Abstract
BRCA1/2 mutation carriers with breast cancer are at high risk of contralateral disease. Such women often elect to have contralateral risk-reducing mastectomy (CRRM) to reduce the likelihood of recurrence. This study considers whether CRRM improves overall survival. 105 female BRCA1/2 mutation carriers with unilateral breast cancer who underwent CRRM were compared to controls (593 mutation carriers and 105 specifically matched) not undergoing CRRM and diagnosed between 1985 and 2010. Survival was assessed by proportional hazards models, and extended to a matched analysis using stratification by risk-reducing bilateral salpingo-oophorectomy (RRBSO), gene, grade and stage. Median time to CRRM was 1.1 years after the primary diagnosis (range 0.0–13.3). Median follow-up was 9.7 years in the CRRM group and 8.6 in the non-CRRM group. The 10-year overall survival was 89 % in women electing for CRRM (n = 105) compared to 71 % in the non-CRRM group (n = 593); p < 0.001. The survival advantage remained after matching for oophorectomy, gene, grade and stage: HR 0.37 (0.17–0.80, p = 0.008)—CRRM appeared to act independently of RRBSO. CRRM appears to confer a survival advantage. If this finding is confirmed in a larger series it should form part of the counselling procedure at diagnosis of the primary tumour. The indication for CRRM in women who have had RRBSO also requires further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
BRCA1 and BRCA2 carriers with breast cancer are at substantially higher risk of contralateral breast cancer (CBC) than non-carriers [1–3]. The 10-year CBC risk of carriers is approximately 30 % (2–3 % annual risk) [1–4] compared to 4–7 % in non-carriers (0.4–0.7 % annual risk) [4–6]. CBC risks in carriers at 20 years are likely to be ~50 % but only one estimate is available [1]. Most, [7–11] but not all studies, [12, 13] show that CBC indicates a greater risk of relapse and breast cancer-specific and all-cause mortality, especially if the interval between the primary surgery and CBC is short. In general, CBC is more likely in women with a family history—if affected young and have large oestrogen receptor (ER) negative high-grade tumours [14]. CBC may be associated with poor prognosis [8, 15, 16] because its development indicates the failure of adjuvant therapy for the primary with a concomitant risk of metastasis [6].
Although endocrine therapy, including risk-reducing bilateral salpingo-oophorectomy (RRBSO), chemotherapy and lifestyle factors reduce CBC risk [6, 17] surgery is by far the most effective intervention [15, 18–20]. CRRM reduces risk of CBC by over 90 % in BRCA1/2 carriers and in non-carriers: thus women may elect for CRRM in order to avoid the trauma of developing CBC and undergoing treatment. This may be part of the reason for the increase in CRRM, not only among BRCA1/2 carriers but also other women at high risk, and even population risk [21–23]. However, it is not clear whether CRRM is associated with reduced relapse and increased all-cause and breast cancer survival. Some studies in women at high risk and population risk indicate that CRRM is associated with a survival advantage [24–26] whereas others do not [19, 27] including one study in carriers [19].
Estimation of survival after CRRM is confounded by the propensity of carriers of BRCA1/2 mutations to undergo RRBSO, which substantially reduces the risks of: ovarian cancer; relapse from the primary breast cancer; and CBC [6, 17]. Thus it may be difficult to distinguish the known protective effects of RRBSO from the putative effects of CRRM on survival from the primary cancer. A Dutch case–control (CC) study in BRCA1/2 carriers reported that CRRM improved survival but this advantage became non-significant after correcting for RRBSO [19].
Here we report the follow-up on a large series of women with BRCA1/2 mutations and a diagnosis of unilateral breast cancer.
Methods
Women diagnosed with a first unilateral breast cancer after first January 1985 and before 31st December 2010 were eligible for the study if they tested positive for a pathogenic mutation in BRCA1 or BRCA2. Details on grade, tumour size, lymph node involvement and ER status of the primary tumour were gathered from pathology and hospital records. Survivorship (Kaplan–Meier) curves were generated from the date of initial primary breast cancer diagnosis to the date of last follow-up or death for women not undergoing CRRM. Women not undergoing CRRM usually had wide local excision and radiotherapy although around 25 % had a full unilateral mastectomy. The curves were compared using proportional hazards regression, adjusting for tumour grade, stage and ER status and investigating CRRM and RRBSO effects. For those undergoing CRRM or RRBSO the survival plots and models were generated from the date of risk-reducing surgery unless RRBSO was before breast cancer primary diagnosis. Survival analysis was also repeated for breast cancer-specific survival. In order to control for ascertainment bias a second analysis was carried out matching each case with CRRM with the closest historic control with the same gene involved, the same stage and grade and, if available, ER status. Matching was performed to within 6 years of age at diagnosis. Controls did not have CBC, ovarian cancer or known metastatic disease. Follow-up for cases was matched to start at the same interval from breast cancer as the length of time from the first breast cancer to CRRM in the control. Plots and models were also generated for CBC from the date of initial primary tumour diagnosis. Time of oophorectomy after breast cancer diagnosis was also modelled—RRBSO was performed in 16 cases before CRRM, six synchronously, and 40 after (total n = 62).
