Abstract
Purpose
Peripancreatic fluid collection (PFC) is a frequent radiological finding on postoperative computed tomography (CT) after distal pancreatectomy (DP). We evaluated the risk factors for drainage of PFC after DP to clarify the optimal management of PFC.
Methods
This study included 85 patients who underwent elective DP between January 2010 and December 2020. PFC was defined as an area of fluid located at the pancreatic resection margin on postoperative routine CT on approximately postoperative day 7 (first CT). We retrospectively investigated the relationship between clinical variables, including CT findings and PFC drainage.
Results
Drainage was performed in 19 patients (22.4%). Drainage for PFC was significantly associated with a longer postoperative hospital stay, higher PFC volume, presence of air bubbles, and higher white blood cell (WBC) count at the time of the first CT. According to the multivariate analyses, a PFC volume ≥ 60 mL and WBC count ≥ 12,400/μL on the day of the first CT were independent risk factors for PFC drainage after DP. The combination of these 2 factors showed 73.7% sensitivity and 90.9% specificity.
Conclusion
The PFC volume and WBC count at the first CT were significantly associated with PFC drainage and may help determine the appropriate treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postoperative pancreatic fistula (POPF) is the most common complication after distal pancreatectomy (DP). Large series of patients who underwent DP have described POPF rates that range from 12 to 33% [1, 2]. POPF may cause serious complications, such as intra-abdominal abscess, hemorrhaging, and sepsis, and requires aggressive intervention in some cases [3]. Therefore, drainage is required at an appropriate time in cases of POPF. Since microbial contamination from the drain fluid is considered to play a potential role in POPF etiology, early drain removal has become standard [4, 5].
Enhanced computed tomography (CT) is the most useful modality for the detection of POPF. Under the strategy of early drain removal, postoperative routine enhanced CT (from this point referred to as “first CT”) is important for the early and accurate detection of POPF. Peripancreatic fluid collection (PFC) is a common radiological finding on the first CT scan [6]. PFC is characterized by septal formation, expansion appearance, and the presence of air bubbles, among other features [7, 8]. Some cases of PFC require invasive treatments, and such cases are often associated with POPF, while other cases of PFC do not result in any clinical problems. In addition, iatrogenic complications, including injuries to other organs, may occur due to PFC drainage. It is also difficult to determine the indication for drainage of PFC at first CT.
Although PFC and DP are equal in terms of frequency, few recommendations have been established for the optimal management of PFC after DP. Therefore, in this study, we retrospectively evaluated the risk factors for drainage of PFC after DP to clarify its optimal management.
Methods
Patients
Between January 2010 and December 2020, in the Department of Surgery at The Jikei University Hospital in Tokyo, Japan, 155 patients underwent primary DP due to a pancreatic tumor. Of these, 70 patients were excluded for the following reasons: postoperative enhanced CT was not performed (34 patients), continuous drain placement occurred at the time of first CT (34 patients), and combined multiple organ resection (2 patients). The remaining 85 patients were enrolled in the current study.
We retrospectively reviewed a prospectively maintained patient database. The following patient information was obtained from the prospective database: gender, age, body mass index, diagnosis of diabetes mellitus, malignancy of primary disease, neoadjuvant chemotherapy, details of surgical procedure, duration of surgery, estimated blood loss, pancreatic thickness, and postoperative hospital stay. The laboratory measurements were routinely obtained immediately before surgery, on postoperative day (POD) 1, and on the day of first CT.
This research was approved by the institutional Ethics Committee of The Jikei University School of Medicine [27-177(8062)] and conformed to the provisions of the Declaration of Helsinki.
