Abstract
Background
The first aim of our study was to evaluate surgical decision-making by BRCA mutation carriers with breast cancer based on the timing of knowledge of their BRCA mutation status. The second aim was to evaluate breast cancer outcome following surgical treatment.
Methods
This was a retrospective study of 164 patients diagnosed with invasive breast cancer, tested for BRCA mutation, and treated with primary surgery between 2004 and 2015 at Samsung Medical Center in Seoul, Korea. We reviewed types of surgery and timing of the BRCA test result. We compared surgical decision- making of BRCA carriers with breast cancer based on the timing of knowledge of their BRCA mutation status.
Results
Only 15 (9.1%) patients knew their BRCA test results before their surgery, and 149 (90.9%) knew the results after surgery. In patients with unilateral cancer, there was a significant difference between groups whose BRCA mutation status known before surgery and groups whose BRCA status unknown before surgery regarding the choice of surgery (p = 0.017). No significant difference was observed across surgery types of risk of ipsilateral breast tumor recurrence (p = 0.765) and contralateral breast cancer (p = 0.69).
Conclusion
Genetic diagnosis before surgery has an impact on surgical decision choosing unilateral mastectomy or bilateral mastectomy in BRCA mutation carriers with breast cancer. Knowledge about BRCA mutation status after initial surgery led to additional surgeries for patients with BCS. Thus, providing genetic counseling and genetic testing before surgical choice and developing treatment strategies for patients with a high risk of breast cancer are important.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of the BRCA mutation in Korea was reported to be 20% of breast cancer patients with a family history, which is consistent with Western populations. In previous study, the estimated cumulative risk of breast and ovarian cancer with BRCA mutations has been calculated as 72 and 25% for BRCA1 and 66 and 11% for BRCA2 in Korea [1]. Several aspects should be considered before these patients make decisions for treatment. The risk of ipsilateral breast tumor recurrence (IBTR) and contralateral breast cancer (CBC) [2], and the potential survival benefit of prophylactic mastectomy, need to be taken into account. A meta-analysis of 18 retrospective and 2 prospective cohorts reported on cumulative risk of secondary–primary contralateral breast cancer in 1324 people carrying BRCA1/BRCA2 mutations. The cumulative 10-year risk of CBC was 27% for BRCA1 mutations and 19% for BRCA2 mutations compared with 5% for people without BRCA mutations [3]. Several studies have tried to clarify these issue but their results are conflicting [4, 5].
Identification of BRCA mutation status can influence surgical treatment decisions for patients with a diagnosis of breast cancer [6, 7]. Surgical treatment for women with BRCA-associated breast cancer remains controversial. Patients who underwent breast-conserving surgery had higher risk of IBTR than patients with mastectomy (15-year cumulative estimated risk 23.5 vs 5.5%) [8]. However, both breast cancer-specific survival (BCSS) and overall survival (OS) were similar between the two groups at 15 years (93.5 vs 92.8 and 91.8 and 89.8%). There was no difference in breast cancer-specific survival (BCSS) between patients with BRCA mutation who underwent contralateral prophylactic mastectomy and patients who did not [9, 10]. Other study concluded that the 10-year overall survival advantage showing 89% in women electing for contralateral risk-reducing mastectomy (CRRM) compared to 71% in the non-CRRM group [11].
There are several studies analyzing the timing of genetic testing and impact on surgical decision-making in patients with breast cancer [12,13,14,15]. However, there are limited studies to evaluate these issues in Korea. The first aim of our study was to evaluate surgical decision-making by BRCA mutation carriers with breast cancer based on the timing of knowledge of their BRCA mutation status. The second aim was to evaluate breast cancer outcome following surgical treatment.
Methods
This was a retrospective study of 823 patients diagnosed with invasive breast cancer. The patients were tested for BRCA mutation and treated with primary surgery or neoadjuvant chemotherapy (NAC) followed by curative surgery at Samsung Medical Center between January 2004 and December 2015. BRCA mutation carriers were categorized into two groups: (1) patients whose BRCA status was known before their surgery; (2) patients whose BRCA status was unknown before their surgery. We compared surgical decision-making based on the timing of knowledge of their BRCA mutation status. We reviewed medical records for age at breast cancer diagnosis, types of surgery, timing of the BRCA test result, clinical and pathologic staging, histology, hormone receptor status, and information about systemic therapy, radiation therapy and patient outcome. Types of initial surgery were classified by breast-conserving surgery (BCS), unilateral mastectomy (UM), or bilateral mastectomy (BM), and we established whether mastectomy was contralateral or/and risk-reducing mastectomy. We also investigated if patients underwent a bilateral oophorectomy.
