Abstract
Objectives
Pancreatic fistula after distal pancreatectomy (DP) remains an unsolved problem, and postoperative CT imaging often demonstrates fluid collection (FC) around the pancreatic remnant. This study sought to clarify the clinical implications of FC.
Methods
This study enrolled 146 patients who underwent DP. FC was defined as a cyst-like lesion ≥ 10 mm in diameter on CT imaging at postoperative day (POD) 7. FC size, irregularity of FC margin, and air bubbles in FC were investigated. In addition, clinical data were retrospectively collected, and useful predictive factors for postoperative pancreatic fistula (POPF) were analyzed.
Results
Clinically relevant POPF was observed in 26 patients (17.8%), and FC was detected in 136 patients (94.4%). Multivariate analysis identified FC size and drain amylase levels on POD3 as significant risk factors for POPF. Cutoff values were determined by ROC analyses, and the levels of the FC size and drain amylase on POD3 were determined as 41 mm and 1026 IU/L, respectively. The sensitivity and specificity of FC diameters > 41 mm were 76.9% and 75.0%, respectively, while those of drain amylase levels > 1026 IU on POD3 were 73.1% and 75.8%, respectively.
Conclusions
While treating some FCs after DP was necessary for the management of POPF, others did not require any intervention since most of them spontaneously disappeared. FC size and drain amylase levels on POD3 were found to be significantly associated with POPF and could potentially help to determine appropriate treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postoperative pancreatic fistula (POPF) is one of the most important complications after pancreatic resection and is associated with several other complications such as intra-abdominal abscess formation, hemorrhage, and sepsis. In particular, POPF after distal pancreatectomy (DP) remains an unsolved problem, with a reported incidence of 12–33% for clinically relevant POPF [1,2,3,4].
POPF requires aggressive intervention in some cases, making early detection vital. Clinical symptoms indicative of POPF cases include abdominal pain, fever, purulent discharge, and inflammatory markers in blood tests. Computed tomography (CT) is the most useful modality for screening of abdominal events during DP including abdominal abscess, free air, inflammation, and bleeding [5,6,7], and has often revealed peripancreatic fluid collection (FC) postoperatively [8,9,10]. Some FCs require invasive treatments if a high amylase level is demonstrated on re-aspiration, because it indicates delayed pancreatic fistula [11], while other FCs do not cause any clinical problems and can go untreated. This retrospective study thus aimed to clarify the clinical implications of FC after DP, focusing in particular on the features of FC on CT imaging.
Materials and methods
Patients and data collection
From January 2006 to December 2017, 183 patients underwent DP at our institute. Of these, one patient was excluded because she had undergone pancreatoduodenectomy before DP. We performed CT scans at postoperative day (POD) 7 after introducing a defined clinical pathway to detect abdominal events such as abscess formation, aneurysm, bleeding, and POPF. Of the 183 patients who underwent DP, 146 were enrolled in this study. The other 37 patients did not receive CT scans at POD7 during the transition period of the prescribed pathway and were therefore excluded from this study.
The following patient information was obtained from a prospective database combined with the hospital’s electronic patient records system: age, gender, body mass index (BMI), primary disease, tumor size, thickness of the pancreas at resection line, operation duration, estimated blood loss, stump closure methods for the pancreatic remnant, grade of POPF, duration of drainage, and postoperative hospital stay. Blood tests were routinely conducted on POD1, 3, and 5. Drain amylase level was examined on POD1 and 3. Informed consent was obtained from all individual participants included in the study. This study was approved by the Human Research Ethics Committee of Tokyo Medical and Dental University (No. 1080).
Surgical procedure
For cases of pancreatic ductal adenocarcinomas and tumors with malignant potential, the pancreas was transected at the level of the portal vein with radical lymph node dissection and splenectomy. The pancreas was transected with a tri-stapler or with a scalpel. After a scalpel resection, the pancreatic remnant was closed by hand-sewn suture using the fish mouth technique, following ligation of the main pancreatic duct. The pancreatic stump was also completely wrapped with an absorbable polyglycolic acid (PGA) sheet and fibrin sealant for some patients at the discretion of the attending surgeon.