Surgical procedure
In cases of pancreatic ductal adenocarcinomas and tumors with malignant potential, the pancreas was transected at the level of the portal vein with radical lymph node dissection and splenectomy, according to the concept of anterior or posterior radical antegrade modular pancreatosplenectomy (RAMPS) [9]. The pancreas was transected with a Tri-Staple™ device (staple height, 3.5–4.2 mm; COVIDIEN, North Haven, CT, USA) or with a scalpel. After resection using a scalpel, the remnant pancreatic stump was closed by hand-sewn suture using the fish mouth technique following ligation of the main pancreatic duct [10]. A surgical drain (small silastic flexible drains; BLAKE® Drains, Ethicon, Somerville, NJ, USA) was placed around the pancreatic stump via the left subphrenic space avoiding contacting the remnant pancreas. For all patients, 1 g of cefmetazole was administered via the intravenous route as a prophylactic antibacterial agent for one day after the surgery.
The assessment of PFC
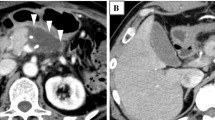
Routine first CT was performed between POD 5 and 7. Imaging was performed using a 64-slice multi-detector row CT (SOMATOM Definition AS + ; Siemens, Erlangen, Germany) at our hospital. Contrast-enhanced CT was performed after a rapid bolus injection of iodinated contrast agent at a rate of 2.5–4.0 mL/s using a power injector. We obtained the arterial, portal, and venous phases at 35, 70, and 180 s, respectively. Slice thickness was 1 mm, and the reconstruction interval was 5 mm. We evaluated fluid collection using the portal phase images. PFC was defined as an area of fluid located at the pancreatic resection margin. We used the volume analyzer SYNAPSE VINCENT software program (Fujifilm Medical, Tokyo, Japan) and its Liver Analysis Application to evaluate the PFC volume (Fig. 1). When the long diameter of the PFC was traced, the computer automatically contoured the PFC in each CT slice and calculated the volume of the PFC in cubic centimeters. The reviewers verified the outlines drawn by the computer for each CT slice. Corrections were made to the computer contours if necessary. We also evaluated other characteristics, including the average CT value of the PFC, septal formation or air bubbles inside the PFC, and expansion appearance of the PFC. Pancreatic stump thickness was measured at the resection line in preoperative CT images. The resection line was assessed based on surgical records, intraoperative photographs, and first CT images. Two reviewers analyzed the CT images (M.T and Y.S), and average values were adopted for the volume and CT value of the PFC and pancreatic thickness.
Representative first computed tomography (CT) images showing peripancreatic fluid collection (PFC) after distal pancreatectomy. A We used the volume analyzer SYNAPSE VINCENT to evaluate the PFC volume; the area within the white dotted line was determined to be PFC. The PFC volume in this case was 146 mL. The white blood cell (WBC) count on the day of first CT was 9400/μL. B The volume of the small area of PFC in this case, which also had high WBC levels (14,900/μL), was 44 mL
Surgical drain management and drainage of PFC
The drain amylase level was examined on POD 1 and 3. POPF was determined according to criteria established by the 2016 update of the International Study Group on Pancreatic Fistula (ISGPF) [11], wherein POPF includes grades B and C. The former grade A POPF is considered biochemical leakage in the updated criteria.
In a previous report, the initial drain was removed on POD 4 [5]. We followed the criterion for removing the drain when the drain amylase level on POD 3 was below 1000 U/L with no signs of infection. We performed a routine culture test of the initial drain tip. If sustained high drain amylase levels with a high fever was observed by POD 5, we placed the drainage tube continuously or carefully changed the tube using fluoroscopy. After removal of the initial surgical drain, drainage of PFC was performed when signs of uncontrollable infection were observed under antibiotics treatment. Antibiotics were selected according to the drug sensitivity of the initial drain tip culture if available. If the results of the culture were negative, the choice was made according to the drug sensitivity of the bile culture. Uncontrollable infection was defined as a fever of ≥ 38 °C for 2 days under antibiotic administration and the presence of PFC on contrast-enhanced CT. We re-evaluated the PFC and determined whether or not it was puncturable. If the PFC was puncturable, PFC drainage was performed.
