Abstract
Background
In patients with persistent (P-PHPT) or recurrent (R-PHPT) primary hyperparathyroidism, preoperative localization is important. Selective parathyroid hormone venous sampling (sPVS) is an invasive technique that can be used to regionalize and/or lateralize the source of PHPT when noninvasive imaging studies are nonlocalizing. The aim of the present study was to assess the role of sPVS in the preoperative evaluation of patients with P-PHPT or R-PHPT and negative, equivocal, or discordant noninvasive imaging localization.
Methods
After IRB-approval a retrospective review of all patients with P-PHPT or R-PHPT and nonlocalizing noninvasive imaging that underwent sPVS from 2000 to 2014 was performed. The location of the source of PHPT at sPVS was predicted by a parathyroid hormone (PTH) gradient and compared to the surgical, pathology, and biochemical follow-up data as the gold standard. Sensitivity and positive predictive value (PPV) were calculated.
Results
Of 30 patients who underwent sPVS, 12 patients did not undergo surgical exploration due to negative or non-localizing PTH gradient (n = 8) or opted for medical management (n = 4). Of the 18 patients who underwent surgical exploration, 17 (94 %) had a positive PTH gradient and pathologic parathyroid tissue identified at surgery. Sensitivity and PPV of sPVS were 93 and 77 %, respectively, for all surgical cases, 86 and 60.0 % for cervical cases (n = 11), and 100 and 100 % for mediastinal cases (n = 7). Sixteen patients (89 %) were surgically cured.
Conclusions
In patients with P-PHPT or R-PHPT and nonlocalizing imaging studies, sPVS is a sensitive test for localizing the source of PHPT when a positive PTH gradient is present.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary hyperparathyroidism (PHPT) is a common cause of endogenous hypercalcemia with an estimated recent overall incidence of 50.4 cases per 100,000 person-years [1, 2]. PHPT is caused by a solitary benign adenoma in 80–85 % of patients, multiglandular hyperplasia in 10–15 % of patients, and parathyroid carcinoma in <1 % of patients [3]. Surgical management is the treatment of choice with cure rates of 88–97 % and low surgical morbidity of 2 % [4, 5].
However, 5–10 % of patients may develop biochemically confirmed recurrent PHPT (R-PHPT; elevated PTH and calcium >6 months after surgical exploration) or persistent PHPT (P-PHPT; elevated PTH and calcium ≤6 months after surgical exploration). Diagnostic considerations may include ectopic parathyroid adenoma, multigland parathyroid disease or parathyroid carcinoma. In patients with R-PHPT or P-PHPT, preoperative localization is critical to guide the surgical approach and optimize the success of remedial surgical exploration [4, 6].
Initial localization with noninvasive imaging may include ultrasonography, parathyroid scan, four-dimensional computed tomography (4D-CT) and/or magnetic resonance imaging (MRI) with sensitivities ranging from 64 to 95 % [7, 8]. However, in patients with negative, equivocal, or discordant noninvasive imaging studies, selective parathyroid venous sampling (sPVS) is an invasive interventional radiologic technique that can be used to regionalize and/or lateralize the source of PHPT to the cervical and/or mediastinal region with a sensitivity of greater than 90 % [6, 8].
The aim of the present study was to assess the role of selective parathyroid hormone venous sampling (sPVS) in the pre-operative evaluation of 30 patients with persistent (P-PHPT) or recurrent (R-PHPT) PHPT and negative, equivocal, or discordant noninvasive imaging localization.
Methods
After approval by the Institutional Review Board, we conducted a Health Insurance Portability and Accountability Act–compliant, retrospective review using the comprehensive electronic medical record system of all patients with biochemically confirmed recurrent or persistent PHPT (above normal PTH with a serum calcium >10.1 mg/dL) who underwent sPVS in the period from 1/1/2000 to 9/1/2014. Although the methods used to measure serum calcium levels changed over time at our institution, the normal range remained unchanged (8.9–10.1 mg/dL) throughout the duration of the study since instrumentation was calibrated against atomic absorption spectrophotometry according to certified references from the National Bureau of Standards. Serum intact PTH was measured by a two-site immunochemiluminometric assay, which changed throughout the course of the study with normal ranges as follows: 1.0–5.2 pmol/L by manual bead immunoassay (in-house assay) in 2000 to December 2, 2003; 10–55 pg/mL (1.1–5.8 pmol/L) by Nichols Advantage (Nichols Institute Diagnostics, San Juan Capistrano, CA U.S.A.) in December 3, 2003 to May 1, 2006; 10–67 pg/mL (1.1–7.1 pmol/L) by Diagnostics Products Corporation assay (Diagnostics Products Corporation, Los Angeles, CA U.S.A.) in March 31, 2005 to July 15, 2007; and 15 to 65 pg/mL (1.59 to 6.90 pmol/L) as performed on the Roche Cobas 6000 (Roche Diagnostics GmbH, Mannheim, Germany) since July 16, 2007. Patients who did not give approval for the use of their medical record for research purposes were excluded. Clinical, laboratory, radiologic, surgical, and pathology data were abstracted from the comprehensive electronic medical record. Postoperative surgical cure was defined as eucalcemia with a serum calcium <10.1 mg/dL at 6-month follow-up. Persistent PHPT was defined as an inappropriate PTH with a serum calcium >10.1 mg/dL at 6-month follow-up.
