tumors meeting any of the following criteria: microscopic extrathyroidal extension, >5 positive lymph nodes at least 0.2 cm in largest dimension or those with clinically positive lymph nodes, vascular invasion, or aggressive histologic variants (tall cell, diffuse sclerosing, solid, oncocytic, clear cell, and columnar variants). High-risk PTMC was defined as tumors with gross extrathyroidal extension, incomplete tumor resection, any positive lymph node ≥3 cm, or distant metastases. The remaining patients were classified as low risk.
The clinical and pathologic features in the three groups were also reviewed, along with the therapeutic outcomes of the patients. Data extracted from patients’ medical records included: age at time of diagnosis, sex, race, ethnicity, family history of thyroid cancer, personal radiation history, incidental carcinoma (noncancerous indication for surgery), date of surgery, extent of operation, radioactive iodine (RAI) therapy, postoperative thyroglobulin level, presence of loco-regional or distant metastases, length of follow-up, and mortality.
Information on the most recent duration of follow-up and the timing of disease recurrence were collected. By adapting the system used by Tuttle et al., biochemical recurrence was defined as a significant rise in nonstimulated serum thyroglobulin levels (≥1 ng/mL) in patients without elevated anti-thyroglobulin antibodies who underwent a total thyroidectomy with or without RAI treatment. In patients with elevated anti-thyroglobulin antibodies, biochemical recurrence was defined as a rise in postoperative levels. Structural tumor recurrence was detected by radiographic or nuclear imaging (ultrasound, computed tomography, whole-body radioactive iodine scan, positron emission tomography) [16].
Data extracted from pathology reports included: tumor size (mm), histologic variant, multifocality, presence of microscopic extrathyroidal extension, presence of vascular invasion, margin status, and presence of lymph node metastases (number and dimension). Operative reports were reviewed to identify gross extrathyroidal extension and incomplete tumor resection.
All data and statistical tests were carried out using Stata (version 13, StataCorp, College Station, TX). Descriptive statistics were used to summarize the data. Quantitative data were expressed as median and interquartile range. Categorical data were represented as a percentage or frequency. Fisher’s exact test was used for categorical variables, and Kruskal–Wallis test was used for parametric continuous data. A two-tailed p value less than 0.05 was considered statistically significant.
Results
A total of 357 patients with PTMC were identified during the study period (Table 2). The majority of patients had PTMCs classified as low risk (n = 246), followed by intermediate risk (n = 93), and high risk (n = 18). There were relatively more female patients in the low-risk group than the intermediate-risk or high-risk groups (208 [84.6%], 64 [68.8%], and 11 [61.1%], respectively, p = 0.001), and the low-risk group had more Hispanic patients. There were no other significant differences in demographics between groups.
Most patients underwent a total thyroidectomy (269, 75.3%). Low-risk patients were more likely to undergo a hemithyroidectomy than intermediate-risk and high-risk patients (75 [30.5%] vs. 12 [12.9%] and 1 [5.6%], respectively, p = 0.002), while high-risk patients were more likely to undergo total thyroidectomy (Table 3). Furthermore, intermediate-risk and high-risk patients underwent a prophylactic central neck dissection as part of their procedure more often than low-risk patients (p = 0.003) and intermediate-risk patients were more likely to get a therapeutic central neck dissection (p < 0.001). High-risk patients were also more likely to undergo a modified lateral neck dissection (p < 0.001). Carcinomas found incidentally were more common in patients with low-risk PTMC (39.8%) than intermediate-risk (16.1%) or high-risk (5.6%) groups (p < 0.001).
Table 4 identifies the prevalence of specific features that classify patients into intermediate-risk and high-risk PTMC groups. The most common feature of intermediate-risk patients was microscopic extrathyroidal extension (41, 44.1%) followed by aggressive histologic subtype (30, 32.2%). The majority of high-risk patients were classified as such due to positive margins (17, 94.4%).
