Abstract
Introduction
Parathyroid venous sampling (PVS) has been reported to be a useful adjunctive test in localizing lesions in elusive cases of primary hyperparathyroidism (PHPT). Conventional cutoff (twofold) is now widely being used, but optimal cutoff threshold for PVS gradient based on discriminatory performance remains unclear.
Materials and methods
Among a total of 197 consecutive patients (mean age 58.2 years, female 74.6%) with PHPT who underwent parathyroidectomy at a tertiary center between 2012 and 2018, we retrospectively analyzed 59 subjects who underwent PVS for persistent or recurrent disease after previous parathyroidectomy, or for equivocal or negative results from conventional imaging modalities including ultrasonography (US) and Tc99m-Sestamibi SPECT-CT (MIBI). True parathyroid lesions were confirmed by combination of surgical, pathological findings, and intraoperative parathyroid hormone (PTH) changes. Optimal PVS cutoff were determined by receiver-operating characteristics (ROC) analysis with Youden and Liu method.
Results
Compared to subjects who did not require PVS, PVS group tends to have lower PTH (119.8 pg/mL vs 133.7 pg/mL, p = 0.075). A total of 79 culprit parathyroid lesions (left 40; right 39) from 59 patients (left 24; right 26; bilateral 9) were confirmed by surgery. The optimal cutoff for PVS gradient was estimated as 1.5-fold gradient (1.5 ×) with sensitivity of 61.8% and specificity of 84%. When 1.5 × cutoff was applied, PVS improved the discrimination for true parathyroid lesions substantially based on area under ROC (0.892 to 0.942, p < 0.001) when added to US and MIBI.
Conclusion
Our findings suggest that PVS with cutoff threshold 1.5 × can provide useful complementary information for pre-operative localization in selected cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical resection is the only curative treatment in patients with primary hyperparathyroidism (PHPT), a common endocrine disorder characterized as hypercalcemia with elevated parathyroid hormone (PTH) level [1,2,3]. However, 5–10% of patients suffer from persistent or recurrent post-operative hypercalcemia [4, 5]. Besides, increase in incidence and prevalence of thyroid cancer in Korea has led to an increase in life-long chance of neck surgery [6]. Correspondingly, sparing favorable surgical field in neck area by performing minimally invasive parathyroidectomy is important in reducing cost, operation time, and complication for both current and future operations [7]. As a result, precise pre-operative localization of the PHPT lesions is pivotal in deliberating optimal intra- and post-operative management.
Conventional pre-operative localization is based on the concordance in non-invasive tests including ultrasonography (US) and 99mTc-sestamibi SPECT/CT (MIBI) [2]. However, US often have limited visualization which is affected by spatial orientation of lesions, while in multiglandular disease, gland size, cell type, and patients’ biochemical status affect MIBI in finding of causative lesions [8]. In this context, parathyroid venous sampling (PVS) is reported to provide useful information in cases with equivocal and negative findings.[9] Positive PVS finding was conventionally defined as twofold (2.0 ×) increase in intact PTH compared with peripheral level [1, 2, 10]. However, the optimal cutoff for PVS gradient remains unclear, while studies regarding PVS cutoff adjustment are relatively scarce and limited with anecdotal cases [7, 11]. In Jones et al. (n = 64), sensitivity and positive predictive value (PPV) in 2.0 × cutoff was 75% and 86%, respectively, while alternative cutoffs were proposed in Ogilvie et al. (n = 27) where PPV at 1.5 × , 2.5 × , 3.0 × cutoff were 72%, 75%, and 83%, respectively, demonstrating that optimal cutoff has not yet been statistically validated and remained arbitrary [7, 12].
In this retrospective longitudinal study, we aim to determine the optimal cutoff for the positive gradient in PVS and evaluate its discriminatory diagnostic performance in comparison with conventional PVS cutoff and imaging techniques.
Materials and methods
Data source
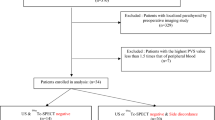
We reviewed a retrospective cohort of all patients with diagnosis of PHPT who underwent parathyroidectomy at Severance Hospital in Seoul, Korea, between 2012 and 2018 (Fig. 1). This study was approved by the Institutional Review Board (IRB) of Severance Hospital (4-2018-0050). The IRB waived the requirement for obtaining informed consent based on study design of retrospective medical record review. Clinical Data Repository System, Electronic Medical Record system, and Picture Archiving and Communication System of Severance Hospital were used to retrieve the clinical and imaging data. All data were analyzed as anonymized.
Study subjects
A total of 197 patients were diagnosed with PHPT and underwent parathyroidectomy at Severance Hospital, Korea, between 2012 and 2018. Diagnosis and surgical treatment for PHPT was performed based on the guidelines from Endocrine Society [2, 3]. PVS was performed in 59 cases of persistent or recurrent disease after previous parathyroid surgery (n = 2), multiple glands disease (n = 12), elusive (n = 8), or non-concordant results (n = 37) in conventional imaging studies, whereas PVS was not needed in 138 subjects (Fig. 1). There were no apparent familial multiple endocrine neoplasia (MEN1) cases in the study population.