Drainage was performed by percutaneous puncture, as described below [12, 13]. Using real-time ultrasound or CT guidance, a 21-gage needle was inserted percutaneously into the PFC. After a guidewire was advanced into the PFC, a 7.5-Fr pigtail drainage catheter was inserted into the collected fluid. Catheter exchange or removal was based on clinical improvement as well as drainage catheter output. Catheters were removed at the surgeon’s discretion.
If the PFC was in contact with the gastric wall, drainage was performed by transgastric puncture under endoscopic ultrasound [14], which was performed using a linear array echoendoscope. The PFC was identified by ultrasound, and a 19-gage needle was used to puncture the gastric wall after which it entered the PFC. After a guidewire was advanced into the PFC, a 5-Fr drainage catheter was placed into the PFC and pulled out through a nostril. A week after the initial drainage catheter was inserted, it was replaced with one to three double-pigtail biliary stents (7 Fr × 5 cm) across the cyst-gastrostomy. Patients who demonstrated clinical improvement were reassessed by CT to confirm resolution of the PFC, at which point these stents were removed. We have used the Hot AXIOS stent (Boston Scientific, Marlborough, MA, USA) for PFC drainage since 2019 [15].
Statistical analyses
First, using univariate analyses, we investigated the relationship between the drainage status and clinical variables of patients who underwent DP. Next, using a multivariate analysis, we investigated the relationship between the drainage status and clinical variables to examine the risk factors associated with drainage of postoperative fluid collection. All statistical analyses were performed using STATA/SE (STATA Statistical Software program, version 14.0; Stata Corp., College Station, TX, USA). Continuous variables are expressed as medians and interquartile ranges, while categorical variables are expressed as absolute numbers. The clinical variables were compared using the Mann–Whitney U-test and χ2 test. Univariate and multivariate analyses were performed using the logistic regression model. For the multivariate analysis, all variables for which p was < 0.05 in the univariate analysis were included. The optimal cut-off values were determined by a receiver operating characteristic (ROC) curve analysis. The areas under the curve were measured and compared to evaluate the discriminative ability. We used the two-sided α level of 0.05.
Results
Patient characteristics and features observed on first CT
The characteristics of the 85 patients are shown in Table 1. The median age of the patients was 63 (range 46–74) years old, and 46 (54%) were male. Forty-two patients (49%) underwent DP with lymph node dissection for invasive ductal adenocarcinoma. Laparoscopic surgery accounted for 46% of DP, and in 66% of cases, the pancreas was resected using a stapler. The median drain fluid amylase level on POD 3 was 260 U/L, and the incidence of pancreatic fistula according to the ISGPF was 25.8%. PFC was observed in all patients. The median PFC volume was 55 (range 30–111) mL on the first CT. Septal formation, expansion appearance, and air bubbles in the PFC ware detected in 16 (18.8%), 11 (12.9%), and 21 (24.7%) cases, respectively.
Results of PFC drainage
The results of microbiologic analyses of the initial drain tip are shown in Supplementary Table 2. No intra-abdominal bacteria were detected (Supplementary Table 1).
In total, 32 patients (38%) received postoperative additional antibiotics. All patients with PFC drainage received antibiotic therapy. The antibiotics used were as follows: Tazobactam/piperacillin, n = 12, 38%; Cefozopran, n = 5, 16%; Cefmetazole, n = 4, 13%; Cefepime, n = 3, 9%; Ciprofloxacin, n = 2, 6%; Sulbactam/cefoperazone, n = 2, 6%; Doripenem, n = 1, 3%; Meropenem, n = 1, 3%; Levofloxacin, n = 1, 3%; and Cefotiam, n = 1, 3%. Nineteen patients (22%) underwent PFC drainage, 7 of whom received endoscopic transgastric drainage. The number of days of PFC drainage was 13 (range 12–16). Microbiologic analyses of the drainage fluid revealed that 11 (58%) patients were infectious (Supplementary Table 2).