Drain management
A drainage tube (Blake drains, Ethicon, Somerville, NJ, USA) was placed around the pancreatic stump via the left subphrenic space, and the postoperative drainage volume was recorded daily. Previous studies reported the importance of early drain removal [12,13,14]; however, they did not mention the risk of delayed pancreatic fistula [11]. We carefully placed the drainage tube for 5 days or more after surgery even if the drain amylase levels were low on POD1 and POD3. If no infection was observed by POD 5–7, the drain was removed.
Definition of POPF
POPF was determined according to criteria established by the 2016 update of the International Study Group on Pancreatic Fistula (ISGPS) [15], wherein POPF includes grade B and C (clinically relevant POPF). The former grade A POPF is regarded as a biochemical leak (BL) in the updated criteria.
Assessment of peripancreatic FC and measurement of pancreatic thickness
Contrast-enhanced CT scans were performed on POD7. FC was defined as a cyst-like lesion ≥ 10 mm in diameter with a typical cyst-like appearance located at the pancreatic resection margin [8]. The maximum diameter of FC was evaluated on the axial viewing, with an FC diameter < 10 mm or lack of FC defined as no FC. In addition to the size of FC, we also evaluated other characteristics including whether the FC margin on CT imaging was smooth or irregular and whether air bubbles were present. Pancreatic thickness of the transection line of the pancreas was measured on postoperative CT imaging. Two reviewers analyzed the CT images (D.B and J.Y), and average values were adopted for the FC diameter and pancreatic thickness.
Statistical analysis
To assess the risk factors for clinically relevant POPF, clinical factors were compared using the Mann–Whitney U test and the Chi-squared test. Variables with a P value < 0.05 were incorporated into a multivariate analysis, which used a logistic regression model to examine the factors associated with clinically relevant POPF and optimal cutoff values determined by receiver operating characteristic (ROC) curve analysis. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software version 25 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
The perioperative characteristics and postoperative outcomes of the 146 patients enrolled in this study are detailed in Table 1. The median patient age was 65 years (range, 19–90 years), and the male-to-female ratio was 70:76. Laparoscopic distal pancreatectomy was performed in 57 patients (39.0%), and the spleen was preserved in 10 patients (6.8%). In terms of primary disease, 37 patients had pancreatic ductal carcinoma (25.3%), 54 had neuroendocrine tumor (37.0%), 24 had intraductal papillary mucinous neoplasm (16.4%), 15 had mucinous cystic neoplasm (10.3%), 7 had serous cystic neoplasm (3.8%), and 12 had other diseases (10.4%). The median tumor size was 20 mm (6–110 mm), and 33 patients (22.6%) had diabetes mellitus before surgery. Clinically relevant POPF developed in 26 patients (17.8%), and all patients had grade B POPF. No patients died within 90 days after surgery.
The features of FC around the pancreatic stump on CT imaging
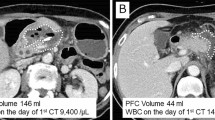
CT imaging revealed FC in 138 patients among the 146 patients (94.5%), and we evaluated the FC characteristics on these scans. The median FC diameter was 34.3 (0–137.6) mm. Figure 1a shows a well-demarcated FC, confined to a limited area by the cystic wall and containing no air bubbles. On the other hand, Fig. 1b shows an FC with poor boundaries and an irregular margin that was unclearly extended into the peripancreatic space with the presence of air bubbles. An irregular margin and air bubbles in the FC were detected in 90 patients (61.6%) and 56 patients (38.3%), respectively.