All cases of breast cancer were confirmed by hospital/pathology records, or from Regional Cancer Registries. Mutation carriers and their close relatives were offered regular follow-up through the regional genetic service and dates of last follow-up were obtained from the clinical genetics notes and from a prevalence check on the cancer registry on 29th July 2011. Dates of death were obtained from the cancer registry or from death certification. Mutation testing was carried out by a combination of whole gene sequencing or targeted sequencing after conformation-sensitive gel electrophoresis (CSGE) and then a dosage test using multiple ligation dependent probe amplification (MLPA) as previously described [28].
Statistical analyses were performed using Stata version 12 (www.stata.com). Log-log plots were examined to assess the proportionality assumption in the proportional hazards models, and stratification was used to construct the matched cohort sub-group analysis [29]. Results are presented as the main effect with a 95 % confidence interval unless otherwise stated.
Results
Seven hundred and eighteen female patients with invasive breast cancer were diagnosed with a pathogenic mutation in either BRCA1 (n = 357) or BRCA2 (n = 361) in the study period 1985–2010. Twenty women were diagnosed with breast cancer in both breasts either synchronously (n = 17) or within 2 months of diagnosis of the primary breast cancer (n = 3) and were excluded from the survival and contralateral risk analyses. One hundred and five (15 %) of the remaining 698 BRCA1/2 carriers with breast cancer elected to have CRRM at a median time after the first cancer of 1.1 years (range 0.0–13.3 years). Of the 105 CRRM patients 62 (59 %) also elected to undergo RRBSO (Table 1). A further group of patients had RRBSO only (n = 120; 17 %). Both operations were performed throughout the time periods but there was a trend for an increase in risk-reducing surgery more recently (Table 1). Median follow-up from breast cancer diagnosis in the CRRM group was 9.7 years (range 2.0–22.0 years) and from CRRM was 7.2 years (range 0.7–21.0 years). In the non-CRRM group median follow-up from breast cancer surgery was 8.6 years (range 0.1–26.0 years).
There was no incidence of recurrent breast cancer in the contralateral reconstructed breast, although six occult cancers (four invasive and two DCIS) were diagnosed at the time of CRRM in the removed breast tissue. In contrast, 25 % (118/473) of women not undergoing CRRM developed metachronous CBC. The 5, 10 and 20 year risks of CBC were in all patients: 10 % (8–14 %), 31 % (25–38 %) and 63 % (48–81 %), respectively (Fig. 1).
The 10-year survival of women who had no risk-reducing surgery (n = 473) was 65 % (59–69 %), whereas (from the time of first risk-reducing surgery): for women who had a CRRM only (n = 43) it was 83 % (60–93 %), HR 0.48 (0.19–1.14, p = 0.104); for women who had RRBSO only (n = 120) 10-year survival was 81 % (68–89 %), HR 0.46 (0.27–0.78, p = 0.004); for women who had a RRBSO and CRRM (n = 62) it was 92 % (75–98 %), HR 0.16 (0.06–0.44, p < 0.001). Figure 2 illustrates the relative survival courses of these groups. In the no risk-reducing surgery group 165/473 (35 %) of women died; 138 (29 %) from breast cancer, 18 (4 %) from ovarian cancer and 9 (2 %) from other causes. In the CRRM only group 5/43 (12 %) died all of which were related to breast cancer. In the RRBSO group 15/120 (12 %) died; 12 (10 %) from breast cancer, two from ovarian cancer (2 %) and one from other causes (1 %). In women who had both operations 4/62 (7 %) died; three (5 %) from breast cancer and one (2 %) from ovarian cancer. Of the 105 women who elected to have CRRM 52 (50 %) were operated within 13 months of initial breast cancer diagnosis and 81 (77 %) within 3 years (51 were BRCA1 carriers and 54 BRCA2 carriers).