The eligibility criteria for the BRCA test were as follows: (1) breast cancer patients with family history of breast or ovarian cancer in any relative; (2) diagnosis of breast cancer at age 40 or younger; (3) bilateral breast cancer; and (4) male with breast cancer. Eligible patients were offered genetic counseling and brief information about the test by investigators at our institution.
BRCA mutation analysis
BRCA1/2 genetic testing was performed using genomic DNA from peripheral blood by full direct sequencing. All BRCA1 and BRCA2 variants were categorized as pathogenic, unknown significance, or polymorphic. Prevalence of mutations was calculated as the proportion of carriers with pathogenic mutations in each subgroup.
Statistical analysis
We used Chi-squared tests and Spearman’s correlation coefficient to compare discrete variables. The Kaplan–Meier method with 95% confidence intervals (CIs) was calculated based on the logarithm of the survival function and used to estimate long-term outcomes of contralateral breast cancer and local–regional recurrence. Differences were defined as significant when p values were less than 0.05. We used SPSS, Version 22 (IBM Corp., Armonk, NY, USA) for the Chi-squared tests and R 3.2.0 package for calculating Cox’s proportional hazard regression and logistic regression.
Results
We retrospectively analyzed records of 836 patients who were surgically treated for primary breast cancer between 2004 and 2015. Among these patients, we identified 171 who carried BRCA mutations and had a breast cancer. Patients who underwent primary surgery outside our institution were excluded, leaving 164 patients eligible for analysis (Fig. 1). The mean age at the time of surgery was 41.2 years (range 25–68 years), and 50% of patients were younger than 40. Mean follow-up time was 53.5 months (range 7–149 months). Among 164 patients, 82 (50%) carried BRCA1 mutations, 81 (49.4%) carried BRCA2 mutations, and 1 (0.6%) had both BRCA1 and BRCA2 mutations. 144 (87.8%) patients had unilateral breast cancer, and 20 (12.2%) had bilateral breast cancer. Only 15 (9.1%) patients knew their BRCA test results before their surgery, and 149 (90.9%) knew the results after surgery. Of patients who were treated with neoadjuvant chemotherapy, only 46.4% (13 of 28) were aware of their mutation status before surgery. The timing of the BRCA test with regard to surgery was not significantly different between patients with BRCA1 and BRCA2 mutations. 9 of 76 (7.4%) patients with BRCA1 mutations had their mutation status identified before surgery, and 5 of 68 (7.4%) patients with BRCA2 mutations knew their status before surgery (p = 0.683). Triple-negative (hormone receptor-, HER2-) invasive cancer was significantly more likely in patients with BRCA1 mutations (55 of 76, 72.4%) than in patients with BRCA2 mutations (14 of 68, 20.6%) (p < 0.001). Pathologic stage did not vary significantly between BRCA1 and BRCA2 carriers (p = 0.883). Demographic, clinicopathologic, and treatment characteristics of patients are in Table 1.
Initial surgical choice by mutation status identified before versus after surgery
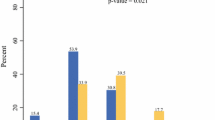
Of patients with unilateral cancer whose BRCA mutation status was known before surgery, 5 (35.7%) chose BCS, 8 (57.1%) chose UM, and 1 (7.2%) chose BM. Of patients with unilateral cancer whose BRCA mutation status was unknown before surgery, 96 (73.8%) chose BCS, 33 (25.4%) chose UM, and 1 (0.8%) chose BM. Difference between the two groups regarding the choice of surgery was significant (p = 0.017) (Fig. 2). Of 130 patients with unilateral cancer whose BRCA status was unknown before surgery and underwent BCS or UM, one patient underwent BM for risk reduction after mutation status was identified, and 2 underwent BM as a result of IBTR or CBC. Overall, 3 (1.5%) patients underwent delayed BM.