Representative CT images showing fluid collections around the pancreatic stump after distal pancreatectomy. CT images on postoperative day 7 show FC at the resection margin after distal pancreatectomy, with doubled-headed arrows showing the FC size. a. The FC was well demarcated and confined to a limited area by the cystic wall, with no apparent air bubbles (white arrowhead). b. The FC had poor boundaries and an irregular margin and was clearly extended into peripancreatic space with air bubbles (white arrowhead)
Analysis of risk factors for POPF
Univariate analysis identified the use of PGA sheet and fibrin sealant, amount of blood loss, drain amylase levels on POD1 and POD3, and the size of FC as significant risk factors for POPF (Table 2). By multivariate analysis, drain amylase level on POD3 and the size of FC were found to be significant independent risk factors for clinically relevant POPF (Table 3). The cutoff values were determined by ROC analyses, and the level of the FC size and drain amylase levels on POD3 were determined as 41 mm and 1026 IU/L, respectively. The area under curves (AUC) of the FC size and drain amylase level on POD3 were 0.76 and 0.72, respectively (Fig. 2).
Receiver operating characteristic (ROC) curves based on the size of fluid collection and drain amylase on POD3 for postoperative pancreatic fistula after distal pancreatectomy. The cutoff values were determined by ROC analyses, and the level of the FC size and the drain amylase on POD3 were determined as 41 mm and 1026 IU/L, respectively. The area under curves (AUC) of the FC size and the drain amylase level on POD3 were 0.76 and 0.72, respectively
Risk classification of patients after DP based on fluid collection and drain amylase levels on POD3
Patients were classified by the cutoff values for the FC size and drain amylase level on POD3 (Fig. 3). The red squares and blue circles indicate patients with and without clinically relevant POPF, respectively. Patients were classified into the following three subgroups based on the risk of POPF according to FC size and drain amylase level on POD3: High-risk patients had an FC size ≥ 41 mm and drain amylase levels ≥ 1026 IU/L on POD3; low-risk patients with an FC size < 41 mm and drain amylase levels < 1026 IU/L on POD3; and, all other patients were defined as intermediate risk. The incidence of POPF was 60.9% (14/23) in the high-risk group, 19.6% (11/56) in the intermediate-risk group, and 1.5% (1/67) in the low-risk group (P < 0.001). We next analyzed sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) based on the FC size and drain amylase level on POD3 for clinically relevant POPF (Table 4). The combination of two factors (FC size ≥ 41 mm and/or ≥ 1026 IU/L of drain amylase level on POD3) showed a 96.2% sensitivity and 55% specificity.
Classification of patients after DP by the size of fluid collection and drain amylase on POD3. The red squares and blue circles indicate patients with and without clinically relevant POPF, respectively. Patients were classified into three subgroups denoting risk of POPF by the FC size and the drain amylase level on POD3: Patients with FC size ≥ 41 mm and ≥ 1026 IU/L of drain amylase level on POD3 were at high risk; patients with FC size < 41 mm and < 1026 IU/L of drain amylase level on POD3 were at low risk; all other patients were defined as intermediate risk
Natural course of FC
Surveillance by CT imaging was performed at 3–9 months and 1 year after surgery. We followed the 80 patients who showed no clinical problems by CT for more than 1 year after surgery. The postoperative long-term course of FC amount showed median FC diameters at 7 days, 3–9 months, and 1 year after surgery of 34.8 (0–112.7) mm, 16 (0–103) mm, and 0 (0–57.2) mm, respectively (Fig. 4). In these patients, 96.3% (77/80) of FCs showed spontaneous regression and 77.5% (62/80) of FCs disappeared within 1 year.
Discussion
Peripancreatic FC is frequently observed after distal pancreatectomy [9, 10, 16], and even in small studies, FC was observed in most patients (~ 95%). We routinely checked the peripancreatic FC on CT imaging to detect a potential pancreatic fistula; however, peripancreatic FC does not necessarily correlate with clinically relevant POPF, and most FCs do not present clinical problems [8]. In this study, spontaneous regression of FC was observed in 96% of patients without any intervention, and complete disappearance was seen in 78% patients within a year. Some authors have also described the spontaneous regression of FC after DP [9, 10]. On the other hand, in the case of clinically relevant POPF, a decision must be made for undertaking conservative or invasive treatments based on various examinations including CT.