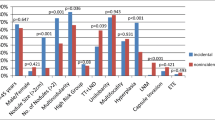
Intermediate-risk and high-risk patients had slightly larger tumors when compared to low-risk patients (7.0 mm [IQR 6–8 mm] and 7.0 mm [IQR 6–8 mm] vs. 6.0 mm [IQR 3–8 mm], p < 0.001). Gross extrathyroidal extension and positive margins were solely found in high-risk patients, as they are high-risk defining characteristics. The prevalence of vascular invasion and microscopic extrathyroidal extension was not different between intermediate-risk and high-risk patients. Interestingly, the frequency of the BRAFV600E mutation was not significantly different between groups (p = 0.761), while intermediate-risk patients were significantly more likely to have multifocal tumors when compared to low-risk tumors (p < 0.001) (Table 5). While the majority of patients in all risk groups had classic PTMC, the histology distribution between the groups varied (p < 0.001). Intermediate-risk patients had the highest frequency of tall cell (16.1%) and oncocytic (7.5%) variants, and both intermediate- and high-risk groups included patients with tall cell and diffuse sclerosing histology (Fig. 1).
Our cohort had a median follow-up of 17.5 months (Table 5). More patients with intermediate-risk and high-risk PTMCs received postoperative RAI compared with low-risk PTMCs (p < 0.001). Of patients who underwent RAI treatment, low-risk patients had an overall lower median dose when compared to intermediate-risk and high-risk patients (p < 0.001). High-risk patients were significantly more likely to have rising thyroglobulin levels when compared with intermediate-risk and low-risk patients (6 [40.0%], 4 [5.1%], 9 [5.7%], respectively, p = 0.001). In contrast, biochemical recurrence was not significantly different between low-risk patients and intermediate-risk patients (p = 1.000). Furthermore, only high-risk patients had structural recurrence (p < 0.001) (Fig. 2).
There was a total of three patients with structural recurrence. All three patients were male and underwent a total thyroidectomy for cancer as their index operation. Two patients also underwent a central neck dissection. There were two patients with local recurrence in their thyroid beds and one patient with distant metastases to the lung. Upon pathologic review, each patient had classical histology, microscopic extrathyroidal extension, and multifocal disease. Two patients were positive for the BRAFV600E mutation, while one did not undergo testing. All three patients underwent postoperative radioactive iodine treatment, and all three had rising thyroglobulin levels (Table 6).
Discussion
PTMC has been increasing in incidence over the past few decades, and the approach to management of subcentimeter tumors has been widely debated. Current guidelines recommend either thyroid lobectomy or active surveillance in properly selected patients. For those patients undergoing lobectomy, it is unclear whether the ATA risk stratification for recurrence risk in larger tumors is applicable to PTMCs. Therefore, in this study we sought to investigate the risk of recurrence in patients with PTMC based on the current 2015 ATA risk stratification in order to evaluate its applicability.
We showed that in our cohort, intermediate-risk patients did not have any structural recurrence with a median follow-up period of 25 months. Furthermore, intermediate-risk patients were less likely to have biochemical recurrence when compared with high-risk patients (4 [5.1%] vs 6 [40%], p = 0.001). This suggests that perhaps patients found to have intermediate-risk characteristics on final pathologic examination are more likely to have indolent tumors amenable to lobectomy alone. Furthermore, the grossly invasive features of high-risk PTMCs may predict an increased risk of recurrence similar to that seen in patients with tumors greater than one centimeter.
Stratifying patients preoperatively is a challenge, as many intermediate- and high-risk features are only apparent at the time of surgery or with final pathology. There have been several studies that have attempted to identify risk factors for developing aggressive disease in PTMC [11, 17,18,19,20,21,22]. Mercante et al. investigated 445 PTMCs and found that microscopic extrathyroidal tumor extension and cervical lymph node metastases at presentation were independent risk factors for structural recurrence. Furthermore, patients who did not exhibit these features and were treated with lobectomy or isthmusectomy alone did not experience structural recurrence [19]. In 1999, Sugitani et al. analyzed 178 patients with PTMC. They noted that gross extrathyroidal invasion, large lymph node metastases (≥2 cm), and poorly differentiated components in the primary tumor were significantly related to adverse outcomes. On the contrary, age, gender, tumor size, multifocality, and number of involved lymph nodes did not affect survival. In 148 patients without clinically apparent lymph node metastases and/or recurrent laryngeal involvement, neither distant metastasis nor cause-specific death was observed [23]. These studies are consistent with our results, as all three structural recurrences in this cohort had high-risk features that were clinically evident at the time of presentation or surgery such as positive margins or lymph node metastases at presentation.
