Abstract
Endocrine venous sampling plays a specific role in the diagnosis of endocrine disorders. In this article, we cover inferior petrosal sinus sampling, selective parathyroid venous sampling, hepatic venous sampling with arterial stimulation, adrenal venous sampling, and ovarian venous sampling. We review their indications and the scientific evidence justifying these indications in the diagnosis and management of Cushing’s syndrome, hyperparathyroidism, pancreatic endocrine tumors, Conn’s syndrome, primary hyperaldosteronism, pheochromocytomas, and androgen-secreting ovarian tumors. For each sampling technique, we compare its diagnostic accuracy with that of other imaging techniques and, where possible, look at how it impacts patient management. Finally, we incorporate venous sampling into diagnostic algorithms used at our institution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Role of Bilateral Inferior Petrosal Sinus Sampling in Cushing’s Syndrome
Cushing’s syndrome is a clinical state resulting from prolonged, inappropriate exposure to glucocorticoids [1]. The most common cause is exogenous steroid use. Among the endogenous causes, ACTH-dependent causes account for 80%, of which 85% are due to pituitary Cushing’s disease and 15% are due to ectopic secretion of ACTH.
The best treatment of pituitary Cushing’s disease is by surgical removal of the causative ACTH-secreting corticotrope adenoma. Therefore, differentiating Cushing’s disease from ectopic ACTH secretion is important to guide treatment. However, this is one of the most difficult challenges facing clinicians.
Although various noninvasive biochemical tests are available to differentiate pituitary Cushing’s disease and ectopic ACTH secretion, none have been validated as providing 100% diagnostic accuracy in large series or meta-analyses [1].
The conventional neuroradiological imaging techniques of CT and MRI have poor sensitivity and specificity for identifying a pituitary mass lesion in patients with Cushing’s disease [1, 2]. Approximately 40%–50% of cases of pituitary Cushing’s disease will have no abnormalities seen on MRI [4–7]. Moreover, the demonstration of a lesion on cross-sectional imaging, especially if it is <5 mm, does not necessary imply functionality, since 10% of the general population in the 30- to 40-year age group harbor a pituitary incidentaloma [3].
For these reasons, central venous sampling was developed to improve the diagnostic accuracy in distinguishing a pituitary from an ectopic source of ACTH.
In this section, we address two common problems facing clinicians when dealing with ACTH-dependent Cushing’s syndrome:
-
1.
How accurate is central venous sampling in differentiating ectopic ACTH syndrome from those 40%–50% of patients with Cushing’s disease who have no abnormalities on MRI [4–7]?
-
2.
Does central venous sampling accurately localize the pituitary adenoma in patients with presumed Cushing’s disease?
Bilateral Inferior Petrosal Sinus Sampling (BIPSS): Radiological Technique
In this technique, originally described by Corrigan et al. [8], two sheaths are inserted via the same or bilateral femoral veins. Catheters are advanced into the internal jugular veins and baseline samples are obtained. The catheters are then advanced into the inferior petrosal sinuses bilaterally. At the authors’ institution, the patients are routinely given 3000 units of heparin to prevent pericatheter thrombosis. The most useful catheter is a multipurpose long 5- to 6-F cobra catheter with a side hole close to the tip. In some instances, a 3-F microcatheter may also be useful. Good catheter position is confirmed by demonstrating crossover flow into the contralateral petrosal sinus upon contrast injection (Fig. 1). Samples are taken simultaneously from both catheters and peripherally. A basal ratio of central-to-peripheral ACTH values of ≥2 is strongly indicative of Cushing’s disease [9].
In a significant minority of patients subsequently shown to have Cushing’s disease histologically, the basal ratio is <2. This is because ACTH secretion is episodic, and sampling may miss the burst of ACTH secretion. For this reason, corticotrophin releasing hormone (CRH) is used as a stimulating agent to increase the sensitivity of the test. Plasma ACTH samples are obtained from both inferior petrosal sinuses and peripherally at 5 and 10 min after intravenous administration of 100 μg of human or ovine CRH. A peak stimulated (5 min post-CRH) central-to-peripheral ratio of ≥3 is strongly indicative of Cushing’s disease [9].
How Accurate Is It?
A systematic analysis by Newell-Price et al. [1], including 21 studies involving 569 patients, found that inferior petrosal sinus sampling with CRH stimulation achieves 96% sensitivity and 100% specificity in discriminating Cushing’s disease from ectopic ACTH secretion.
Does It Predict Tumor Lateralization?
An interpetrosal sinus ratio of ≥1.4 has been suggested as being indicative of ipsilateral localization of a microadenoma [10]. Using this ratio, Newell-Price et al. [1] reviewed 19 lateralization studies using pituitary surgery as the gold standard. The overall diagnostic accuracy was only 78% (range, 50%–100%).
Cavernous Sinus Sampling
Cavernous sinus sampling was first described by Teramoto and coworkers in 1993 [11]. The cavernous sinuses receive blood directly from the pituitary gland before draining into the inferior petrosal sinuses. Therefore, in theory, it might be possible to predict the exact tumor location within the pituitary gland using cavernous sinus sampling, and obviate the need for CRH administration. However, cavernous sinus sampling is technically more demanding, requires the routine use of microcatheter technique, and is rarely used.
Cavernous Sinus Sampling Versus BIPSS: Which Is Better?
Graham et al. [12] prospectively studied 93 consecutive patients with ACTH-dependent Cushing’s syndrome who underwent bilateral simultaneous cavernous sinus sampling before and after CRH administration. They reported that cavernous sinus sampling was as efficacious as published IPSS results in achieving 93% diagnostic accuracy in distinguishing pituitary Cushing’s disease from ectopic ACTH secretion without CRH stimulation, and 100% with CRH. They also found cavernous sinus sampling to be highly accurate in predicting intrapituitary tumor lateralization, with a positive predictive value of 85% for locating a lateral tumor in all patients and 90% if there was good catheter position and symmetric venous flow. This is superior to published results for inferior petrosal sinus sampling, where the average diagnostic accuracy for tumor lateralization is 78%.
However, these excellent lateralization results obtained by Graham et al. [12] were not replicated in a more recent study by Liu and coworkers [7]. They retrospectively analyzed 95 patients who had both inferior petrosal sinus sampling with CRH stimulation and cavernous sinus sampling without CRH administration. As for inferior petrosal sinus sampling, they found a 97% diagnostic accuracy for inferior petrosal sinus sampling with CRH stimulation, compared to 86% for cavernous sinus sampling, in line with other published results. They did not have data for cavernous sinus sampling after CRH stimulation because they found it technically infeasible to withdraw adequate blood volume through the microcatheters quickly enough during the CRH stimulation period.
Another finding from this study was that in patients with suspected Cushing’s disease based on central venous sampling, and in whom an adenoma was not found on MRI or located by the surgeon intraoperatively, hemihypophysectomy based on lateralization data was successful in only 10 of the 18 patients (56%) with asymmetric venous drainage, and in 5 of 10 patients with symmetrical drainage.
Children and Adolescents
The pediatric and adolescent population appear to present particular difficulties to cross-sectional imaging of the pituitary gland. At the authors’ institution, inferior petrosal sinus sampling has been found to be accurate in localization of pituitary adenomas and guiding transsphenoidal surgery in this patient group [13, 14].
Complications
Most patients experience transient pain or discomfort in the ear while the catheters are being placed. Other, more adverse complications include brainstem stroke [15, 16], venous subarachnoid hemorrhage [17], cavernous sinus thrombosis, cranial nerve palsies, groin hematomas, pulmonary emboli, and deep vein thromboses.
Pitfalls
Central venous sampling simply identifies the pituitary as the source of ACTH secretion and does not distinguish Cushing’s disease from those with pseudo-Cushing’s states, those with normal hypothalamic-pituitary-adrenal function, or patients with the rare syndrome of ectopic CRH secretion. Hence, for candidates undergoing any selective venous samplings, the general diagnosis should be firmly established before embarking on localization procedures.