Analyses of risk factors for drainage
The clinical characteristics of the patients in the groups are shown in Table 2. PFC drainage was associated with a higher PFC volume, the presence of air bubbles, and a higher white blood cell (WBC) count or serum CRP on the day of first CT. No significant difference was observed in the CT values of PFC, septal formation, or the expansion appearance between the two groups. In the multivariate analysis, a PFC volume ≥ 60 mL and a WBC ≥ 12,400/μL on the day of first CT were independent risk factors for PFC drainage (Table 3). The cut-off values were determined by ROC analyses (Fig. 2). All patients who underwent drainage for PFC recovered from their infectious symptoms. The postoperative hospital stay was extended by 18 days in the PFC drainage group.
Risk classification of patients after DP based on fluid collection and WBC count on the day of first CT
Patients were classified according to cut-off values for the volume of the PFC and WBC count on the day of first CT (Fig. 3). The squares and white circles indicate patients with and without PFC drainage, respectively. The incidence of PFC drainage was 68% (13/19) in the high-risk patients whose PFC was ≥ 60 mL and whose WBC count was ≥ 12,400/μL on the day of first CT. The combination of these two factors showed a sensitivity and specificity of 73.7% and 90.9%, respectively (Supplementary Table 3).
Classification of patients after distal pancreatectomy according to the volume of peripancreatic fluid collection (PFC) and white blood cell count (WBC) on the day of first computed tomography (CT). The black squares and white circles indicate patients with and without drainage for PFC, respectively. The cut-off values were PFC ≥ 60 mL and WBC count ≥ 12,400/μL on the day of first CT
The 6 patients who had these two risk factors but did not undergo drainage received antibiotics for 12 (range 8–16.5) days.
Discussion
In the present study, multivariate analyses showed that a PFC volume ≥ 60 mL and a WBC count ≥ 12,400/μL on the day of first CT were independent risk factors of PFC drainage after DP. The combination index of these 2 factors, which was the best predictive indicator of PFC drainage, showed 73.7% sensitivity and 90.9% specificity.
The usefulness of CT for the identification of postoperative abdominal fluid collection has been reported in several studies [7, 8]. Previous studies reported fluid collection in 25–69% of patients after DP [6, 16, 17]. Not all cases of fluid collection require drainage, and thus, it is important to extract cases that require PFC drainage at the time of first CT. Although routine CT requires high medical cost and radiation exposure, several studies have reported that PFC measured using routine CT is accurate for predicting clinically significant POPF after pancreatic resection [5,6,7,8]. In this study, even in the absence of uncontrollable sings of infection, patients with a high PFC volume on first CT required later drainage. Routine CT can be useful for extracting this patient group. We measured PFC using the volume analyzer SYNAPSE VINCENT software program, assuming that PFC occurs in all patients with early drain removal. The proportion of cases with PFC drainage was 22.4% (19/85). The criterion for determining whether or not PFC drainage was necessary was the presence of infection. Fluid attenuation measurements, septal formation, stranding of the surrounding fat, and the presence of air bubbles within the collection have been proposed as helpful for differentiating infected and non-infected collections [18]. However, in terms of POPF after DP, it was difficult to detect infected PFC using these features, and only the size of the PFC was reported to be a useful finding in diagnosing POPF [19]. In the current study, although the presence of air bubbles was significantly associated with PFC drainage, the PFC volume was the only independent risk factor for drainage based on CT findings. A previous study reported that continuous drain placement led to 72.2% of cases of bacterial contamination [19]. Early drain removal may contribute to reduced infection of PFC because of a decreased incidence of retrograde infection. However, another previous study reported that the timing of PFC development was important [6]. The size of the postoperative initial PFC was not significantly associated with treatment, but the size of delayed PFC was significantly associated with treatment. In that report, the mean size of PFC in the drainage group was 7 cm, which was much larger than in the present study. The reason for this is that the PFC, for which drainage could not be determined by first CT, increased over time. In our study, we established a combination index that included the WBC count and PFC volume to extract cases that required PFC drainage at the time of first CT.