The usefulness of CT for diagnosing intra-abdominal abscess has been reported in some studies, with CT attenuation, wall enhancement, wall thickness, fat stranding, and entrapped gas in the collection identified as indicative factors [17, 18]. At the beginning of this research, we expected that detailed analyses of CT imaging would reveal some characteristics of a clinically relevant POPF that required intervention and provide predictive features to help determine the appropriate management of FC. Consequently, we investigated FC findings including the margin, the presence of air bubbles, CT attenuation, and irregularity of content on CT imaging; however, the heterogeneity of FC appearance rendered it difficult to classify FCs into certain patterns, suggesting that various CT findings of FC are not related to clinically relevant POPF, and only the size of FC is a useful finding in diagnosing POPF.
The drain amylase level has also been cited as a risk factor for POPF, although the superiority of drain amylase on POD1 or POD3 is controversial [19,20,21,22,23,24]. Our data showed that drain amylase levels on POD3 were similarly valuable as a risk factor for POPF to those recorded on POD1 (Table 2). We thus utilized a drain amylase on POD3 for further analyses in accordance with the ISGPF criteria. Predictive cutoff values of drain amylase on POD3 ranged from 1000 to 3000 [25], supporting our initial data.
In a clinical setting, how to treat pancreatic fistula is comprehensively determined by physical findings such as fever and abdominal pain, inflammatory findings of blood test, and imaging such as by CT examination. To this end, Supplementary Table S1 demonstrates the clinical features of 26 patients who needed some intervention for POPF. Of these, 16 patients (61.5%) and 15 patients (57.7%) showed abdominal pain and fever, respectively. These physical manifestations are essential for decision making, alongside subjective findings. CT imaging could therefore reveal a potential POPF and make it possible to initiate suitable early intervention. Although CT imaging provides various useful information, we often get lost in the decision based on the detailed findings such as appearance of FC, when in this work, the size of FC provided a more reliable index than the detailed appearance of FC on CT imaging with a sensitivity of 76.9% and specificity of 75% for this index only. Importantly, combining the two factors (FC size and drain amylase level) produced an even higher sensitivity (96.2%).
This study provides unique information about the FC after DP; however, several limitations should be described. The data were retrospectively collected at one facility, and thus, the criteria of intervention for clinically relevant POPF depended on the attending surgeons. In addition, the number of POPF cases was relatively small. The cutoff value of drain amylase on POD3 and the FC size should be validated in future prospective studies.
In conclusion, we found that while some FCs after DP required treatment as part of the POPF management, others needed no intervention and most of them spontaneously disappeared. Further, FC size and drain amylase level on POD3 were revealed to be significantly associated with POPF, potentially facilitating decisions about appropriate treatment.
References
Hackert T, Werner J, Buchler MW (2011) Postoperative pancreatic fistula. Surgeon 9:211–217
Rodriguez JR, Germes SS, Pandharipande PV et al (2006) Implications and cost of pancreatic leak following distal pancreatic resection. Arch Surg 141:361–365
Pannegeon V, Pessaux P, Sauvanet A et al (2006) Pancreatic fistula after distal pancreatectomy: predictive risk factors and value of conservative treatment. Arch Surg 141:1071–1076
Ferrone CR, Warshaw AL, Rattner DW et al (2008) Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg 12:1691–1697
Chincarini M, Zamboni GA, Pozzi Mucelli R (2018) Major pancreatic resections: normal postoperative findings and complications. Insights Imaging 9:173–187
Scialpi M, Scaglione M, Volterrani L et al (2005) Imaging evaluation of post pancreatic surgery. Eur J Radiol 53:417–424