Survival analysis for BRCA1/2 mutation carriers who underwent contralateral risk-reducing mastectomy (CRRM), RRBSO without CRRM and both surgeries compared to those with no risk-reducing surgery. Median survival time for no surgery is 15.44 years. Hazard ratios and Confidence intervals compared to no surgery: RRBSO only HR 0.46 (0.27–0.78) p = 0.004; CRRM only HR 0.48 (0.19–1.14), p = 0.104; CRRM + RRBSO HR 0.16 (0.06–0.435), p < 0.001
Although women with CRRM had apparently reduced breast cancer and non-breast cancer mortality this result is potentially confounded by several factors including: (1) the trend for risk-reducing operations to be performed more recently over the period of study; (2) concomitant RRBSO; (3) differences in median follow-up (8.8 years for the CRRM group and 7.3 years for the non-CRRM group) and (4) differences in time to BRCA1/2 mutation testing (median of 3.6 years from the primary surgery in the CRRM group and of 7.1 years in the non-CRRM group).
After adjusting for potential confounders only CRRM [HR 0.28 (0.14–0.55)] and RRBSO [HR 0.34 (0.21–0.55)] were independently predictive of improved survival. However, grade was missing on 218 cases and ER status on 328 (mainly pre-2000). CRRM remained a significant predictor after adjusting for RRBSO [HR 0.42 (0.21–0.85, p = 0.016)].
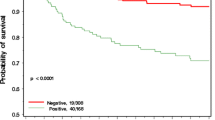
In order to circumvent the potential confounding of the effect of RRBSO, stage at diagnosis and tumour characteristics we performed a CC study where one control without CRRM was matched to the 105 cases with CRRM (Table 2). The mean age of the cases was 40.3 years (range 20.7–62.6) and of controls was 41.5 years (range 27.6–68.1 years). RRBSO was performed in 62/105 (59 %) in the CRRM group and 54/105 (51 %) in the non-CRRM group. The two groups were also otherwise well matched (Table 2). There were 25 % (26 deaths in 105) in the matched cohort without CRRM of which 24 (23 %) were due to breast cancer (Table 2) whereas there were 9 % (nine deaths in 105) in the CRRM group of which eight were due to breast cancer. Eleven deaths (69 % of the excess) in the controls including the two stage 0 cancers were almost certainly due to invasive contralateral cancers that had occurred 1.67–15 (median 5.5) years post the first primary. Survival was significantly greater from matched date of CRRM for CRRM cases [HR 0.37 (0.17–0.80, p = 0.008, Fig. 3). 10-year survival in these matched CRRM women was 89 % (76–95 %) compared to the 73 % (60–82 %) who had no CRRM. There was no significant difference in deaths between the patients who also had RRBSO, whereas there were 5/43 (12 %) deaths in the CRRM group and 20/51 (39 %) deaths in the non-CRRM group who did not have RRBSO, although this did not reach significance [HR 0.41(0.15–1.13, p = 0.085)]. CRRM nonetheless remained significant when adjusted for RRBSO [HR 0.43 (0.20–0.95, p = 0.036)] (Table 3).
Discussion
In this study, women who chose to undergo CRRM (15 %) had a significantly greater survival (breast-specific and all-cause) than women who did not undergo CRRM. The survival advantage was similar whether all carriers were included in the analysis or just carefully matched controls versus the CRRM group. This study also confirms the very high rate of CBC in BRCA1/2 mutation carriers who do not undergo CRRM (31 % at 10 years and 60 % at 20 years of follow-up) [1–4]. The contralateral risk is almost constant at 3 % per year. Only 105 (14 %) of women in this series elected to have CRRM which is partly reflected in the long-term nature of the study: for women diagnosed between 1985 and 2000, 10 % eventually elected to have a CRRM, whereas 22 % elected to have CRRM in the period 2001–2010. Recently published data indicate a generally increasing uptake of CRRM, which may now be as high as 50 % in mutation carriers, although there are clear cultural differences in either the offer or uptake of the operation [21–23]. The potential bias of operation at different time periods was addressed in the CC analysis since cases were matched with controls to within 5 years of diagnosis.