False-positive results have been reported in patients with pseudo-Cushing’s syndrome [18] and in two patients with intermittent ectopic secretion of ACTH due to bronchial carcinoid tumors when central venous sampling happened to be performed during the eucortisolemic phase [19]. It is therefore imperative that hypercortisolism is confirmed in all cases before central venous sampling is performed.
False-negative results in inferior petrosal sinus sampling mainly relate to technical difficulties in obtaining optimal catheter position. One cause of this is the hypoplastic inferior petrosal sinus as reported by Doppman et al. [20] in 4 of 591 patients, since the accuracy of inferior petrosal sinus sampling in predicting lateralization is dependent on both the catheter position and the symmetry of venous drainage [21, 22].
Another pitfall in using central venous sampling to lateralize tumor is that in approximately 5%–10% of cases, a reversal of the intersinus gradient is seen from the pre- to the post-CRH values [7, 9]. The reason for this is not clear. This can confound the neurosurgeon when deciding on the side of hemihypophysectomy if no adenoma is evident on MRI or during pituitary exploration. These factors limit the use of central venous sampling as a tool to predict tumor location.
Indication for BIPSS
At our institution, patients with ACTH-dependent Cushing’s disease and a pituitary macroadenoma proceed straight to surgery without inferior petrosal sinus sampling, whereas patients with a normal scan or a pituitary microadenoma undergo sampling first (Fig. 2).
Key Points
-
1.
Since transsphenoidal surgery offers the best chance of cure for Cushing’s disease, the clinician must be absolutely certain that Cushing’s disease is accurately differentiated from ectopic ACTH secretion when dealing with ACTH-dependent Cushing’s syndrome.
-
2.
Bilateral inferior petrosal sinus sampling is a powerful technique and is the most accurate method to distinguish Cushing’s disease from ectopic ACTH secretion (Table 1).
-
3.
The use of central venous sampling to predict tumor localization is more controversial, with varying success rates in the literature. It cannot be relied on to guide the neurosurgeon in locating the tumor.
Role of Selective Venous Sampling in Hyperparathyroidism
Primary hyperparathyroidism has an estimated prevalence of 1 in 1000 in outpatient surveys [23] and an annual incidence rate of 0.04 per 1000 [24]. It is twice as common in women and occurs rarely before the age of 45. The majority of cases are detected incidentally during investigation for other conditions through routine serum calcium measurement in multichannel biochemistry screening.
The diagnosis is confirmed when the following criteria [25] are met: (1) corrected calcium >2.65 mmol/L on two occasions, (2) inappropriately high or normal parathyroid hormone (PTH) level, and (3) urine fractional excretion ratio >0.01 calculated by the calcium (Ca)/creatinine (Cr) ratio = [urine Ca × plasma Cr] ÷ [plasma Ca × urine Cr] to exclude familial hypocalciuric hypercalcemia.
The majority of cases of primary hyperparathyroidism are due to a solitary adenoma (85%) [26]. The remainder are due to diffuse hyperplasia (10%), two or more adenomas (5%), and carcinoma (<1%).
Surgery currently offers the only definitive cure for primary hyperparathyroidism. Bilateral neck exploration is the “gold standard” against which other surgical techniques are compared. In experienced hands, it has a success rate of >95% and a complication rate of 1%–2%.
In recent years, there has been a trend toward minimally invasive surgery such as unilateral neck exploration and focused parathyroidectomy. These newer techniques offer the advantage of shorter operative time and length of in-hospital stay. Their success is critically dependent on the ability to identify a solitary adenoma preoperatively with accuracy. Preoperative localization is therefore mandatory if minimally invasive surgery is planned.
In approximately 5%–15% of cases [27], the initial bilateral neck exploration fails to cure the condition. In the majority of these cases, the failure is due to a missed single adenoma [26]. The rest are due to primary nonfamilial multiglandular hyperplasia, familial hyperplasia due to multiple endocrine neoplasia (MEN) syndromes, or parathyroid carcinoma [28]. Reoperation carries a substantially higher morbidity, as scarring from the original operation distorts the neck anatomy leading to increased risk of recurrent laryngeal nerve injury, permanent hypoparathyroidism, bleeding, and anesthetic complications [29]. It is thus vital that meticulous efforts are made to localize the source of abnormal PTH production when a repeat operation is required.
Available Imaging Techniques for Preoperative Localization
Parathyroid Ultrasound
Ultrasound provides a cheap, noninvasive, nonionizing way of locating parathyroid lesions, but it is operator-dependent. When used with Sestamibi scan, its key strength is in providing spatial resolution to distinguish enlarged parathyroid glands, when they are located behind and close to the thyroid gland, from Sestamibi avid thyroid nodules [33].
It is less sensitive in locating ectopic parathyroid glands, and may miss glands in the substernal, retrotracheal, or retro-esophageal space. False-positive examinations occur because other structures in the neck may mimic the enlarged parathyroid, such as enlarged lymph nodes, muscles, vessels, and esophagus [33].
Perhaps the greatest use of ultrasound (US) is in identifying thyroid nodular disease, which, when present, makes differentiation of an intrathyroid parathyroid adenoma impossible from a similar looking thyroid nodule on MIBI scanning, thereby giving a false-positive MIBI scan result. Thus detection of thyroid nodular disease on US should exclude patients for minimally invasive surgery [34].
Parathyroid Scintigraphy
99mTc-Sestamibi (MIBI) imaging relies on the fact this radioactive tracer accumulates quickly in both thyroid and parathyroid tissues within minutes of intravenous administration but is released much faster from thyroid tissue. This differential washout rate enables scintigraphic localization of the parathyroid gland [33].
False-negative scans relate to low metabolic activity in the parathyroid nodule such as in necrotic or cystic areas. False-positive scans are seen in Hashimoto’s thyroiditis, benign or malignant thyroid neoplasms, and inflammatory lymph nodes. Use of a dual isotope technique using MIBI and iodine-123, which is also taken up by thyroid nodules but not parathyroid glands, can reduce the false-positive rate.
SPECT (single-photon emission computed tomography) techniques can improve localization of ectopic glands in the mediastinum or in posterior locations in the neck [35]. SPECT also provides additional information on the depth of the lesion and the topographic correlation with other anatomic structures.
CT and MRI
CT and MRI can be particularly useful in detecting ectopic glands in the mediastinum. The imaged area should extend to the carina inferiorly. They provide topographic information to help plan the surgical approach. The absence of contrast enhancement, the small size (<1 cm), and the ellipsoid shape of abnormal parathyroid glands reduces the sensitivity of CT and MRI [33, 36]. As with US and MIBI scans, thyroid nodules or lymph nodes can give rise to false-positive results.
Indication for Selective Venous Sampling for PTH
Each preoperative localization procedure has its own merits and disadvantages, and its use very much depends on the expertise and resources of the institution. Selective venous sampling (SVS) for PTH is a technique used in conjunction with other imaging modalities.
At our institution (Fig. 3), it is indicated if the patient has
-
(a)
had previous neck surgery,
-
(b)
known or newly diagnosed multiple endocrine neoplasia type 1 (MEN-1) or other familial hyperparathyroidism syndromes,
-
(c)
evidence of nonconcordant or unhelpful scans, or
-
(d)
evidence of more than one area of involvement on Sestamibi scanning.
Selective Venous Sampling Technique
A sheath is placed in the femoral vein. Samples are obtained from veins in the neck and mediastinum. In the authors’ experience, the most useful catheters are long multipurpose and cobra-shaped catheters with side holes close to the tip. Cannulation of the thymic mediastinal vein can be technically demanding but invaluable in confirming a mediastinal tumor, which will alter the surgical approach.