Ma Y, Liu G, Zhang L (2017) CT findings and features of postoperative abdominal infection patients with pancreatic carcinoma. Pak J Med Sci 33:695–698
Tjaden C, Hinz U, Hassenpflug M et al (2016) Fluid collection after distal pancreatectomy: a frequent finding. HPB (Oxford) 18:35–40
Uchida Y, Masui T, Sato A et al (2018) Computer tomographic assessment of postoperative peripancreatic collections after distal pancreatectomy. Langenbecks Arch Surg 403:349–357
Chang YR, Kang MJ, Kim H et al (2016) The natural course of pancreatic fistula and fluid collection after distal pancreatectomy: is drain insertion needed? Ann Surg Treat Res 91:247–253
Barreto G, D’Souza MA, Shukla PJ et al (2008) The gray zone between postpancreaticoduodenectomy collections and pancreatic fistula. Pancreas 37:422–425
Bassi C, Molinari E, Malleo G et al (2010) Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg 252:207–214
Kawai M, Tani M, Terasawa H et al (2006) Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg 244:1–7
Yeo CJ (2010) Pancreatic surgery 101: drain, no drain, early drain removal, or late drain removal. What are the data? Where do we go from here? Ann Surg 252:215–216
Bassi C, Marchegiani G, Dervenis C et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161:584–591
Sierzega M, Kulig P, Kolodziejczyk P et al (2013) Natural history of intra-abdominal fluid collections following pancreatic surgery. J Gastrointest Surg 17:1406–1413
Allen BC, Barnhart H, Bashir M et al (2012) Diagnostic accuracy of intra-abdominal fluid collection characterization in the era of multidetector computed tomography. Am Surg 78:185–189
Gnannt R, Fischer MA, Baechler T et al (2015) Distinguishing infected from noninfected abdominal fluid collections after surgery: an imaging, clinical, and laboratory-based scoring system. Invest Radiol 50:17–23
Kawai M, Kondo S, Yamaue H et al (2011) Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 18:601–608
Cirocchi R, Graziosi L, Sanguinetti A et al (2015) Can the measurement of amylase in drain after distal pancreatectomy predict post-operative pancreatic fistula? Int J Surg 21(Suppl 1):S30–S33
Noji T, Nakamura T, Ambo Y et al (2012) Clinically relevant pancreas-related infectious complication after pancreaticoenteral anastomosis could be predicted by the parameters obtained on postoperative day 3. Pancreas 41:916–921
Yan JF, Kuang TT, Ji DY et al (2015) Laparoscopic versus open distal pancreatectomy for benign or premalignant pancreatic neoplasms: a two-center comparative study. J Zhejiang Univ Sci B 16:573–579
Vass DG, Hodson J, Isaac J et al (2018) Utility of drain fluid amylase measurement on the first postoperative day after distal pancreatectomy. HPB (Oxford) 20:803–808
Giglio MC, Spalding DR, Giakoustidis A et al (2016) Meta-analysis of drain amylase content on postoperative day 1 as a predictor of pancreatic fistula following pancreatic resection. British J Surg 103:328–336
Yang J, Huang Q, Wang C (2015) Postoperative drain amylase predicts pancreatic fistula in pancreatic surgery: a systematic review and meta-analysis. Int J Surg 22:38–45
Acknowledgements
This study was supported by a grant from the Japan Society for the Promotion of Science (JSPS KAKENHI #17K10689).
Author information
Authors and Affiliations
Contributions
Author contributions to the study and manuscript preparation include the following: Jun Yoshino, Daisuke Ban, and Minoru Tanabe conceived the design. Jun Yoshino, Toshiro Ogura, Kosuke Ogawa, and Hiroaki Ono collected and assembled the clinical data. Jun Yoshino and Daisuke Ban reviewed CT imaging. Jun Yoshino, Daisuke Ban, Atsushi Kudo, and Shinji Tanaka analyzed and interpreted the data. Jun Yoshino, Daisuke Ban, and Yusuke Mitsunori wrote the manuscript. Jun Yoshino, Daisuke Ban, Toshiro Ogura, Kosuke Ogawa, Hiroaki Ono, Yusuke Mitsunori, Atsushi Kudo, Shinji Tanaka, and Minoru Tanabe finally approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoshino, J., Ban, D., Ogura, T. et al. The Clinical Implications of Peripancreatic Fluid Collection After Distal Pancreatectomy. World J Surg 43, 2069–2076 (2019). https://doi.org/10.1007/s00268-019-05009-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05009-8