CRRM is highly effective in reducing the incidence of CBC. No contralateral cancers were detected in the operated group after a median follow-up of 7.2 years, whereas 33 % of women developed CBC after a median follow-up of 8.6 years in the CC group. Most studies report that CRRM reduces the risk of CBC by over 90 % [15, 18–20]. These studies also show occult invasive and non-invasive breast cancer in the removed breast similar to the 5 % detected in this report.
The reduction in mortality seen in this study was mainly related to reduction in breast cancer relapse and breast cancer-specific deaths in the CRRM group. Breast cancer deaths were reduced from 29.2 to 11.6 % in the overall study and from 22.8 to 7.6 % in the CC analysis. Four studies show evidence of breast cancer-specific survival after CRRM in women at high risk and in the general population. Peralta et al. [24] in a retrospective study described 64 patients who underwent CRRM compared to a control group of 182 patients not undergoing CRRM matched for age, stage and treatment; and compared for CBC rate, disease-free survival and overall survival. Only three breast cancers occurred (at initial surgery) in the CRRM group compared to 36 in controls in follow-up (p = 0.005). Disease-free survival at 15 years in the CRRM group was 55 % versus 28 % in the control group (p = 0.01). Overall survival at 15 years was 64 % compared to 48 % (39–58 %) in controls, but this was not significant (p = 0.26). A North American study used the Surveillance, Epidemiology, and End Results database to identify 107,106 women with breast cancer who had been treated with mastectomy between 1998 and 2003, including 8,902 women having CRRM [25]. CRRM was associated with improved disease-specific survival (HR 0.63, p < 0.001) largely related to a reduction in breast cancer-specific mortality in women aged 18–49 years with stages I–II ER-negative cancer (HR 0.68, p = 0.004). 5-year-adjusted breast cancer survival for the CRRM group was 88.5 versus 83.7 %, for those not having CRRM. This effect in ER-negative cancer was supported by 3,889 women from Texas [26]. A study from the Mayo clinic published further long-term results from their CRRM familial cohort [30]. Some 385 women with stage I/II familial breast cancer CRRM between 1971 and 1993 were compared to 385 patient controls matched for age at diagnosis, tumour stage and year of diagnosis who underwent unilateral mastectomy only. In the CRRM group 128 women had died compared to 162 women in the control group giving 10-year overall survival estimates of 83 and 74 % (HR 0.68, p = 0.001) [30].
Most women (77 %) in the current study undertook CRRM within 3 years of diagnosis of the primary tumour. Examination of the survival curves in Figs. 2 and 3 indicated that deaths are reduced during the first and also the second 5-year period thus reducing early and later deaths after the primary surgery. A CBC arising in the first 5 years after the primary diagnosis is a marker of poor survival from the primary although one occurring in the second 5 years is also associated with increased deaths compared to unilateral cancer only [7–11].
Women with BRCA1/2 mutations are at increased lifetime risk of ovarian cancer and increasing numbers elect to reduce ovarian cancer risk by undergoing RRBSO [2]. Removal of the ovaries in premenopausal women is known to reduce the risk of subsequent breast cancer by approximately 50 % [2]. More relevant to the present study, oophorectomy or drug-induced ovarian suppression after the primary surgery: reduces the risk of systemic relapse; improves breast cancer-specific survival and reduces the incidence of CBC [6, 17]. Consistent with these data, RRBSO in this study improved breast cancer-specific survival from 71.0 % in those who did not undergo surgery to 90.5 % in those with RRBSO indicating the dual effects of RRBSO in reducing ovarian cancer and breast relapse.
A confounding factor in our study is that 59 % of women also had RRBSO, either before or after CRRM. However, although the numbers were relatively small (n = 42), CRRM alone was associated with an improvement of breast-specific and overall survival both in the analysis of all cases and in the CC analysis. Interestingly, in the CC analysis, CRRM only was associated with improved survival whereas there was no significant improvement when CRRM was combined with RRBSO (compared to RRBSO alone) suggesting that the effect of RRBSO on CBC reduction is equivalent to CRRM. This is consistent with a recent study in BRCA1/2 carriers which showed no consistent survival advantage to CRRM when adjusted for RRBSO [19].
It is clear that RRBSO also influences survival in BRCA1/2 carriers with breast cancer. This is at least in part due to prevention of ovarian cancer, but may also be influenced by an adjuvant effect on the primary breast cancer and also by reducing the second primary risk. Nevertheless, in this study, CRRM alone was as effective as RRBSO alone at improving survival and the combination of both procedures gave the best overall survival.