A positive catheter is defined as a gradient of twice the peripheral PTH measurement [30]. Peripheral serum measurement is calculated using the average of the subclavian vein samples.
How Does Selective Venous Sampling Help in Localization?
The single largest group of patients who underwent venous sampling (n = 98) was from the National Institutes of Health (NIH) [26]. They reported in 1996 on 222 patients with persistent or recurrent hyperparathyroidism who underwent surgical re-explorations. Ninety-eight patients went on to have venous sampling if two or more noninvasive studies (US, nuclear medicine, CT, or MRI) were negative, disconcordant, or equivocal, and if US/CT-guided aspiration or angiography were negative. For selective venous sampling, they found a 76% true-positive rate and 4% false-positive rate. They found venous sampling to have the highest sensitivity and specificity among all the preoperative localization procedures they used. Their results were replicated in 2002 in another large group (n = 64) by Jones et al. [31], who found venous sampling to have a sensitivity of 76% and specificity of 88%.
At our institution, Ogilvie et al. [32] recently reviewed our 10-year experience (1994–2004; n = 27) in the use of parathyroid venous sampling in complicated primary hyperparathyroidism. Of the 23 patients who underwent operation, 13 catheters (57%) were completely consistent with surgical and histological findings. There were three false-positive catheters, giving a positive predictive value of 81% in this analysis.
A recent review of the literature of 31 publications by Seehofer et al. [37], in 2004, found selective venous sampling to have the highest sensitivity for localization (Table 2).
Key Points
-
1.
The use of preoperative techniques is dependent on the skills and resources of the center.
-
2.
Preoperative localization is mandatory in planning minimally invasive surgery.
-
3.
Selective venous sampling is a powerful technique with the highest accuracy in detecting abnormal PTH production. Its main indication is in redo operations when two or more preoperative imaging investigations are disconcordant or unhelpful
Role of Arterial Selective Venous Sampling in Insulinomas and Gastrinomas
Pancreatic endocrine tumors (PETs) are rare islet cell tumors. Eighty-five percent of PETs are functional, and they are classified according to the predominant hormone they secrete. Insulinoma is the most common islet cell tumor, accounting for 60% of all cases. Gastrinoma is the second most common type, accounting for 20%. The rest are due to VIPoma (3%) (VIP, vasoactive intestinal polypeptide), glucagonoma (1%), somatostatinoma (<1%), and nonfunctioning islet cell tumors (15%).
Insulinomas are mostly solitary, and 90% of them are located within the pancreas. Roughly a third each are distributed among the head, body, and tail of the pancreas, and 40% are <1 cm in size. Only 4% of insulinomas are associated with MEN-1, and these tend to be multiple [38].
In contrast, gastrinomas are more often multiple and extrapancreatic, and tend to be smaller than insulinomas, making them even more difficult to localize than insulinomas. About 25% of gastrinomas are associated with MEN-1 [38].
CT or MRI is often the first investigation the clinician uses when a PET is suspected. Both allow evaluation of the whole abdomen to assess local tumor invasion or hepatic metastases. Advances in helical CT technology have allowed faster scans with images acquired in arterial and venous phases, improving lesion detection [39, 40].. The development of multidetector CT scanners is likely to improve detection rates further.
Development of fast spin echo and fat saturation sequences and dynamic contrast-enhanced imaging has improved the sensitivity of MRI for detection of pancreatic lesions, with sensitivities of >90% [41, 42]. Studies comparing CT and MRI for detection of islet cell tumors show a greater sensitivity for MRI, particularly for smaller lesions [43, 44].
Endoscopic US [45] has the advantage of visualizing tumors as small as 2–3 mm and localizing them to precise regions in the pancreas. However, its sensitivity is lower for lesions in the pancreatic tail and for extrapancreatic gastrinomas. Lymph nodes, splenunculi, focal pancreatic nodules, and the heterogeneous nature of chronic pancreatitis may give false-positive results.
Somatostatin-receptor scintigraphy [46] makes use of the fact that somatostatin receptors are expressed at a high density in many PETs. These receptors provide a target for scintigraphy using radiolabeled somatostatin analogues such as indium-111 pentetreotide (OctreoScan). This technique is more sensitive for detecting gastrinomas, glucagonomas, nonfunctioning PETs, and carcinoid tumors, but less so for insulinomas, 40% of which do not express somatostatin receptors.
One advantage of somatostatin-receptor scintigraphy over other imaging techniques is its ability to correlate an anatomical abnormality with functional evidence of probable neuroendocrine function. Not only does it help to identify the neuroendocrine nature of primary tumors, but it can detect previously unsuspected metastases and, thus, guide medical therapy. It also opens up possible therapeutic options with targeted radionuclide therapy.
Transhepatic portal venous sampling [47] is a highly invasive technique involving direct percutaneous transhepatic puncture of the right portal vein and selective catheterization via this route to access the portal venous tributaries. Blood is sampled from the splenic, pancreatic, pancreaticoduodenal, and superior mesenteric veins, and the tumor is localized based on a rise in hormone concentration from the specific region of the pancreas. However, this technique carries a 0.7% mortality and 9.2% morbidity [38] and, for this reason, has been superseded by arterial stimulation and venous sampling.
Angiography and Arterial Stimulation and Venous Sampling (ASVS) Radiological Technique
For this technique, a sheath is placed in the femoral artery and another in the femoral vein. A catheter, usually a cobra catheter with a side hole close to the tip, is placed in a hepatic vein, usually the right or middle hepatic vein, which provides a stable catheter position. A second catheter is then placed selectively into one of four arterial sites: the hepatic artery, the superior mesenteric artery, the gastroduodenal artery, or the splenic artery, and selective angiography performed. Angiography is useful in demonstrating vascular islet cell tumors (Fig. 4) and is now commonly used in conjunction with ASVS.
After taking two baseline samples from the hepatic vein, with a 3-min interval between baseline samples, calcium is injected into the arterial catheter and hepatic venous samples taken every 30 s for 3 min. The arterial catheter is then repositioned into the next arterial site and the process repeated, until all four arterial sites have been injected. A time interval of at least 5 min is allowed between calcium injections, so that hormone levels can return to baseline before the next injection. If noninvasive imaging is suggestive of an insulinoma at a particular site, for instance, the pancreatic tail, then cannulating the artery supplying that area—in this instance, the splenic artery—last may decrease the chance of symptomatic hypoglycemia occurring during the investigation. Some workers have modified the technique to include five arterial sites by subdividing the splenic artery sites into proximal and distal in an attempt at more precise regional localization.
Evidence for ASVS to Localize Insulinomas
ASVS was first described by Imamura et al. [48] in 1987 in localizing gastrinomas by using secretin as the stimulating agent. Later, Doppman et al. [49], in 1991, modified the technique by using calcium as the secretagogue to localize insulinomas in four patients. ASVS has now been used successfully in many studies [50–57] to localize insulinomas.
The largest study using ASVS to localize insulinomas is a recent study by Wiesli et al. [57] in 2004 involving 27 patients, comparing the diagnostic accuracy of ASVS to CT and/or MR imaging. They found ASVS to be superior, with a sensitivity of 96%, versus 59% for CT/MR imaging.
Another study [55] looked at the use of ASVS to localize insulinomas in 7 of 24 patients in whom US, CT, MRI, and endoscopic US were negative or equivocal. In all seven patients, ASVS accurately localized the pathological source of insulin secretion. In three of these seven patients, the results of ASVS directly affected surgical management because no lesions were palpable during the operation, but knowledge of the results of ASVS enabled the surgeon to perform the correct operation successfully.
Evidence for ASVS to Localize Gastrinomas
The technique of ASVS for localizing primary gastrinomas is the same as that used for insulinomas. Calcium is used as the secretagogue and gastrin is measured in the hepatic venous effluent. A 50% rise in gastrin level in the 30-s sample from the hepatic vein is considered positive [58], localizing the region of the pancreas with pathological gastrin secretion depending on which artery the calcium was injected into.