Microscopic extrathyroidal extension, which is not clinically apparent preoperatively or at the time of surgery, was a commonly found pathologic factor in the intermediate-risk cohort (44.1%) where no patients developed structural recurrence. While this was found to be associated with an increased risk of recurrence by Mercante, microscopic extrathyroidal extension alone, without other aggressive features, has been shown in other studies to not confer the increased risk of recurrence seen with gross extrathyroidal extension [24].
Thirty patients in the intermediate-risk PTMC cohort had the intermediate-risk feature of aggressive histology, and fifteen of these were upgraded from low risk solely due their histologic subtype. When comparing the high-risk patients who exhibited recurrence, all three had classical variant PTMC, and there were no recurrences in intermediate-risk patients with aggressive histology. This could suggest that histology without invasive features may not increase risk of recurrence in these small tumors. Utilizing the SEER database, Kazure et. al. found that aggressive histologic variants of PTC were associated with higher rates of extrathyroidal extension, multifocality, and nodal/distant metastases. Furthermore, they observed that aggressive histology was associated with a significantly reduced survival, and that surgery plus RAI improved survival for these patients. However, they did not subdivide their classes into histology with and without invasive features [25]. In another study utilizing the SEER database, Kuo et al. specifically compared classical variant, tall cell variant, and diffuse sclerosing variant PTMC. They found that there were higher rates of invasive features, such as extrathyroidal extension, multifocality, and nodal metastasis, in the aggressive histology group when compared to classical PTMC. However, there was no association between histologic variant alone and disease-specific survival [26]. Further evaluation of the independent impact of aggressive histology alone on prognosis is warranted.
PTMC has excellent outcomes with a low disease-specific mortality (<1%) and low rate of recurrence (2–6%). This is most likely a result of the indolent nature of the disease rather than current treatment regimens [4,5,6, 15, 27]. Ito et al. have pioneered the active surveillance approach to PTMCs. Their study prospectively followed 1465 patients with PTMC who underwent active surveillance. Observation was offered to patients who had a favorable anatomic position of their cancer, FNA without high-risk features, and no lymph node metastases or signs of progression at follow-up. Most patients exhibited a stable tumor size, while just 5% showed tumor enlargement by ultrasound at 5 years. In addition, 191 underwent surgical treatment for tumor enlargement and lymph node metastases and only one patient developed tumor recurrence [5, 6, 13]. In a prospective study by Sugitani et al., 230 patients with asymptomatic PTMC underwent nonsurgical observation over a mean follow-up of 5 years. No patients during this time developed extrathyroidal invasion or distant metastasis, while only 22 lesions (7%) had increased in size. Only younger age was noted to be related to increases in size. Other factors, such as serum thyroglobulin at diagnosis, serum anti-thyroglobulin antibodies, and multifocality, were not associated with outcome [28]. Thus, active surveillance may be an acceptable choice in patients with intermediate-risk PTMC with features such as aggressive histology or microscopic extrathyroidal extension that would not be evident preoperatively. However, more aggressive treatment is advisable in patients with gross lymph node metastases or gross extrathyroidal extension, both high-risk defining features that may be evident on preoperative ultrasound. Alternatively, these would be evident intraoperatively or at final pathology, in which case a completion thyroidectomy may be advisable.