Although not significantly different, the pancreas thickness tended to be greater in the PFC drainage group than in the non-drainage group. Pancreatic thickness is generally the strongest risk factor for POPF. Since the measurement of pancreas thickness largely depends on the subjectivity of the measurer, it is necessary to standardize the measurement method and accumulate cases. In the previous report, the drain amylase level was useful as a predictor of POPF [20]. In contrast, the drain amylase level was not a significant risk factor for PFC drainage. The pancreatic thickness and proportion of cases requiring a stapler for closing the pancreatic stump may be the reasons for the varied conclusion among different studies. There was a tendency for laparoscopic DP to be less frequently associated with drainage than open surgery (p = 0.052). Previous studies have shown that blood loss is associated with POPF [8, 20]. The blood loss in patients who underwent laparoscopic DP and those who underwent open DP in our study was 30 (5–162.5) and 440 (197.5–811.3) mL, respectively (p < 0.001). In addition, the use of laparoscopy may have helped protect the pancreas. In the previous study, concomitant splenectomy for DP was associated with an increased rate of PFC after laparoscopic DP [16]. There was no correlation between PFC drainage and the volume of the drain before its removal. Although there were many missing values, the drainage volume (ml) in the drainage and non-drainage groups was 80 (50–125) and 75 (60–110), respectively (data not shown in Table). According to a recent study [21], the drainage volume on POD 3 was significantly lower in the patients with clinically relevant postoperative pancreatic fistula than in those without clinically relevant postoperative pancreatic fistula. The drainage efficiency may affect the postoperative pancreatic fistula, but this has not been investigated. Although a previous study reported that reinforced triple-row stapler reduced POPF [22], we did not use that approach during the present study period. There are many issues to be discussed in the future regarding PFC drainage after DP.
Several limitations associated with the present study warrant mention. First, this was a single-center retrospective study that included a relatively small number of patients. In addition, there was heterogeneity in this study with regard to the surgical type and transection of pancreas, operation time, duration of hospital stay, and incidence of POPF. Second, data were acquired over 10 years, during which treatment of PFC and POPF has evolved considerably. Based on the results, we believe that early re-drainage should be performed with the administration of antibiotics if first CT and blood test findings meet the criteria. However, the current cut-off value (a PFC volume ≥ 60 mL and a WBC count of ≥ 12,400/μL) should be validated using a different cohort or a different period. We are currently planning a prospective multicenter study to validate the cut-off values. Third, percutaneous PFC drainage is considered to be associated with a longer hospital stay, while transgastric drainage needs a shorter hospital stay. While external fistulas are difficult to manage and thus a substantial amount of time is required to educate patients on managing their own fistulas, internal fistulas do not require patients’ self-management. Since we selected the safest and shortest access route to the PFC, the access route may have contributed to the length of the hospital stay.
In conclusion, the PFC volume and WBC count on the day of first CT were revealed to be significantly associated with PFC drainage in patients whose drain had already been removed.
References
Hackert T, Werner J, Büchler MW. Postoperative pancreatic fistula. Surgeon. 2011;9:211–7.
Kleeff J, Diener MK, Z’graggen K, Hinz U, Wagner M, Bachmann J, et al. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 2007;245:573–82.
Shirai Y, Furukawa K, Ashida H, Gocho T, Onda S, Hamura R, et al. Endovascular micro-arterial stenting for arterial pseudoaneurysm after pancreatic surgery. Surg Today. 2021;2021(51):1232–6.
Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, et al. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010;252:207–14.
Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244:1–7.
Tjaden C, Hinz U, Hassenpflug M, Fritz F, Fritz S, Grenacher L, et al. Fluid collection after distal pancreatectomy: a frequent finding. HPB (Oxford). 2016;18:35–40.