There are relatively few studies evaluating the usefulness of ASVS in localizing gastrinomas. One prospective study [59] (n = 36) compared ASVS to portal venous sampling and found ASVS to have a higher sensitivity, 89%, and fewer complications. Other studies [48, 58, 60–62] have reported sensitivities of between 77% and 100%, but in very small group of patients (n = 2 to 13).
Due to the rarity of PETs and local bias in the expertise for a particular localization modality, there has yet to be a large prospective study which compares the use of CT/MRI, SRS, endoscopic US, intraoperative US, and ASVS together. Thus, the indications for ASVS will very much depend on the local expertise available.
Indications for Arterial Stimulation and Venous Sampling
-
1.
ASVS is indicated when other imaging modalities have failed to localize the tumor.
-
2.
It should be considered when multiple tumors exist, as is often the case in MEN-1 syndrome, to help discriminate the dominant functional lesion(s) from the nondominant ones [52].
-
3.
The findings of ASVS also predict the tumor site within the pancreas to the right or left of the superior mesenteric artery. This information can be extremely useful to the surgeon in planning the surgical approach, for example, enucleation, distal pancreatectomy, or pancreatoduodenectomy.
Complications and Pitfalls
Most patients experience a transient warm feeling in the stomach during the procedure. Complications specific to ASVS include hypoglycemia from systemic release of insulin during calcium stimulation, and hypercalcemia from injection of calcium gluconate, but these complications are rare.
False-negative results have been reported in patients on diazoxide therapy [57], as diazoxide reduces the responsiveness of tumor cells to acute rise of extracellular calcium. Therefore diazoxide should be stopped prior to ASVS.
False-negative results have also been described in patients with high basal insulin levels prior to calcium stimulation, presumably as a result of mechanical stimulation of the tumor during catheter placement [57]. This is the rationale for taking two basal samples 3 min apart before intra-arterial calcium injection.
One case has been reported in the literature of an insulinoma unresponsive to calcium, resulting in a false-negative result [57]. Another case of false-negative ASVS was described in a patient with glucose-sensitive insulinoma, where exceptionally, the administration of glucose induced an enormous release of insulin [57].
Key Points
-
1.
Localization of PETs requires a combination of techniques depending on the availability, cost, and expertise at the local center.
-
2.
ASVS is highly sensitive in localizing occult tumors and can help the surgeon plan the operative approach.
-
3.
ASVS is particularly valuable in deciding on the dominant functional lesion(s) in cases of hyperplasia or multiple insulinomas associated with MEN-1 syndrome.
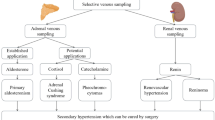
Role of Adrenal Venous Sampling in Primary Hyperaldosteronism
Primary hyperaldosteronism is a state characterized by long-standing aldosterone excess and suppressed plasma renin activity, resulting in hypertension and hypokalemia. It is the most commonest endocrine cause of hypertension and affects 5%–13% of all patients with hypertension [63].
Aldosterone-producing adenoma (APA) and bilateral adrenal hyperplasia (BAH) are the most common subtypes of primary hyperaldosteronism [66] (Table 3). A much less common subtype is primary adrenal hyperplasia [64, 65], which has biochemical features suggestive of unilateral adenoma but histological characteristics of nodular hyperplasia predominantly involving one adrenal gland. Primary adrenal hyperplasia is similar to APA in that surgical removal of the affected gland leads to remission of hyperaldosteronism.
APA and primary adrenal hyperplasia can be treated by adrenalectomy, whereas BAH is best treated medically. Unilateral adrenalectomy in patients with aldosterone producing adenoma or primary adrenal hyperplasia leads to cure in 30%–60%, and improvement in hypertension and hypokalemia in all of these patients [67]. Factors which determine persistent hypertension after adrenalectomy are older age, duration of hypertension, use of two or more antihypertensive agents preoperatively, family history of more than one first-degree relative with hypertension, and raised serum creatinine [60, 68]. It is most likely that these patients have coexistent essential hypertension.
For patients in whom surgery is indicated, it is vital that APA/primary adrenal hyperplasia is distinguished from BAH, as unilateral or bilateral adrenalectomy in the latter seldom leads to cure. This is usually done by a combination of postural stimulation test (PST), CT adrenals, and adrenal vein sampling.
Postural Stimulation Test
In the 4-h PST, renin and aldosterone levels are taken at 0900 h with the patient in the supine position and repeated after 4 h of upright posture. The test is considered positive for APA when the aldosterone level falls, or fails to rise by 30% after 4 h of ambulation [69].This method makes use of an ACTH-responsive property of APA such that a fall in ACTH levels with diurnal variation at 1300 h usually leads to a fall in aldosterone level in patients with APA [70]. In contrast, for patients with BAH, the adrenal sensitivity to angiotensin II is enhanced such that a small increase in angiotensin II that occurs with standing usually leads to a large increase in aldosterone levels.
However, not all APAs are ACTH responsive. A variant of APA which is renin responsive has been described such that the aldosterone level rises with upright posture in these patients [65]. Moreover, in about 30% of patients [69, 71, 72] when ACTH does not fall with diurnal variation as reflected by a rise in cortisol level at 1300 h, the PST becomes invalid. These two factors considerably reduce the sensitivity of the test in detecting APA. The sensitivity of 4-h PST is about 65% to 85% [69, 71, 72].
Despite the low sensitivity of the 4-h PST, when the test is positive, it has a high positive predictive value for APA. In a comprehensive review of 146 patients by Fontes et al. [69], an aldosterone rise of <30% as a positive test for APA gave a positive predictive value of 87% for PST, but if all surgically curable cases were included (aldosterone-producing adenoma plus primary adrenal hyperplasia), this reached 98%. In another retrospective study of 49 patients with primary hyperaldosteronism, the 4-h PST had a positive predictive value of 100% [72].
Adrenal CT
How about the role of adrenal CT scanning in subtyping primary hyperaldosteronism? Many APAs are small, and the longest dimension is <1 cm in a fifth of 143 surgically treated cases at Mayo Clinic [66]. The small size of APAs can make them difficult to detect or interpret on CT. The presence of a nodule, however large or small, does not imply functionality, as adrenal incidentalomas are common. In three recent studies, CT has a low sensitivity, between 53 to 73%, in picking up surgically confirmed APAs [72–74].
Adrenal Venous Sampling
Adrenal venous sampling is added to the diagnostic algorithm because of the relatively low sensitivity of PST and CT.
Radiological Technique
After insertion of a 7-F sheath in the right femoral vein, a curved catheter with a side hole close to the tip, usually a cobra, is used to obtain samples from the right atrium, suprarenal and infrarenal inferior vena cava (IVC), hepatic vein, right renal vein, and left renal vein proximally and distally. Selective catheters are then used to cannulate the adrenal veins.
Regrettably, commercial pressure has resulted in the withdrawal of specialized adrenal vein catheters in recent years. The left adrenal vein, a tributary of the left renal vein, can often be accessed with a Sidewinder II catheter with a side hole close to the tip. Care should be taken not to advance the catheter too distally into the inferior phrenic vein, which may join the adrenal vein to form a common trunk draining into the left renal vein. The right adrenal vein drains directly into the IVC posteriorly. Cannulation of this vein is technically demanding. A reverse catheter such as a Sidewinder I with a side hole close to the tip is the authors’ first choice, while others have found a cobra catheter more efficacious. Confusion may arise from cannulation of a separate vein draining the caudate lobe of liver.
Interpretation of Adrenal Venous Sampling Data
Successful cannulation is verified if the cortisol level in the adrenal vein is an order of magnitude greater than that in the IVC, or the epinephrine level is higher than the norepinephrine level, i.e., the reverse of the catecholamine ratio seen in the periphery.