Selective parathyroid venous sampling (sPVS)
sPVS was performed as an outpatient procedure by a single interventional radiologist. Under conscious sedation, a 5Fr sheath was inserted into the right femoral vein using the Seldinger technique. Selective catheterization was performed of the lower inferior vena cava and multiple cervical and mediastinal veins and five milliliters of blood was obtained at each site for PTH measurement.
sPVS and noninvasive imaging review and interpretation
The location of the source of PHPT at sPVS was predicted by a PTH gradient, defined as a ≥2.0-fold PTH elevation relative to the inferior vena cava PTH level in at least one dominant vein. The preoperative localization by noninvasive imaging modalities was collected from the radiology reports. The preoperative localization by sPVS and noninvasive imaging modalities was compared to the surgical, pathology and biochemical follow-up data as the gold standard and the sensitivity and positive predictive value (PPV) were calculated. A diagnostic test was deemed true positive if the study accurately displayed the location of the single abnormal gland (i.e. right side of neck, mediastinum, etc.) or all regions of overactivity in the case of multigland disease. The study was deemed false positive if it indicated an abnormal gland at a site different from found intraoperatively, and false negative if at least one abnormal gland was not found from the study [4]. A negative noninvasive localization was defined as no identification of a suspicious lesion on any non-invasive imaging modality. An equivocal noninvasive localization was defined as identification of a suspicious lesion on one non-invasive imaging modality but not confirmed on one or more other non-invasive imaging modalities. A discordant noninvasive localization was defined as two or more conflicting lesions identified with noninvasive imaging studies.
Data management and statistical methods
Study data were collected and managed using REDCap electronic data capture tools [9]. Descriptive statistics were generated. Statistical analysis was performed using JMP 10.0 (SAS Institute Inc., Raleigh, NC). Change in serum calcium, phosphorus, and PTH before and after surgery was compared using a paired t test.
Results
Patient characteristics
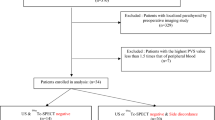
Thirty-two patients who had a biochemical diagnosis of PHPT (above normal PTH with a serum calcium >10.1 mg/dL), history of at least one non-curative prior surgery and underwent sPVS for their P-PHPT and R-PHPT were identified. Two patients were excluded due to a final diagnosis of metastatic parathyroid carcinomatosis. Our final review included 30 patients. Their clinical and surgical details prior to sPVS are summarized in Table 1. There were no significant postoperative complications or deaths.
Remedial parathyroidectomy
Of the 30 patients, 18 (60 %) were treated surgically. Twelve patients did not undergo surgical exploration due to negative or non-localizing PTH gradient at sPVS (n = 8) or opted for medical management (n = 4). Clinical, laboratory, radiologic, surgical, and pathology data on the surgically managed patients are summarized in Table 2. Of these 18 patients, 17 (94 %) had a positive PTH gradient from sPVS and pathologic parathyroid tissue identified at subsequent surgery [solitary adenoma (n = 15), multiglandular hyperplasia (n = 2)]. One patient with a negative PTH gradient was surgically explored without resection and continued to have P-PHPT (false negative sPVS).
Postoperatively, serum calcium, serum phosphorus, and serum PTH levels were significantly changed (p < 0.0001, 0.001, and 0.01, respectively). Postoperative follow-up indicated that sixteen patients (89 %) were cured and two (11 %) had P-HPT, one of whom had no resection from the surgery. There was only one minor post-operative complication: an asymptomatic cervical hematoma that was managed non-operatively.