Of 20 patients with bilateral cancer, 11 (55.0%) underwent bilateral BCS or unilateral BCS and UM, and 9 (45.0%) underwent BM. None of the 20 patients had their BRCA mutation status identified before surgery.
In patients with unilateral breast cancer, 10 patients had UM with reconstruction and 2 patients had BM with reconstruction as risk-reducing mastectomy (RRM). In patients with bilateral breast cancer, 5 of 20 had BM with reconstruction.
Oncologic outcomes of patients with BRCA mutations
During follow-up period, 8 instances of IBTR, 12 of CBC, and 11 of distant metastases occurred. Of all 164 patients, 23 experienced one of these recurrences. Of 144 patients with unilateral breast cancer, 4 had local–regional recurrences, 12 had CBC, and 10 had distant recurrences. The estimate for 5-year local–regional recurrence-free survival was 96.4% (95% CI 94.1–100.0%) for patients who had BCS and 96.2% (95% CI 90.5–103.1%) for patients with mastectomy (Table 2). No significant difference was observed across surgery types of risk of IBTR (p = 0.765) and CBC (p = 0.69, Fig. 3). No significant difference was observed across surgery types of risk of IBTR between patients with BRCA1 and BRCA2 mutations (p = 0.362), and no significant difference was observed for risk of CBC (p = 0.284).
Discussion
In this study, 15 (9.1%) patients knew their BRCA test results before their surgery, and 149 (90.9%) knew the results after surgery. Identification of BRCA mutation status influenced the choice of surgical types, 66.7% of patients knew their BRCA mutation status underwent UM or BM, and 30.2% of patients with unknown mutation status underwent UM or BM. In patients with unilateral breast cancer, no significant difference was observed across surgery types of IBTR (log-rank test, p = 0.765), and no significant difference was observed across surgery types of risk of contralateral breast cancer (log-rank test, p = 0.69). No difference in risk of IBTR was seen between patients with BRCA1 and BRCA2 mutations (log-rank test, p = 0.691), and no significant difference was observed in risk of CBC (log-rank test, p = 0.202).
In this study, two patients were initially treated with BM, but 3 treated with BCS or UM were treated with BM for RRM or for contralateral breast later in patients with unilateral cancer. This delay was most likely the result of being unaware of their mutation status before their surgery. Recent study demonstrates that when patients and surgeons are aware of a BRCA mutation status prior to surgery, they more often choose bilateral mastectomy [16]. Patients with unknown mutation status underwent BCS that will most likely be offered bilateral mastectomy after the results of genetic testing [17, 18], which occur additional cost and morbidity. Preoperative genetic testing has several benefits which are reducing the need for additional surgeries and favor survival outcome [19, 20]. The concept of treatment-focused genetic testing (TFGT) has been addressed, and breast cancer patients with high-risk factors are offered genetic counseling and testing at diagnosis for appropriate timed treatment decisions [21]. Recent study showed that streamlined genetic education is effective in informing women newly diagnosed with breast cancer about TFGT [22].
In this study, only 15 (9.1%) patients knew their BRCA test results before their surgery, and 149 (90.9%) knew their results after surgery. Of patients who were treated with neoadjuvant chemotherapy, only 46.4% (13 of 28) were aware of their mutation status before surgery. This result differs from a study in which 41% of patients with BRCA mutations found to have BRCA mutation before their surgery [14]. In Korea, newly diagnosed patients with high risk for hereditary breast cancer are offered genetic counseling at diagnosis of breast cancer, and BRCA1/2 mutation testing is carried out. High-risk factors for hereditary breast cancer are as follows: (1) any breast cancer patient that has a family history of breast or ovarian cancer in any relative; (2) breast cancer without a family history of breast or ovarian cancer, subjects 40 years or younger at diagnosis, with bilateral breast cancer, male gender, or diagnosed with another primary malignancy. Family members of BRCA1/2 mutation carriers are offered genetic counseling as well [23]. Process of management for patients with high risk is similar to Western countries. However, social and cultural factors in Asia are more conservative than in Western countries. Therefore, social stigma, including the perception of cancer and genetic variations, may remain a barrier to address breast cancer and genetic risks [24]. Patients and family members tend to hesitate about testing for BRCA mutations. This tendency might lead to a delay of knowledge about BRCA mutation status [25].