Bilateral adrenal hyperplasia is diagnosed if the aldosterone/cortisol (A/C) ratio in each adrenal vein is higher than the A/C ratio in the IVC. To diagnose unilateral APA or primary adrenal hyperplasia, there has to be an A/C ratio in the vein of the affected side four times higher than the corresponding ratio in the contralateral adrenal vein and/or a suppressed A/C ratio on the contralateral side [66, 75]. Suppressed A/C ratio is defined as an A/C ratio lower than the A/C ratio in the IVC.
In the event of cannulation of only one vein, the finding of a suppressed A/C ratio may still be used to indicate an APA or primary adrenal hyperplasia on the contralateral side [72, 76, 77], but this finding is not 100% accurate. In a large series from the Mayo Clinic in 2004 [78], the contralateral adrenal vein A/C ratios were lower than the ratios in the IVC in 95 of 102 patients (93.1%) with APA. Furthermore, in the same series, 27 of 84 patients (32%) with BAH had a nondominant A/C ratio lower than the ratio in the IVC.
Therefore, in patients who have failed cannulation of one vein, the finding of a suppressed A/C ratio should be used with caution but may be used as a secondary data point in conjunction with other clinical data to support a preoperative diagnosis of aldosterone producing adenoma.
Evidence for Adrenal Venous Sampling
Adrenal venous sampling is undoubtedly the most accurate way to differentiate the subtypes of primary hyperaldosteronism, with an accuracy of 92%–100% in most series [72, 76, 78, 79] using surgical confirmation as the gold standard. However, other workers have questioned whether it needs to be to be performed in all patients with primary hyperaldosteronism, or whether other tests obviate the need for venous sampling.
In one retrospective review [72], 49 patients with primary hyperaldosteronism underwent CT, 4-h PST, and adrenal venous sampling. A positive posture test and a single focal adrenal macroadenoma on CT successfully identified 39 of 41 cases of surgically proven APA, without the need for venous sampling. This approach has not yet been confirmed in prospective studies.
In another retrospective study, Phillips et al. [74] analyzed their 7-year series of 48 patients with primary hyperaldosteronism to evaluate the predictive value of preoperative PST and CT compared with venous sampling. Only 10 of 41 patients had both positive PST and CT scan, who could then have proceeded directly to adrenalectomy without adrenal venous sampling. They concluded that sampling is still required in patients with a negative CT or a negative PST.
Impact on Patient Management
Young et al. [78] studied the impact of adrenal venous sampling on patient management in 194 patients who underwent sampling after equivocal CT findings. They concluded that without adrenal venous sampling, and using CT findings alone, 42 (22%) patients would have been incorrectly excluded as candidates for adrenalectomy, and 48 (25%) might have had unnecessary or inappropriate adrenalectomy.
For example, of the 32 patients with a unilateral macronodule (>10 mm) apparent on CT, only 21 (65.6%) had evidence of hypersecretion from the ipsilateral gland, and 1 had unilateral aldosterone hypersecretion from the contralateral adrenal [78]. Therefore, 1 patient (3%) would have had inappropriate adrenalectomy (wrong side), and 11 patients (34%) might have had unnecessary operation for nonfunctioning nodules.
In other words, patients with CT examinations that were normal or compatible with BAH, but were subsequently found on adrenal venous sampling to have unilateral aldosterone hypersecretion, might have been excluded for surgery. Also, patients who had a characteristic single macronodule on CT but were subsequently found at adrenal venous sampling to be non-aldosterone-secreting on that side, or hypersecreting from the contralateral gland, may have undergone unnecessary or inappropriate operation. Therefore adrenal venous sampling is essential to direct treatment in these patients. We illustrate this point with the following two case scenarios.
Case scenario 1: A 52-year-old woman

CT results were as reported below.
Right adrenal | Left adrenal | |
|---|---|---|
Nodule | None | None |
Enhancement | NA | NA |
Thickness | ||
Body | 10.1 mm | 8.5 mm |
Medial limb | 5 mm | 6.2 mm |
Lateral limb | 5.2 mm | 5.7 mm |
The radiological diagnosis was BAH.
The adrenal venous sampling results were as follows.
Site | Cortisol (nmol/L) | Aldosterone (pmol/L) | A/C ratio |
|---|---|---|---|
High IVC | 742 | 5,390 | 7.26 |
Right adrenal | 18,890 | 41,500 | 2.20 |
Leftt adrenal | 9,855 | 178,300 | 18.09 |
A/C ratio | Interpretation | |
|---|---|---|
A/C left:A/C right | 8.2 | APA if >4 |
A/C right:A/C IVC | 0.3 | APA if ≤4 |
Adrenal vein sampling diagnosis was left-sided aldosterone secretion.
The final diagnosis was as follows. The adrenal vein sampling diagnosis of left-sided aldosterone secretion was incongruent with the CT diagnosis. This case illustrates the impact of adrenal vein sampling on patient management. Without adrenal vein sampling, this patient may have missed out on potentially curative surgery.
Case scenario 2: A 64-year-old man

The CT results were as reported below.
Right adrenal | Left adrenal | |
|---|---|---|
Nodule | None | 19 × 13 mm in body |
Enhancement (Hounsfield units) | NA | Pre: 46 HU |
Immediate: 130 HU | ||
Delayed: 88 HU | ||
Thickness | ||
Body | 6.2 mm | 6.6 mm |
Medial limb | 7.5 mm | 6.5 mm |
Lateral limb | 7.5 mm | 3.2 mm |
The radiological diagnosis was left adenoma that was very atypically hypervascular and dense on precontrast scan.
The adrenal venous sampling results were as follows.
Site | Cortisol (nmol/L) | Aldosterone (pmol/L) | AC ratio |
|---|---|---|---|
High IVC | 1,116 | 2,274 | 2.04 |
Right adrenal | 54,760 | 163,900 | 2.99 |
Left adrenal | 58,320 | 166,300 | 2.85 |
A/C ratio | Interpretation | |
|---|---|---|
A/C left:A/C IVC | 1.4 | BAH if >1 |
A/C right:A/C IVC | 1.5 | BAH if >1 |
Adrenal vein sampling diagnosis was bilateral aldosterone secretion.
The final diagnosis was as follows: the adrenal vein sampling diagnosis of bilateral aldosterone secretion was incongruent with the CT diagnosis. This patient may have erroneously had surgery for presumed APA if he had not undergone adrenal vein sampling.
Indication for Adrenal Vein Sampling
To our knowledge, there has been no prospective study so far which compares the use of PST, CT, and adrenal venous sampling. One approach used by the Mayo Clinic is to proceed straight to surgery in younger patients (<40 years) with a solitary unilateral macronodule (>1 cm) and normal contralateral adrenal morphology [78]. In cases where CT reveals normal adrenals, minimal unilateral adrenal limb thickening, unilateral micronodules (≤1 cm), or bilateral macronodules, adrenal venous sampling is indicated [78]. As unilateral nonfunctioning adrenal macroadenomas are also not uncommon in older patients (>40 years) [80], hormonal hyperfunction cannot be inferred and adrenal venous sampling is also required in this group of patients [78].
Complications and Pitfalls
The most common cause for adrenal venous sampling being noncontributory is the failure to cannulate the right adrenal vein due to its very short length (5–8 mm) and the acute angle at which it enters the IVC. The general failure rate for cannulating the right adrenal vein is about 36% [81], but success varies across centers.
Complications specific to adrenal venous sampling are rare and include adrenal vein dissection (0.5%), adrenal hemorrhage (Fig. 5), and infarction (0.5%) in one series [78].
Key Points
-
1.
Adrenal vein sampling is the most accurate way of differentiating the subtypes of primary hyperaldosteronism and is an essential diagnostic investigation in patients seeking surgical treatment.
-
2.