A potential weakness of the present study is the need for the majority of patients to have been alive after their breast cancer diagnosis for genetic testing, which could introduce a survival bias. Nonetheless, overall survival especially in the non-CRRM group did not suggest a substantial bias and this group was tested on average longer after diagnosis than cases with CRRM. A perfect study would define mutation status at, or just after, diagnosis—this was only available for 40 (5 %) women in our study.
It is possible that new targeted treatments for cancers in BRCA1/2 carriers could dramatically reduce the new primary risk and make CRRM redundant. Nonetheless, for the time being CRRM remains an important option in the decision-making process for BRCA carriers with breast cancer. In particular we are now beginning to see the mortality reduction predicted in this journal 2 years ago [31]. The evidence that CRRM improves survival in the general population, in those with familial (mostly non-BRCA1/2) risks and in BRCA1/2 carriers, is likely to continue to push up CRRM rates. The CRRM rates in breast cancer patients increased 12 fold in the 10 years to 2007 [21].
The reasons for women choosing to undergo CRRM were not assessed directly in this study. However, the primary motivating factors appear to be: not wanting to be diagnosed and not wanting to go through more breast cancer treatment. A number of women were denied the opportunity to have CRRM as the primary treatment and were only able to take this up when their mutation status was confirmed and as reported previously by us if the option is not discussed by surgeons uptake even in mutation carriers is low [32]. Many women not offered CRRM as the primary treatment do not want to undergo further treatment when diagnosed as a mutation carrier some years later. There are obviously complications from CRRM including: wound infection, loss of implant and poor cosmetic results from reconstruction.
There are some limitations to the present study. It was not an entirely prospective analysis, and although follow-up information is good, survival biases cannot be completely eliminated even in the matched set. Survival advantage cannot be explained for CRRM by prevention of CBC alone and an explanation for reduced death rates from the original ipsilateral breast cancer is required. Perhaps elimination of nearly all breast tissue prevents some deaths by prevention of locoregional and distant (contralateral breast) recurrence.
Conclusion
There is now convincing evidence that CRRM confers a survival advantage. Women at high risk of contralateral disease should at least have this option explored at the time of deciding on the treatment of their first primary. In addition, the option of RRBSO should be discussed with women at increased risk of ovarian cancer as this is a potentially adjuvant treatment.
References
Graeser MK, Engel C, Rhiem K et al (2009) Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 27(35):5887–5892
Metcalfe K, Gershman S, Lynch HT et al (2011) Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 104(9):1384–1392
Malone KE, Begg CB, Haile RW et al (2010) Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol 28(14):2404–2410
Robson ME, Chappuis PO, Satagopan J et al (2004) A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 6(1):R8–R17
Peto J, Mack TM (2000) High constant incidence in twins and other relatives of women with breast cancer. Nat Genet 26(4):411–414
Schaapveld M, Visser O, Louwman WJ et al (2008) The impact of adjuvant therapy on contralateral breast cancer risk and the prognostic significance of contralateral breast cancer: a population based study in the Netherlands. Breast Cancer Res Treat 110(1):189–197
Abdalla I, Thisted RA, Heimann R (2000) The impact of contralateral breast cancer on the outcome of breast cancer patients treated by mastectomy. Cancer J 6(4):266–272
Rubino C, Arriagada R, Delaloge S, Le MG (2010) Relation of risk of contralateral breast cancer to the interval since the first primary tumour. Br J Cancer 102(1):213–219
Vichapat V, Garmo H, Holmqvist M et al (2012) Tumor stage affects risk and prognosis of contralateral breast cancer: results from a large swedish-population-based study. J Clin Oncol 30(28):3478–3485
Alkner S, Bendahl PO, Ferno M, Manjer J, Ryden L (2011) Prediction of outcome after diagnosis of metachronous contralateral breast cancer. BMC Cancer 11:114
Shi YX, Xia Q, Peng RJ et al (2012) Comparison of clinicopathological characteristics and prognoses between bilateral and unilateral breast cancer. J Cancer Res Clin Oncol 138(4):705–714