Selective parathyroid venous sampling
sPVS was positive in 21 of 30 patients (70 %). No complications occurred from the catheterization. Details on presence of PTH gradient, value and location of the maximum PTH, and the exact location during exploration are shown in Table 3. Table 4 provides details of sPVS on the 12 non-surgical candidates. Sensitivity and positive predictive value of sPVS were 93 and 77 %, respectively, for all surgical cases, 86 and 60.0 %, respectively, for cervical cases (n = 11), and 100 and 100 %, respectively, for mediastinal cases (n = 7). These values exceeded the sensitivity and positive predictive value of noninvasive diagnostic tests (Table 5) with sensitivities of ultrasound, parathyroid subtraction scanning (99mTechnetium-sestamibi/123Iodine single-photon emission computed tomography and computed tomography—SPECT/CT), and conventional CT from this cohort of patients of 23, 33, and 25 %, respectively. Moreover, even considering the eight patients with negative sPVS who did not go to surgical exploration as false negative exams, the overall sensitivity for sPVS still exceeds these other noninvasive studies at 59 % (13 of 22 patients).
Discussion
In patients with persistent or recurrent PHPT, preoperative localization can be challenging. Given the increased risk of complications and cost associated with reoperation for PHPT, effective preoperative localization is critical [10–12]. Ultrasonography and parathyroid scanning are considered first line localization studies in patients with PHPT. A prior study has shown that CT with 99mTechnetium-sestamibi single-photon emission computed tomography (CT-MIBI-SPECT image fusion) is a sensitive preoperative localization test (88 %) in patients with PHPT for first time operations [13]. However, the accuracy of ultrasonography and parathyroid scanning has been shown to be significantly lower among patients undergoing reoperative parathyroidectomy for PHPT and the data from the present series support these prior findings [14]. For example, 15 of 18 surgical patients in the present study underwent parathyroid subtraction scanning (99mTechnetium-sestamibi/123Iodine SPECT/CT) which showed a sensitivity of 33 % and an overall accuracy of 20 %. On the other hand, from the present cohort, sPVS serves as a sensitive test (>90 %) to localize the ectopic or non-ectopic culprit glands among the reoperated patients with a positive PTH gradient. The present data agree with previous studies regarding the superior sensitivity of sPVS to noninvasive imaging modalities in this challenging patient population [6, 15–17].
According to the present data, sPVS can be useful in guiding surgical exploration in patients with diverse etiologies for recurrent or persistent PHPT-missed single adenoma, persistent multigland disease, and multiple endocrine neoplasia [4, 18, 19]. Nonetheless, there were four false positive and one false negative sPVS localizations. Interestingly, all of these false localizations occurred in the cervical region. One hypothesis is that prior cervical operations may alter the standard anatomic venous drainage of the cervical region, thereby making interpretations on regionalization or lateralization particularly challenging. This hypothesis is supported by the fact that the sensitivity and PPV were 100 and 100 %, respectively, for mediastinal lesions, but only 86 and 60 %, respectively, for cervical lesions. For example, in one patient with a prior cervical exploration, an inferior thyroid vein was positive at sPVS, but the vein drained both sides of neck. Consequently, sPVS could regionalize the pathology to the cervical region but not lateralize the pathology. Taken together, sPVS should be undertaken by an interventional radiologist experienced with endocrine venous sampling and interpreted in collaboration with an endocrine surgeon familiar with the patients’ prior operative anatomy.
Use of parathyroid scans and intraoperative PTH have not changed cure rates and operative morbidity appreciably in patients with R-PHPT or P-PHPT; integrating sPVS in the pre-operative course can assist in careful patient selection [4, 19]. However, should sPVS yield a negative result, the present data argue against blind surgical exploration. From the cohort of 30 patients, there were 8 such patients who were advised not to undergo surgical exploration due to negative or non-localizing PTH gradient at sPVS and rather were managed medically. It is unclear why these eight patients with biochemical evidence of PHPT had a completely negative PTH gradient in either the cervical or mediastinal region. Of the surgically treated 18 patients in our study, four had negative non-invasive imaging results but were indicated for surgery solely based on sPVS, and all of them were successfully cured by the surgery. Among the 14 patients with equivocal or discordant noninvasive imaging, all but two patients were cured. The sPVS findings agreed with the noninvasive imaging findings in 7 of 14 patients, with both exams correctly positive in 5 of 7, both falsely positive in 1 of 7 (FP) and both falsely negative in 1 of 7 (FN). Among the 7 patients in whom the sPVS findings disagreed with noninvasive imaging findings, sPVS was correct in 4 of 7 while the original noninvasive imaging was correct in 3 of 7 patients. Taken together, sPVS alone or as supplementary information to the discordant or equivocal noninvasive imaging findings may provide useful lateralization and/or regionalization information to help guide surgical management.