Success from the technique is mainly limited by the technical failure in cannulating the right adrenal vein.
Adrenal Venous Sampling for Pheochromocytomas and Paragangliomas
The diagnosis of pheochromocytoma or paraganglioma (extradrenal pheochromocytoma) is usually suggested by the presence of hypertension and classic symptoms of episodic headaches, sweats and palpitations, the discovery of an incidental adrenal mass, or the family history.
These chromaffin cell tumors are diagnosed when there is biochemical evidence of catecholamine release or metabolism. The tumors are localized by CT or MRI and should be confirmed on functional studies such as meta-iodobenzylguanide scintigraphy or positron emission tomography [82]. With advances in imaging technology, these techniques have become highly sensitive (>90%) such that adrenal venous sampling is rarely needed.
Radiological Technique
In the clinical context of a suspected pheochromocytoma, if imaging is suggestive of an adrenal tumor, a technique of adrenal venous sampling identical to that used for hyperaldosteronism may be used to confirm the functioning nature of any lesion seen on imaging. In the rare situation of a patient in whom plasma catecholamines or urinary catecholamines levels are persistently elevated but there is no localization on the imaging methods described above, a whole-body sampling technique may be employed to try to localize the source of catecholamine production. A central norepinephrine-to-epinephrine ratio of >1, which is the reverse of the catecholamine ratio in the periphery, points toward the site of the putative lesion [83].
Indications for Adrenal Vein Sampling
There are circumstances when adrenal vein sampling comes into its own:
Ovarian Venous Sampling for Andogren-Secreting Ovarian Tumors
Androgen-secreting Sertoli-Leydig cell tumors are rare, constituting <0.5% of all ovarian tumors [86]. The diagnosis is usually suggested in women with a short history of amenorrhea, rapidly progressing hirsutism and virilization, and a single testosterone level of >7.5 nmol/L. However, some tumors run a more indolent course and their presentation overlaps with the more subtle presentation of patients with nontumorous hyperandrogenism [87].
The challenge in a patient presenting with hyperandrogensim is not to overinvestigate in discriminating the rarer tumors from the vastly more common nontumorous causes such as polycystic ovarian syndrome.
The 48-h low-dose dexamethasone-suppression test (LDDST) relies on the property that androgen secretion in adrenal and ovarian tumors is characteristically autonomous and is not suppressed by abolishing ACTH secretion with dexamethasone. In one study, the failure of a >40% reduction in the previously elevated testosterone levels after 48-h low-dose dexamethasone-suppression test was associated with 100% sensitivity and 88% specificity in distinguishing patients with ovarian and adrenal androgen-secreting tumors from patients with nontumorous hyperandrogenism [87].
Looking at this approach in a different way, 88% of hyperandrogenic patients who showed suppression of >40% or normalization of the testosterone levels can be excluded from having tumors biochemically. This test may thus help to screen out patients who require further imaging studies to detect tumors.
Hyperandrogenic women in whom the suspicion of an androgen-secreting tumor is high should have adrenal CT and ovarian US to detect such a tumor. Adrenal androgen-secreting tumors are usually >1.5 cm in size and easily detected by CT, which has a sensitivity of between 70% and 90% [88]. Ovarian androgen-secreting tumors, on the other hand, are usually small (<2 cm in diameter) [89, 90] and can escape detection by pelvic examination, diagnostic laparoscopy, or ovarian US.
Indications for Ovarian Venous Sampling
In the event that imaging fails to localize the putative tumor, ovarian and adrenal venous sampling for androgens can be useful. Small case series [89, 91] have reported success in localizing such tumors where US and/or diagnostic laparoscopy have failed.
This technique can also help to exclude tumors. For example, in one series of 16 patients [91], bilateral ovarian-adrenal vein catheterization not only helped to identify 5 occult androgen-producing ovarian tumors and 1 virilizing adrenal mass, but also identified 10 nontumoral causes of hyperandrogenism such as severe polycystic ovarian syndrome in 6 premenopausal women and ovarian stromal and hilus cell hyperplasia in 4 menopausal patients.
Radiological Technique
The techniques for cannulation of the adrenal veins are as described above. The left ovarian vein enters the left renal vein and is best cannulated with a cobra catheter with side holes close to the tip inserted into the left renal vein and withdrawn until the tip engages. The right ovarian vein enters the IVC anteriorly close to and just below the right renal vein. Cannulation is best achieved with either a reverse catheter such as a sidewinder I or a cobra catheter.
Pitfalls
Ovarian venous sampling is technically demanding and the successful cannulation rate of all four veins (right /left ovarian and adrenal veins) is reported to be only 27% [92] to 45% [93]. This low success rate is partly due to the anatomical variation in venous size and drainage and the difficulty in entering a competent valve at the orifice of the ovarian vein in nulliparous women [92]. This limits the interpretation of the results and usefulness of the technique.
A further limitation is the lack of consensus in interpreting catheterization results. Moltz et al. [94] and Surrey et al. [90], by comparing the results of patients with neoplastic hyperandrogenism to those with nonneoplastic hyperandrogenism, used the criteria of an ovarian-to-peripheral gradient of >9.5 nmol/L for testosterone to indicate a unilateral source of testosterone production. However, this guideline has not been replicated by Kaltsas et al. [92], who found that five of eight patients who were shown to have polycystic ovarian syndrome on histology had an ovarian-to-peripheral gradient >9.5 nmol/L. Moltz et al. [94] also found that the ovarian-to-peripheral gradients for testosterone exceeded 9.5 nmol/L in 4 of 10 catheterized women with histologically proven hyperthecosis.
Conclusion
Searching for the source of hormone production in patients with clinical evidence of hormonal excess is one of the most challenging problems facing the endocrinologist. Advances in imaging have aided hugely but cross-sectional imaging does not provide any functional information of the putative lesion(s), while nuclear medicine scintigraphy only provides indirect evidence of hormonal production. There remains a definite and important role for venous sampling in the differential diagnosis and preoperative localization of endocrine diseases to guide and plan surgery.