In this study, we saw a significant difference between two groups regarding choice of surgery (p = 0.017). The probability of choosing a BM was much higher for patients who know their BRCA mutation status at the time of the initial surgery [7]. However, proportion of bilateral RRM of this study at initial surgical choice was less than a previous study, even though our patients knew their BRCA mutation status before surgery [14]. In this study, in patients with unilateral breast cancer, 7.2% with known BRCA mutation status underwent bilateral RRM, whereas 82.5% of patients who were aware of their BRCA mutation status underwent bilateral RRM in a study by Chiba et al. [14]. A study about perceptions of prophylactic mastectomy in Korea showed that patients have less basic information than Western patients about benefit and complications of prophylactic mastectomy. The reasons for refusing to undergo prophylactic mastectomy were aesthetic concerns, followed by surgical risk [26]. Expense of the procedures was one of the reasons to refuse prophylactic mastectomy. However, the National Health Insurance System has promoted coverage for the cost of reconstruction surgery after mastectomy since 2015. Angelina Jolie announced having a BRCA1 mutation and receiving bilateral risk-reducing mastectomy in 2013, and interest in hereditary breast cancer increased [27]. After her disclosure, contralateral prophylactic mastectomy also increased from four cases per year in 2012 to 20 cases per year in 2015 in affected carriers in Korea [28]. These factors may affect increase of BM and RRM in patients with BRCA carriers in the future.
In this study, no significant difference was seen across surgery types of IBTR (log-rank test, p = 0.765) in patients with unilateral breast cancer. This result was consistent with a study by Chiba et al. [14], in which no significant difference was observed across surgery types of local–regional recurrence (p = 0.57). However, another study showed that patients who underwent BCS had a higher risk of IBTR than patients with mastectomy [21], and recent study showed 15-year cumulative estimated risk of IBTR was 23.5% in patients underwent BCS, whereas 5.5% in patients with mastectomy [8].
In this study, no significant difference was observed for risk of CBC in patients with unilateral breast cancer (p = 0.69). This result was inconsistent with a previous study that showed a significantly reduced risk of CBC in patients undergoing BM [14]. This difference may be the result of relatively short follow-up time or small numbers of events. We started bilateral RRM in 2010, so the follow-up time for CBC for patients receiving BM was relatively short.
This study had some limitations. It was a retrospective study from a single institution, so the small sample size and the relatively short median follow-up limited some outcome estimates. Second, patients’ preference and Korean-specific environment could make influence on surgical decision and may not be generalizable to other condition with a similar level of clinical excellence. Last, we started bilateral RRM in 2010, so the follow-up time for CBC for patients receiving BM was short, lower the power for comparison across surgery groups.
In conclusion, genetic diagnosis before surgery has an impact on surgical decision choosing unilateral mastectomy or bilateral mastectomy in BRCA mutation carriers with breast cancer. Knowledge about BRCA mutation status after initial surgery led to additional surgeries for patients with BCS. Thus, providing genetic counseling and genetic testing before surgical choice and developing treatment strategies for patients with a high risk of breast cancer are important.