Tilanus-Linthorst MM, Bartels KC, Alves C et al (2006) Selection bias influences reported contralateral breast cancer incidence and survival in high risk non-BRCA1/2 patients. Breast Cancer Res Treat 95(2):117–123
Wang T, Liu H, Chen KX, Xun P, Li HX, Tang SC (2011) The risk factors and prognosis of bilateral primary breast cancer: a comparative study with unilateral breast cancer. Oncol Res 19(3–4):171–178
Kurian AW, McClure LA, John EM, Horn-Ross PL, Ford JM, Clarke CA (2009) Second primary breast cancer occurrence according to hormone receptor status. J Natl Cancer Inst 101(15):1058–1065
Yi M, Meric-Bernstam F, Middleton LP et al (2009) Predictors of contralateral breast cancer in patients with unilateral breast cancer undergoing contralateral prophylactic mastectomy. Cancer 115(5):962–971
Ciatto S, Houssami N, Martinelli F, Bonardi R, Cafferty FH, Duffy SW (2008) Second breast cancers in a Tuscan case series: characteristics, prognosis, and predictors of survival. Br J Cancer 99(3):539–544
Gronwald J, Tung N, Foulkes WD et al (2006) Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: an update. Int J Cancer 118(9):2281–2284
McDonnell SK, Schaid DJ, Myers JL et al (2001) Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. J Clin Oncol 19(19):3938–3943
van Sprundel TC, Schmidt MK, Rookus MA et al (2005) Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer 93(3):287–292
Kaas R, Verhoef S, Wesseling J et al (2010) Prophylactic mastectomy in BRCA1 and BRCA2 mutation carriers: very low risk for subsequent breast cancer. Ann Surg 251(3):488–492
Yao K, Stewart AK, Winchester DJ, Winchester DP (2010) Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the national cancer data base, 1998–2007. Ann Surg Oncol 17(10):2554–2562
Tuttle TM, Abbott A, Arrington A, Rueth N (2010) The increasing use of prophylactic mastectomy in the prevention of breast cancer. Curr Oncol Rep 12(1):16–21
Graves KD, Peshkin BN, Halbert CH, DeMarco TA, Isaacs C, Schwartz MD (2007) Predictors and outcomes of contralateral prophylactic mastectomy among breast cancer survivors. Breast Cancer Res Treat 104(3):321–329
Peralta EA, Ellenhorn JD, Wagman LD, Dagis A, Andersen JS, Chu DZ (2000) Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. Am J Surg 180(6):439–445
Bedrosian I, Hu CY, Chang GJ (2010) Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst 102(6):401–409
Brewster AM, Bedrosian I, Parker PA et al (2012) Association between contralateral prophylactic mastectomy and breast cancer outcomes by hormone receptor status. Cancer 118(22):5637–5643
Chung A, Huynh K, Lawrence C, Sim MS, Giuliano A (2012) Comparison of patient characteristics and outcomes of contralateral prophylactic mastectomy and unilateral total mastectomy in breast cancer patients. Ann Surg Oncol 19(8):2600–2606
Maurice A, Evans DG, Affen J, Greenhalgh R, Duffy SW, Howell A (2012) Surveillance of women at increased risk of breast cancer using mammography and clinical breast examination: further evidence of benefit. Int J Cancer 131(2):417–425
Cummings P, McKnight B (2004) Analysis of matched cohort data. The Stata Journal 4(3):274–281
Boughey JC, Hoskin TL, Degnim AC et al (2010) Contralateral prophylactic mastectomy is associated with a survival advantage in high-risk women with a personal history of breast cancer. Ann Surg Oncol 17(10):2702–2709
Narod SA (2011) The impact of contralateral mastectomy on mortality in BRCA1 and BRCA2 mutation carriers with breast cancer. Breast Cancer Res Treat 128(2):581–583
Evans DGR, Lalloo F, Hopwood P, Maurice A, Baildam A, Brain A, Barr L, Howell A (2005) Surgical decisions made by 160 women detected with breast cancer <50 years of age. Eur J Surg Oncol 31(10):1112–1118
Acknowledgments
FL and DGE are supported by the Manchester NIHR Biomedical Research Centre; DGE, AH and AB by the Genesis Breast Cancer Prevention Appeal. AH is supported by Breakthrough Breast Cancer, DGE is an NIHR Senior Investigator. IB is supported by the MRC Health eResearch Centre. The study sponsor had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The corresponding author confirms that he had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All aspects of this study comply with the current laws of the United Kingdom of Great Britain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evans, D.G.R., Ingham, S.L., Baildam, A. et al. Contralateral mastectomy improves survival in women with BRCA1/2-associated breast cancer. Breast Cancer Res Treat 140, 135–142 (2013). https://doi.org/10.1007/s10549-013-2583-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2583-1