Introduction of new diagnostic imaging modalities can affect the use of older tests, particularly if the new tests are less invasive. The use of sPVS at our institution has decreased by half, from an average of 3.5 cases per year (median 3, range 2–8) in the period of 2003–2008 to 1.7 cases per year (median 1.5, range 0–4) from 2009 to 2014. One explanation for the reduced use of sPVS was the introduction of 4D-CT at our institution in 2008. 4D-CT is a multiphasic, cross-sectional imaging study that captures the rapid uptake and washout of contrast from parathyroid adenomas. 4D-CT has emerged as a sensitive noninvasive imaging modality for preoperative localization of parathyroid pathology, both in the initial and re-operative setting with a sensitivity ≥88 % [20–22]. However, there is an increase in total radiation dose (10.4 mSv) with 4D CT and it is not widely available [8]. According to a recent study on 28 patients with P-PHPT and R-PHPT and negative sestamibi and ultrasound examinations, the sensitivity with the use of 4D-CT by itself was 50 %, and it was increased to 95 % with the additional use of sPVS on the same cohort [23]. As such, 4D-CT may be considered prior to sPVS given that it is sensitive, noninvasive, and less operator dependent than sPVS.
Fine needle aspiration (FNA) with PTH washout is another localization consideration when a suspicious lesion is present on ultrasound. Bancos et al. showed that FNA with PTH washout had a sensitivity of 84 %, specificity of 100 %, positive predictive value of 100 %, and accuracy of 84 % [24]. However, while the PTH washout levels may be useful in distinguishing parathyroid tissue from thyroid, lymphatic or other soft tissue, this requires visualization of a lesion on ultrasound which may be more challenging in the re-operative setting. Additionally, there is a small risk of inflammation, abscess or hematoma that may impact the option of minimally invasive parathyroidectomy [24]. In the present series, four patients had US-guided FNA with PTH washout with an overall accuracy of 25 %. Last, 11C-methionine positron emission tomography/computed tomography (Met-PET/CT) has shown to be a sensitive test (≥86 %) for preoperative localization of single-gland parathyroid adenomas and may be considered as an additional noninvasive localization test prior to sPVS, depending on institutional availability [25–27]. Taken together, sPVS may be an important adjunct to 4D-CT, US-guided FNA with PTH washout and/or Met-PET/CT when the results are negative or equivocal.
The current study has multiple strengths, including its focus on a specific cohort of P-PHPT or R-PHPT patients who underwent sPVS during their pre-operative diagnostic workup, the availability of detailed medical and surgical history, including demographics, presenting symptoms/signs, imaging data, operative characteristics, and follow-up. This study is limited by the cohort primarily consisting of a referral population, retrospective study design, and variable histopathological diagnoses. Regardless of the heterogeneous etiology, the patients worked up with sPVS demonstrated a high rate of operative success despite the challenges in management of their P-PHPT and R-PHPT including previous operation(s).
The present study assessed the diagnostic workup, operative characteristics, and outcome of 30 patients undergoing selective venous parathyroid sampling for persistent or recurrent hyperparathyroidism. These data support the hypothesis that for patients with negative, equivocal, or discordant noninvasive imaging studies, sPVS can serve as a sensitive test for localizing the source of PHPT when a positive PTH gradient is present. This invasive yet sensitive modality effectively helped determine surgical candidacy and helped to guide surgical exploration. Given that sPVS is an invasive test, the decision to undertake sPVS should only be undertaken after biochemical confirmation of PHPT and review of available diagnostic quality noninvasive imaging studies by a multidisciplinary team with expertise with endocrinology, endocrine surgery and endocrine venous sampling.