References
Newell-Price J, Trainer P, Besser M, et al. (1998) The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev 19(5):647–672
Kaye TB, Crapo L (1990) The Cushing syndrome: an update on diagnostic tests. Ann Intern Med 112:434
Hall WA, Luciano MG, Doppman JL, et al. (1994) Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 120:817–820
Findling JW, Doppman JL (1994) Biochemical and radiological diagnosis of Cushing’s syndrome. Endocrinol Metab Clin North Am 23:511–537
Dwyer AJ, Frank JA, Doppman JL, et al. (1987) Pituitary adenomas in patients with Cushing disease: initial experience with Gd-DTPA enhanced MR imaging. Radiology 163:421–426
Doppman JL, Frank JA, Dwyer AJ, et al. (1988) Gadolinium DTPA enhanced MR imaging of ACTH-secreting microadenomas of the pituitary gland. J Comput Assist Tomogr 12:728–735
Liu C, Lo JC, Dowd CF, et al. (2004) Cavernous and inferior petrosal sinus sampling in the evaluation of ACTH-dependent Cushing’s syndrome. Clin Endocrinol 61:478–486
Corrigan DF, Schaaf M, Whaley RA, et al. (1977) Selective venous sampling to differentiate ectopic ACTH secretion from pituitary Cushing’s syndrome. N Engl J Med 296:861–862
Oldfield EH, Doppman JL, Nieman LK, et al. (1991) Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med 325:897–905
Oldfield EH, Chrousos GP, Schulte HM, et al. (1985) Preoperative lateralization of ACTH-secreting pituitary microadenomas by bilateral and simultaneous inferior petrosal venous sinus sampling. N Engl J Med 312:100–103
Teramoto A, Nemoto S, Takakura K, et al. (1993) Selective venous sampling directly from cavernous sinus in Cushing’s syndrome. J Clin Endocrinol Metab 76:637–641
Graham KE, Samuels MH, Nesbit GM, et al. (1999) Cavernous sinus sampling is highly accurate in distinguishing Cushing’s disease from the ectopic adrenocorticotropin syndrome and in predicting intrapituitary tumor location. J Clin Endocrinol Metab 84(5):1602–1610
Lienhardt A, Grossman AB, Dacie JE, et al. (2001) Relative contributions of inferior petrosal sinus sampling and pituitary imaging in the investigation of children and adolescents with ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab 86(12):5711–5714
Storr HL, Afshar F, Matson M, et al. (2005) Factors influencing cure by transsphenoidal selective adenectomy in paediatric Cushing’s disease. Eur J Endocrinol 52(6):825–833
Sturrock ND, Jeffcoate WJ (1997) A neurological complication of inferior petrosal sinus sampling during investigation for Cushing’s disease: a case report. J Neurol Neurosurg Psychiatry 62:527–528
Miller DL, Doppman JL, Peterman SB, et al. (1992) Neurologic complications of petrosal sinus sampling. Radiology 185:143–147
Bonelli FS, Huston J III, Carpenter PC, et al. (2000) Adrenocorticotropic hormone-dependent Cushing’s syndrome: sensitivity and specificity of inferior petrosal sinus sampling. Am J Neuroradiol 21:690–696
Yanovski JA, Cutler GB Jr, Doppman JL, et al. (1993) The limited ability of inferior petrosal sinus sampling with corticotrophin-releasing hormone to distinguish Cushing’s disease from pseudo-Cushing states or normal physiology. J Clin Endocrinol Metab 77:503–509
Yoshihiro Y, Davies DH, Nippoldt TB, et al. (1995) False-positive inferior petrosal sinus sampling in the diagnosis of Cushing’s disease. J Neurosurg 83:1087–1091
Doppman JL, Chang R, Oldfield EH, et al. (1999) The hypoplastic inferior petrosal sinus: a potential source of false-negative results in petrosal sampling for Cushing’s disease. J Clin Endocrinol Metab 84(2):533–540
Lefournier V, Martinie M, Vasdev A, et al. (2003) Accuracy of bilateral inferior petrosal or cavernous sinuses sampling in predicting the lateralization of Cushing’s disease pituitary microadenoma: influence of catheter position and anatomy of venous drainage. J Clin Endocrinol Metab 88(1):196–203
Mamelak AN, Dowd CF, Tyrrell JB, et al. (1996) Venous angiography is needed to interpret inferior petrosal sinus and cavernous sinus sampling data for lateralizing adrenocorticoptropin-secreting adenomas. J Clin Endocrinol Metab 81(2):475–481
Boonstra CE, Jackson CE (1971) Serum calcium survey for hyperparathyroidism: results in 50000 clinic patients. Am J Clin Pathol 55:523–526
Wermers RA, Khosla S, Atkinson EJ, et al. (1997) The rise and fall of primary hyperparathyroidism: a population-based study in Rochester, Minnesota, 1965–1992. Ann Intern Med 126:433–440
Davies M, Fraser WD, Hosking DJ (2002) The management of primary hyperparathyroidism. Clin Endocrinol 57:145–155
Jaskowiak N, Norton JA, Alexander HR, et al. (1996) A prospective trial evaluating a standard approach to reoperation for missed parathyroid adenoma. Ann Surg 224(3):308–322
Grant CS, van Heerden JA, Charboneau JW, et al. (1986) Clinical management of persistent and/or recurrent primary hyperparathyroidism. World J Surg 10:555–565
Brennan MF, Marx SJ, Doppman JL, et al. (1981) Results of reoperation for persistent and recurrent hyperparathyroidism. Ann Surg 94(6):671–676
Saxe AW, Brennan MF (1981) Strategy and technique of reoperative parathyroid surgery. Surgery 89(4):417–423
Sugg SL, Fraker DL, Alexander RH, et al. (1993) Prospective evaluation of selective venous sampling for parathyroid hormone concentration in patients undergoing reoperations for primary hyperparathyroidism. Surgery 114:1004–1010
Jones JJ, Brunaud L, Dowd CF, et al. (2002) Accuracy of selective venous sampling for intact parathyroid hormone in difficult patients with recurrent or persistent hyperparathyroidism. Surgery 132:944–951
Ogilvie CM, Brown PL, Matson M, et al. (2005) Selective parathyroid venous sampling in patients with complicated hyperparathyroidism. 87th Annual Meeting of the Endocrine Society, San Diego, OR 30–2
Mariani G, Gulec SA, Rubello D, et al. (2003) Preoperative localisation and radioguided parathyroid surgery. J Nucl Med 44:1443–1458
Udelsman R (2000) Is unilateral neck exploration for parathyroid adenoma appropriate? Adv Surg 34:319–329
Billotey C, Sarfati E, Aurango A, et al. (1996) Advantages of SPECT in technetium-99m-Sestamibi parathyroid scintigraphy. J Nucl Med 37(11):1773–1778
De Feo ML, Colagrande S, Biagini C, et al. (2000) Parathyroid glands: combination of 99mTc MIBI scintigraphy and US for demonstration of parathyroid glands and nodules. Radiology 214:393–402
Seehofer D, Steinmuller T, Rayes N, et al. (2004) Parathyroid hormone venous sampling before reoperative surgery in renal hyperparathyroidism: comparison with non-invasive localisation procedures and review of the literature. Arch Surg 139:1331–1338
King CM, Reznek RH, Dacie JE, et al. (1994) Imaging islet cell tumours. Clin Radiol 49:295–303
King AD, Ko GT, Yeung VT, et al. (1998) Dual phase spiral CT in the detection of small insulinomas of the pancreas. Br J Radiol 71:20–23
Van Hoe L, Gryspeerdt S, Marchal G, et al. (1995) Helical CT for the preoperative localization of islet cell tumors of the pancreas: value of arterial and parenchymal phase images. AJR 165:1437–1439
Kelekis NL, Semelka RC (1997) MRI of pancreatic tumours. Eur J Radiol 7:875– 886
Owen N, Sohaib SA, Peppercorn PD, et al. (2001) MRI of neuroendocrine tumours of the pancreas. Br J Radiol 74:968–973
Semelka RC, Cumming MJ, Shoenut JP, et al. (1993) Islet cell tumors: comparison of dynamic contrast-enhanced CT and MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology 196:799–802
Moore NR, Rogers CE, Britton BJ (1995) Magnetic resonance imaging of endocrine tumours of the pancreas. Br J Radiol 68:341–347
Anderson MA, Carpenter C, Thompson NW, et al. (2000) Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol 95:2271–2277
Virgolini I, Traub-Weidinger T, Decristoforo C (2005) Nuclear medicine in the detection and management of pancreatic islet-cell tumours. Best Pract Res Clin Endocrinol Metab 19(2) 213–227
Jackson JE (2005) Angiography and arterial stimulation venous sampling in the localization of pancreatic neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab 19(2):229–239