Our study has several limitations. In addition to being a retrospective review at a single institution, we had a limited median follow-up of 17.5 months which may not be long enough to identify long-term recurrence and survival. Furthermore, as expected, the sample size for the high-risk PTMC subtype is small relative to the other subtypes. This restricts our ability to identify other clinicopathologic factors that may be significantly different between the three subtypes or characterize independent risk factors for recurrence. Additionally, the small number of structural recurrences (3/357, <1% of patients) makes it difficult to elucidate specific conclusions about recurrence risk as a whole. Furthermore, as the guidelines for treatment have changed over time, more patients underwent lobectomies and fewer patients were treated with RAI therapy during the course of the study period, and thus, treatment was not uniform among our cohort of patients. Moreover, intermediate-risk PTMC patients received RAI therapy at higher doses when compared to low-risk PTMC patients, which may play a role in treatment outcomes. However, Kim et al. showed that total thyroidectomy and RAI therapy did not necessarily prevent tumor recurrence in intermediate-risk PTMC patients (defined as microscopic extrathyroidal extension, cervical lymph node metastases, or multifocality) [29]. Additionally, in 2016 Hu et al. performed a systemic review and meta-analysis of studies examining the effectiveness of RAI in patients with PTMC. Their analysis concluded that there was almost no positive treatment effect for PTMC patients, and thus, RAI ablation may not be beneficial at decreasing ten-year recurrence rate or disease-specific mortality in patients undergoing near total or total thyroidectomy [30]. Finally, although biochemical recurrences were identified through measurement of thyroglobulin and anti-thyroglobulin antibodies, there is little evidence in measuring thyroglobulin levels in patients who underwent total thyroidectomy without RAI treatment. The 2015 ATA guidelines suggest that periodic serum thyroglobulin measurements while on hormone therapy can be considered during follow-up and rising thyroglobulin values over time can be suspicious for cancer [13, 31, 32].
In conclusion, in this study cohort, patients with ATA high-risk PTMC had an increased risk of both biochemical and structural recurrence when compared to those with low-risk or intermediate-risk microcarcinomas. Patients with low-risk and intermediate-risk PTMCs did not have any structural recurrence. High-risk PTMC should be treated similarly to tumors larger than one centimeter due to the increased risk of structural recurrence. While the results may suggest low- and intermediate-risk PTMC should be treated similarly, further prospective studies correlating long-term recurrence rates in patients undergoing current treatment recommendations of thyroid lobectomy alone should be conducted.
References
Lim H, Devesa SS, Sosa JA et al (2017) Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317:1338–1348. https://doi.org/10.1001/jama.2017.2719
Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167. https://doi.org/10.1001/jama.295.18.2164
Vigneri R, Malandrino P, Vigneri P (2015) The changing epidemiology of thyroid cancer: why is incidence increasing? Curr Opin Oncol 27:1–7. https://doi.org/10.1097/CCO.0000000000000148
Leboulleux S, Tuttle RM, Pacini F, Schlumberger M (2016) Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol 4:933–942. https://doi.org/10.1016/S2213-8587(16)30180-2
Ito Y, Tomoda C, Uruno T et al (2004) Papillary microcarcinoma of the thyroid: how should it be treated? World J Surg 28:1115–1121. https://doi.org/10.1007/s00268-004-7644-5
Ito Y, Uruno T, Nakano K et al (2003) An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 13:381–387. https://doi.org/10.1089/105072503321669875
Sorrentino F, Atzeni J, Romano G et al (2010) Differentiated microcarcinoma of the thyroid gland. G Chir 31:277–278
Mazzaferri EL (2007) Management of low-risk differentiated thyroid cancer. Endocr Pract 13:498–512. https://doi.org/10.4158/EP.13.5.498
Frangos S, Iakovou IP, Marlowe RJ et al (2015) Difficulties in deciding whether to ablate patients with putatively “low-intermediate-risk” differentiated thyroid carcinoma: do guidelines mainly apply in the centres that produce them? Results of a retrospective, two-centre quality assurance study. Eur J Nucl Med Mol Imaging 42:2045–2055. https://doi.org/10.1007/s00259-015-3124-4
Dhir M, McCoy KL, Ohori NP et al (2018) Correct extent of thyroidectomy is poorly predicted preoperatively by the guidelines of the American Thyroid Association for low and intermediate risk thyroid cancers. Surgery 163:81–87. https://doi.org/10.1016/j.surg.2017.04.029