References
Griebeler ML, Kearns AE, Ryu E et al (2015) Secular trends in the incidence of primary hyperparathyroidism over five decades (1965–2010). Bone 73:1–7
Yeh MW, Ituarte PH, Zhou HC et al (2013) Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab 98:1122–1129
Rao DS, Honasoge M, Divine GW et al (2000) Effect of vitamin D nutrition on parathyroid adenoma weight: pathogenetic and clinical implications. J Clin Endocrinol Metab 85:1054–1058
Thompson GB, Grant CS, Perrier ND et al (1999) Reoperative parathyroid surgery in the era of sestamibi scanning and intraoperative parathyroid hormone monitoring. Arch Surg 134:699–704
Udelsman R, Lin Z, Donovan P (2011) The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg 253:585–591
Lebastchi AH, Aruny JE, Donovan PI et al (2015) Real-time super selective venous sampling in remedial parathyroid surgery. J Am Coll Surg 220:994–1000
Cheung K, Wang TS, Farrokhyar F et al (2012) A meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism. Ann Surg Oncol 19:577–583
Kunstman JW, Kirsch JD, Mahajan A et al (2013) Clinical review: parathyroid localization and implications for clinical management. J Clin Endocrinol Metab 98:902–912
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381
Morris LF, Zanocco K, Ituarte PH et al (2010) The value of intraoperative parathyroid hormone monitoring in localized primary hyperparathyroidism: a cost analysis. Ann Surg Oncol 17:679–685
Sosa JA, Powe NR, Levine MA et al (1998) Cost implications of different surgical management strategies for primary hyperparathyroidism. Surgery 124:1028–1035
Doherty GM, Weber B, Norton JA (1994) Cost of unsuccessful surgery for primary hyperparathyroidism. Surgery 116:954–957
Prommegger R, Wimmer G, Profanter C et al (2009) Virtual neck exploration: a new method for localizing abnormal parathyroid glands. Ann Surg 250:761–765
Witteveen JE, Kievit J, Stokkel MP et al (2011) Limitations of Tc99 m-MIBI-SPECT imaging scans in persistent primary hyperparathyroidism. World J Surg 35:128–139
Udelsman R, Aruny JE, Donovan PI et al (2003) Rapid parathyroid hormone analysis during venous localization. Ann Surg 237:714–719
Witteveen JE, Kievit J, van Erkel AR et al (2010) The role of selective venous sampling in the management of persistent hyperparathyroidism revisited. Eur J Endocrinol 163:945–952
Seehofer D, Steinmuller T, Rayes N et al (2004) Parathyroid hormone venous sampling before reoperative surgery in renal hyperparathyroidism: comparison with noninvasive localization procedures and review of the literature. Arch Surg 139:1331–1338
Lau JH, Drake W, Matson M (2007) The current role of venous sampling in the localization of endocrine disease. Cardiovasc Intervent Radiol 30:555–570
Richards ML, Thompson GB, Farley DR et al (2008) Reoperative parathyroidectomy in 228 patients during the era of minimal-access surgery and intraoperative parathyroid hormone monitoring. Am J Surg 196:937–942
Mortenson MM, Evans DB, Lee JE et al (2008) Parathyroid exploration in the reoperative neck: improved preoperative localization with 4D-computed tomography. J Am Coll Surg 206:888–895
Rodgers SE, Hunter GJ, Hamberg LM et al (2006) Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 140:932–940
Day KM, Elsayed M, Beland MD et al (2015) The utility of 4-dimensional computed tomography for preoperative localization of primary hyperparathyroidism in patients not localized by sestamibi or ultrasonography. Surgery 157:534–539
Ginsburg M, Christoforidis GA, Zivin SP et al (2015) Adenoma localization for recurrent or persistent primary hyperparathyroidism using dynamic four-dimensional CT and venous sampling. J Vasc Interv Radiol 26:79–86
Bancos I, Grant CS, Nadeem S et al (2012) Risks and benefits of parathyroid fine-needle aspiration with parathyroid hormone washout. Endocr Pract 18:441–449
Weber T, Maier-Funk C, Ohlhauser D et al (2013) Accurate preoperative localization of parathyroid adenomas with C-11 methionine PET/CT. Ann Surg 257:1124–1128
Traub-Weidinger T, Mayerhoefer ME, Koperek O et al (2014) 11C-methionine PET/CT imaging of 99mTc-MIBI-SPECT/CT-negative patients with primary hyperparathyroidism and previous neck surgery. J Clin Endocrinol Metab 99:4199–4205
Lenschow C, Gassmann P, Wenning C et al (2015) Preoperative (1)(1)C-methionine PET/CT enables focused parathyroidectomy in MIBI-SPECT negative parathyroid adenoma. World J Surg 39:1750–1757
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no potential or real conflicts of interest or financial support related to the content of this manuscript.
Rights and permissions
About this article
Cite this article
Sun, P.Y., Thompson, S.M., Andrews, J.C. et al. Selective Parathyroid Hormone Venous Sampling in Patients with Persistent or Recurrent Primary Hyperparathyroidism and Negative, Equivocal or Discordant Noninvasive Imaging. World J Surg 40, 2956–2963 (2016). https://doi.org/10.1007/s00268-016-3621-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3621-z