Imamura M, Takahashi K, Adachi H, et al. (1987) Usefulness of selective arterial injection test for localisation of gastrinoma in the Zollinger-Ellison syndrome. Ann Surg 205:230–239
Doppman JL, Miller DL, Chang R, et al. (1991) Insulinomas: localization with selective intraarterial injection of calcium. Radiology 178:237–241
Doppman JL, Miller DL, Chang R, et al. (1993) Intraarterial calcium stimulation test for detection of insulinomas. World J Surg 17:439–443
Doppman JL, Chang R, Fraker DL, et al. (1995) Localization of insulinomas to regions of the pancreas by intra-arterial stimulation with calcium. Ann Intern Med 123:269–273
O’Shea D, Rohrer-Theus AW, Lynn JA, et al. (1996) Localisation of insulinomas by selective intraarterial calcium injection. J Clin Endocrinol Metab 81(4):1623–1627
Tsagarakis S, Kaskarelis J, Malagari C, et al. (1997) Regionalization of occult pancreatic insulinomas with the arterial stimulation venous sampling (ASVS) technique. Clin Endocrinol (Oxf) 47:753–757
Kuzin NM, Egorov AV, Kondrashin SA, et al. (1998) Preoperative and intraoperative topographic diagnosis of insulinomas. World J Surg 22:593–598
Pereira PL, Roche AJ, Maier GW, et al. (1998) Insulinoma and islet cell hyperplasia: Value of the calcium intraarterial stimulation test when findings of other preoperative studies are negative. Radiology 206:703–709
Brandle M, Pfammatter T, Spinas GA, et al. (2001) Assessment of selective arterial calcium stimulation and hepatic venous sampling to localise insulin-secreting tumours. Clin Endocrinol 55:357–362
Wiesli P, Brandle M, Schmid C, et al. (2004) Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinaemic hypoglycaemia: potential and Limitations. J Vasc Interv Radiol 15:1251–1256
Doppman Jl, Miller DL, Chang R, et al. (1990) Gastrinomas: localisation by means of selective intraarterial injection of secretin. Radiology 174(1):25–29
Thom Ak, Norton JA, Doppman JL, et al. (1992) Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery 112:1002–1009
Rosata FE, Bonn J, Shapiro M, et al. (1990) Selective arterial stimulation of secretin in localization of gastrinomas. Surg Gynecol Obstet 171:196–200
Cohen MS, Picus D, Lairmore TC, et al. (1997) Prospective study of provocative angiograms to localize functional islet cell tumours of the pancreas. Surgery 122:1091–1100
Turner JJ, Wren AM, Jackson JE, et al. (2002) Localisation of gastrinomas by selective intra-arterial calcium injection. Clin Endocrinol (Oxf) 57:821–825
Young WF (2003) Minireview: primary aldosteronism-changing concepts in diagnosis and treatment. Endocrinology 144(6):2208–2213
Biglieri EG, Irony I, Kater CE, et al. (1989) Identification and implication of new types of mineralocorticoid hypertension. J Steroid Biochem 32:199–204
Irony I, Kater CE, Biglieri EG, et al. (1989) Correctable subsets of primary aldosteronism: primary adrenal hyperplasia and renin responsive adenoma. Am J Hypertension 3:576–582
Young WF, Stanson AW, Grant CS, et al. (1996) Primary aldosteronism: adrenal venous sampling. Surgery 120:913–920
Sawka AM, Young WF, Thompson GB, et al. (2001) Primary aldosteronism: factors associated with normalisation of blood pressure after surgery. Ann Intern Med 135:258–261
Celen O, O’Brien MJ, Melby JC, et al. (1996) Factors influencing outcome of surgery for primary aldosteronism. Arch Surg 131:646–650
Fontes RG, Kater CE, Biglieri EG, et al. (1991) Reassessment of predictive value of the postural stimulation test in primary aldosteronism. Am J Hypertens 4:786–791
Ganguly A, Dowdy AJ, Luetscher JA, et al. (1973) Anomalous postural response of plasma aldosterone concentration in patients with aldosterone-producing adrenal adenoma. J Clin Endocrinol Metab 36:401–404
Feltynowski T, Ignatowska-Switalska H, Wocial B, et al. (1994) Postural stimulation test in patients with aldosterone-producing adenomas. Clin Endocrinol 41:309–414
Espiner EA, Ross DG, Yandle TG, et al. (2003) Predicting surgically remedial primary aldosteronism: role of adrenal scanning, posture testing, and adrenal vein sampling. J Clin Endocrinol Metab 88:3637–3644
Harper R, Ferrett CG, McKnight JA, et al. (1999) Accuracy of CT scanning and adrenal vein sampling in the pre-operative localisation of aldosterone-secreting adrenal adenomas. Q J Med 92:643–650
Phillips JL, Walther MM, Pezzullo JC, et al. (2000) Predictive value of preoperative tests in discriminating bilateral adrenal hyperplasia from an aldosterone-producing adrenal adenoma. J Clin Endocrinol Metab 85:4526–4533
Doppman JL, Gill JR (1996) Hyperaldosteronism: sampling the adrenal veins. Radiology 198:309–312
Doppman JL, Gill JR, Miller DL, et al. (1992) Distinction between hyperaldosteronism due to bilateral hyperplasia and unilateral aldosteronoma: reliability of CT. Radiology 184:677–682
Magill SB, Raff H, Shaker JL, et al. (2001) Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. J Clin Endocrinol Metab 86:1066–1071
Young WF, Stanson AW, Thompson GB, et al. (2004) Role for adrenal venous sampling in primary aldosteronism. Surgery 136:1227–1235
Sheaves R, Golding J, Reznek RH, et al. (1996) Relative value of computed tomography scanning and venous sampling in establishing the cause of primary hyperaldosteronism. Eur J Endocrinol 134:308–313
Kloos RT, Gross MD, Francis IR, et al. (1995) Incidentally discovered adrenal masses. Endocr Rev 16:460–484
Young WF, Klee GG (1998) Primary aldosteronism: diagnostic evaluation. Endocrinol Metab Clin North Am 17:367–395
Ilias I, Pacak K (2004) Clinical problem solving: current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. J Clin Endocrinol Metab 89(2):479–491
Newbould EC, Ross GA, Dacie JE, et al. (1991) The use of venous catheterization in the diagnosis and localization of bilateral phaeochromocytomas. Clin Endocrinol (Oxf) 35(1):55–59
Pacak K, Goldstein DS, Doppman JL, et al. (2001) A “pheo” lurks: novel approaches for locating occult pheochromocytoma. J Clin Endocrinol Metab 86(8):3641–3646
Chew SL, Dacie JE, Reznek RH, et al. (1994) Bilateral phaeochromocytomas in von Hippel-Lindau disease: diagnosis by adrenal vein sampling and catecholamine assay. Q J Med 87(1):49–54
DiSaia PJ, Creasman WT. (1997) Germ cell, stromal and other ovarian tumors. In: Clinical gynecologic oncology. Mosby-Yearbook, New York, p 351
Kaltsas GA, Isidori AM, Kola BP, et al. (2003) The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab 88(6):2634–2643
Reznek RH, Armstrong P (1994) The adrenal gland. Clin Endocrinol 40:561–576
Moltz L, Pickartz H, Sorensen R, et al. (1984) Ovarian and adrenal vein steroids in seven patients with androgen-secreting ovarian neoplasms: selective catheterization findings. Fertil Steril 42(4):585–593
Surrey ES, De Zielger D, Cambone JC, et al. (1988) Preoperative localization of androgen-secreting tumours: clinical, endocrinologic and radiologic evaluation of ten patients. Am J Gynecol 158:1313–1322
Bricaire C, Raynaud A, Benotmane A, et al. (1991) Selective venous catheterization in the evaluation of hyperandrogenism. J Endocrinol Invest 14(11):949–956
Kaltsas GA, Mukherjee JJ, Kola BP, et al. (2003) Is ovarian and adrenal venous catheterization and sampling helpful in the investigation of hyperandrogenic women? Clin Endocrinol 59:34–43
Sorensen R, Moltz L, Schwartz U (1986) Technical difficulties of selective venous blood sampling in the differential diagnosis of female hyperandrogenism. Cardiovasc Interv Radiol 9:75–82
Moltz L, Schwartz U, Sorensen R, et al. (1984) Ovarian and adrenal vein steroids in patients with nonneoplastic hyperandrogenism: selective catheterization findings. Fertil Steril 42:69–75
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lau, J.H.G., Drake, W. & Matson, M. The Current Role of Venous Sampling in the Localization of Endocrine Disease. Cardiovasc Intervent Radiol 30, 555–570 (2007). https://doi.org/10.1007/s00270-007-9028-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-007-9028-3