Allen BC, Barnhart H, Bashir M, Nieman C, Breault S, Jaffe TA. Diagnostic accuracy of intra-abdominal fluid collection characterization in the era of multidetector computed tomography. Am Surg. 2012;78:185–9.
Uchida Y, Masui T, Sato A, Nagai K, Anazawa T, Takaori K, et al. Computer tomographic assessment of postoperative peripancreatic collections after distal pancreatectomy. Langenbecks Arch Surg. 2018;403:349–57.
Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery. 2003;133:521–7.
Minarich MJ, Schwarz RE. Simplicity and safety: minimized pancreatic fistula rate after distal pancreatectomy through pancreas stump sutured fish-mouth closure. Am Surg. 2018;84:1734–40.
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–91.
Kwon YM, Gerdes H, Schattner MA, Brown KT, Covey AM, Getrajdman GI, et al. Management of peripancreatic fluid collections following partial pancreatectomy: a comparison of percutaneous versus EUS-guided drainage. Surg Endosc. 2013;27:2422–7.
Cronin CG, Gervais DA, Castillo CF, Mueller PR, Arellano RS. Interventional radiology in the management of abdominal collections after distal pancreatectomy: a retrospective review. AJR Am J Roentgenol. 2011;197:241–6.
Varadarajulu S, Wilcox CM, Christein JD. EUS-guided therapy for management of peripancreatic fluid collections after distal pancreatectomy in 20 consecutive patients. Gastrointest Endosc. 2011;74:418–23.
Patil R, Ona MA, Papafragkakis C, Anand S, Duddempudi S. Endoscopic ultrasound-guided placement of AXIOS stent for drainage of pancreatic fluid collections. Ann Gastroenterol. 2016;29:168–73.
Song KB, Kwon J, Lee YJ, Hwang DW, Lee JH, Shin SH, et al. The treatment indication and optimal management of fluid collection after laparoscopic distal pancreatectomy. Surg Endosc. 2019;33:3314–24.
Sierzega M, Kulig P, Kolodziejczyk P, Kulig J. Natural history of intra-abdominal fluid collections following pancreatic surgery. J Gastrointest Surg. 2013;17:1406–13.
Gnannt R, Fischer MA, Baechler T, Clavien PA, Karlo C, Seifert B, et al. Distinguishing infected from noninfected abdominal fluid collections after surgery: an imaging, clinical, and laboratory-based scoring system. Invest Radiol. 2015;50:17–23.
Osakabe H, Nagakawa Y, Kozono S, Takishita C, Nakagawa N, Nishino H, et al. Causative bacteria associated with a clinically relevant postoperative pancreatic fistula infection after distal pancreatectomy. Surg Today. 2021. https://doi.org/10.1007/s00595-021-02287-5.
Yoshino J, Ban D, Ogura T, Ogawa K, Ono H, Mitsunori Y, et al. The clinical implications of peripancreatic fluid collection after distal pancreatectomy. World J Surg. 2019;43:2069–76.
Fukami Y, Saito T, Osawa T, Hanazawa T, Kurahashi T, Kurahashi S, et al. Which is the best predictor of clinically relevant pancreatic fistula after pancreatectomy: drain fluid concentration or total amount of amylase? Ann Gastroenterol Surg. 2021;5:844–52.
Matsumoto I, Kamei K, Satoi S, Murase T, Matsumoto M, Kawaguchi K, et al. Efficacy of the slow firing method using a reinforced triple-row stapler for preventing postoperative pancreatic fistula during laparoscopic distal pancreatectomy. Surg Today. 2021. https://doi.org/10.1007/s00595-021-02344-z.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 20K17665 (Dr. Yoshihiro Shirai).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsunematsu, M., Shirai, Y., Hamura, R. et al. The clinical management of peripancreatic fluid collection after distal pancreatectomy. Surg Today 52, 1524–1531 (2022). https://doi.org/10.1007/s00595-022-02483-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-022-02483-x