References
Kang E, Seong MW, Park SK et al (2015) The prevalence and spectrum of BRCA1 and BRCA2 mutations in Korean population: recent update of the Korean Hereditary Breast Cancer (KOHBRA) study. Breast Cancer Res Treat 151:157–168
Valachis A, Nearchou AD, Lind P (2014) Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat 144:443–455
Molina-Montes E, Pérez-Nevot B, Pollán M et al (2014) Cumulative risk of second primary contralateral breast cancer in BRCA1/BRCA2 mutation carriers with a first breast cancer: a systematic review and meta-analysis. Breast 23:721–742
Metcalfe K, Lynch HT, Ghadirian P et al (2011) Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 127:287–296
Garcia-Etienne CA, Barile M, Gentilini OD et al (2009) Breast-conserving surgery in BRCA1/2 mutation carriers: are we approaching an answer? Ann Surg Oncol 16:3380–3387
Lokich E, Stuckey A, Raker C et al (2014) Preoperative genetic testing affects surgical decision making in breast cancer patients. Gynecol Oncol 134:326–330
Weitzel JN, McCaffrey SM, Nedelcu R et al (2003) Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis. Arch Surg 138:1323–1328 (discussion 1329)
Pierce LJ, Phillips K-A, Griffith KA et al (2010) Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat 121:389–398
Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C et al (2007) Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer 43:867–876
van Sprundel TC, Schmidt MK, Rookus MA et al (2005) Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer 93:287–292
Evans DG, Ingham SL, Baildam A et al (2013) Contralateral mastectomy improves survival in women with BRCA1/2-associated breast cancer. Breast Cancer Res Treat 140:135–142
Schwartz MD, Lerman C, Brogan B et al (2004) Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol 22:1823–1829
Evans DG, Lalloo F, Hopwood P et al (2005) Surgical decisions made by 158 women with hereditary breast cancer aged <50 years. Eur J Surg Oncol 31:1112–1118
Chiba A, Hoskin TL, Hallberg EJ et al (2016) Impact that timing of genetic mutation diagnosis has on surgical decision making and outcome for BRCA1/BRCA2 mutation carriers with breast cancer. Ann Surg Oncol 23:3232–3238
Yi M, Hunt KK, Arun BK et al (2010) Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res 3:1026–1034
Yadav S, Reeves A, Campian S et al (2017) Preoperative genetic testing impacts surgical decision making in BRCA mutation carriers with breast cancer: a retrospective cohort analysis. Hered Cancer Clin Pract 15:11
Giuliano AE, Boolbol S, Degnim A et al (2007) Society of Surgical Oncology: position statement on prophylactic mastectomy. Approved by the Society of Surgical Oncology Executive Council, March 2007. Ann Surg Oncol 14:2425–2427
Paluch-Shimon S, Cardoso F, Sessa C et al (2016) Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol 27:v103–v110
Heemskerk-Gerritsen BA, Rookus MA, Aalfs CM et al (2015) Improved overall survival after contralateral risk-reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. Int J Cancer 136:668–677
Metcalfe K, Gershman S, Ghadirian P et al (2014) Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ 348:g226
Trainer AH, Lewis CR, Tucker K et al (2010) The role of BRCA mutation testing in determining breast cancer therapy. Nat Rev Clin Oncol 7:708–717
Quinn VF, Meiser B, Kirk J et al (2017) Streamlined genetic education is effective in preparing women newly diagnosed with breast cancer for decision making about treatment-focused genetic testing: a randomized controlled noninferiority trial. Genet Med 19:448–456
Han SA, Park SK, Ahn SH et al (2011) The Korean Hereditary Breast Cancer (KOHBRA) study: protocols and interim report. Clin Oncol (R Coll Radiol) 23:434–441
Cheung EL, Olson AD, Yu TM et al (2010) Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiol Biomarkers Prev 19:2211–2219
Nakamura S, Kwong A, Kim SW et al (2016) Current status of the management of hereditary breast and ovarian cancer in Asia: first report by the Asian BRCA consortium. Public Health Genomics 19:53–60
Yoon HY, Shim JS, Lee JW (2016) Perceptions of prophylactic mastectomy in Korea. Arch Plast Surg 43(1):53–58. https://doi.org/10.5999/aps.2016.43.1.53
Evans DG, Wisely J, Clancy T et al (2015) Longer term effects of the Angelina Jolie effect: increased risk-reducing mastectomy rates in BRCA carriers and other high-risk women. Breast Cancer Res 17:143
Lee J, Kim S, Kang E et al (2017) Influence of the Angelina Jolie announcement and insurance reimbursement on practice patterns for hereditary breast cancer. J Breast Cancer 20:203–207
Acknowledgements
The study was approved by the Institutional Review Board of the Samsung Medical Center, Seoul, Korea (Approval Numbers: 2017-01-142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in this manuscript, including financial, consultant, institutional, or other relationships that might lead to bias.
Rights and permissions
About this article
Cite this article
Park, S., Lee, J.E., Ryu, J.M. et al. Genetic Diagnosis before Surgery has an Impact on Surgical Decision in BRCA Mutation Carriers with Breast Cancer. World J Surg 42, 1384–1390 (2018). https://doi.org/10.1007/s00268-017-4342-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4342-7