Gao R, Jia X, Liang Y et al (2019) Papillary thyroid micro carcinoma: the incidence of high-risk features and its prognostic implications. Front Endocrinol (Lausanne) 10:74. https://doi.org/10.3389/fendo.2019.00074
Yu X-M, Wan Y, Sippel RS, Chen H (2011) Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg 254:653–660. https://doi.org/10.1097/SLA.0b013e318230036d
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133. https://doi.org/10.1089/thy.2015.0020
Kwon H, Jeon MJ, Kim WG et al (2017) A comparison of lobectomy and total thyroidectomy in patients with papillary thyroid microcarcinoma: a retrospective individual risk factor-matched cohort study. Eur J Endocrinol 176:371–378. https://doi.org/10.1530/EJE-16-0845
Kim TY, Shong YK (2017) Active surveillance of papillary thyroid microcarcinoma: a mini-review from Korea. Endocrinol Metab (Seoul) 32:399–406. https://doi.org/10.3803/EnM.2017.32.4.399
Tuttle RM, Tala H, Shah J et al (2010) Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 20:1341–1349. https://doi.org/10.1089/thy.2010.0178
Cho J-K, Kim J-Y, Jeong C-Y et al (2012) Clinical features and prognostic factors in papillary thyroid microcarcinoma depends on age. J Korean Surg Soc 82:281–287. https://doi.org/10.4174/jkss.2012.82.5.281
Zheng W, Wang X, Rui Z et al (2019) Clinical features and therapeutic outcomes of patients with papillary thyroid microcarcinomas and larger tumors. Nucl Med Commun 40:477–483. https://doi.org/10.1097/MNM.0000000000000991
Mercante G, Frasoldati A, Pedroni C et al (2009) Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid 19:707–716. https://doi.org/10.1089/thy.2008.0270
Cheng F, Chen Y, Zhu L et al (2019) Risk factors for cervical lymph node metastasis of papillary thyroid microcarcinoma: a single-center retrospective study. Int J Endocrinol 2019:8579828. https://doi.org/10.1155/2019/8579828
Jeon MJ, Kim WG, Choi YM et al (2016) Features predictive of distant metastasis in papillary thyroid microcarcinomas. Thyroid 26:161–168. https://doi.org/10.1089/thy.2015.0375
Siddiqui S, White MG, Antic T et al (2016) Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid 26:807–815. https://doi.org/10.1089/thy.2015.0429
Sugitani I, Fujimoto Y (1999) Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J 46:209–216
Arora N, Turbendian HK, Scognamiglio T, et al. (2008) Extrathyroidal extension is not all equal: Implications of macroscopic versus microscopic extent in papillary thyroid carcinoma. Surgery 144:942–7; discussion 947. doi: 10.1016/j.surg.2008.07.023
Kazaure HS, Roman SA, Sosa JA (2012) Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol 19:1874–1880. https://doi.org/10.1245/s10434-011-2129-x
Kuo EJ, Goffredo P, Sosa JA, Roman SA (2013) Aggressive variants of papillary thyroid microcarcinoma are associated with extrathyroidal spread and lymph-node metastases: a population-level analysis. Thyroid 23:1305–1311. https://doi.org/10.1089/thy.2012.0563
Brito JP, Ito Y, Miyauchi A, Tuttle RM (2016) A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid 26:144–149. https://doi.org/10.1089/thy.2015.0178
Sugitani I, Toda K, Yamada K et al (2010) Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 34:1222–1231. https://doi.org/10.1007/s00268-009-0359-x
Kim HJ, Kim NK, Choi JH et al (2013) Radioactive iodine ablation does not prevent recurrences in patients with papillary thyroid microcarcinoma. Clin Endocrinol (Oxf) 78:614–620. https://doi.org/10.1111/cen.12034
Hu G, Zhu W, Yang W et al (2016) The effectiveness of radioactive iodine remnant ablation for papillary thyroid microcarcinoma: a systematic review and meta-analysis. World J Surg 40:100–109. https://doi.org/10.1007/s00268-015-3346-4
Durante C, Montesano T, Attard M et al (2012) Long-term surveillance of papillary thyroid cancer patients who do not undergo postoperative radioiodine remnant ablation: is there a role for serum thyroglobulin measurement? J Clin Endocrinol Metab 97:2748–2753. https://doi.org/10.1210/jc.2012-1123
Ibrahimpasic T, Nixon IJ, Palmer FL et al (2012) Undetectable thyroglobulin after total thyroidectomy in patients with low- and intermediate-risk papillary thyroid cancer–is there a need for radioactive iodine therapy? Surgery 152:1096–1105. https://doi.org/10.1016/j.surg.2012.08.034
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
The study was approved by the institutional review board at Weill Cornell Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stefanova, D.I., Bose, A., Ullmann, T.M. et al. Does the ATA Risk Stratification Apply to Patients with Papillary Thyroid Microcarcinoma?. World J Surg 44, 452–460 (2020). https://doi.org/10.1007/s00268-019-05215-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05215-4