Imaging modalities
US
Ultrasound scans of parathyroid glands with both longitudinal and transverse planes were detected using ultrasound system device (iU22 xMatrix, Philips Medical Systems, Best, The Netherlands) with 5–12 MHz linear transducer by experienced radiologists. Images were acquired from posterior or inferior region of thyroid and further extended from hyoid bone and thoracic inlet for potential ectopic or multiple glands, as appropriate.
MIBI
After injection of 740 ± 20 MBq of 99mTc-SestaMIBI, early and delayed parathyroid scan was obtained after 10 min and 2 h after injection, respectively, using 99mTc-sestamibi SPECT/CT scan (Symbia TruePoint SPECT/CT, Siemens Healthcare, Knoxville, TN, USA). At 2 h, SPECT imaging was performed based on standard protocol and reconstructed into axial, sagittal, and coronal planes and acquired along with low-dose, non-contrast-enhanced CT scan. Positive parathyroid lesions were defined as focal delayed washout of radiotracers with higher uptake than background, thyroid parenchyma, on the delayed SPECT/CT images. Two experienced nuclear medicine physicians reviewed all planar and SPECT/CT images individually on Syngo workstation (Siemens, VA60A software) without informed patients’ clinical background. All discrepancies in opinion were settled by consensus.
PVS
Two dedicated radiologists with more than 10 years of experiences performed the procedure. After local anesthesia with 1% lidocaine, venous access was acquired via the right femoral vein using Seldinger method with the insertion of four-French sheath, four-French nontapered catheter, and 035 Terumo guidewire. Blood samples were collected from eight predefined venous locations at upper (above cervical vertebrae, C3 level), middle (between C3 and C5 level), and lower (between C5 and C7 level) bilateral internal jugular vein (IJV) and from bilateral brachiocephalic vein (below C7 level at convergence of IJV and subclavian vein), respectively. (Supplementary Fig. 1) Peripheral blood sample was retrieved directly from femoral vein sheath. All blood samples were collected in 5 mL serum-separating tubes (SST) and immediately placed in ice before transfer to laboratory for PTH analysis. Increase in fold of PTH gradient at respective sites with regard to peripheral PTH level was calculated. Informed consents were obtained before procedure for all subjects. Hemostasis of the puncture site was achieved by manual compression. No complications including hematoma, bleeding, pain, or delay of hospital discharge reported for all cases. For lateralization, maximum value of PTH gradient was defined as PTHmax value at left side and right side, respectively (Supplementary Fig. 2).
Surgical exploration
We decided surgical approach based on multidisciplinary discussion between surgeons, endocrinologists, and radiologists. In case of inconclusive evidences in non-invasive preoperative localization (e.g., MIBI: left lesion suspected but confounded by diffuse uptake; US: not revealing any lesion on both sides), even slight unilateral PTHmax elevation below 2.0 × threshold at corresponding area was deemed potentially significant to decide focused surgery (when consistent with any results from imaging modalities) or unilateral exploration (usually elusive cases in imaging modalities). Successful removal of hyperfunctioning gland was defined as a 50% or higher decline of intraoperative PTH compared to highest pre-incisional PTH level [13]. If bilateral, multiglandular disease was suspected or no suspicious lesions were detected in preoperative imaging without any gradient differences of PTHmax between bilateral lesions and without PTHmax above 2.0 × fold in PVS, Surgical procedure was performed using two-step approach to avoid unnecessary bilateral exploration. First, unilateral neck exploration was planned at the side with highest PTHmax value. Intraoperative PTH monitoring was performed after removal of suspicious gland to determine successful exploration. When successful removal of culprit lesions was not supported by intraoperative PTH monitoring, bilateral exploration was performed.
Adjudication of presence of culprit parathyroid lesions by side
Successful preoperative localization was defined based on correct preoperative lateralization for culprit lesion. In every individual, cervical region was divided into left side and right side based on the midline of the neck. The presence of culprit parathyroid lesions at each side was adjudicated as ‘positive’ or ‘negative’ by two expert endocrinologists (NH, YR) based on surgical findings (the “gold standard”), more than 50% intraoperative PTH declination, postoperative biochemical normalization, pathology findings, and imaging study results according to the Guideline of the 4th International Workshop [2].
Laboratory measurements
Blood samples were drawn at first visit or second visit of subjects under overnight fasting status before initiation of any intervention to assess biochemical parameters including serum calcium, phosphate, creatinine, serum total 25-hydroxyvitamin D [25(OH)D], 1,25-dihydroxycholecalciferol [1,25(OH)2D3], and intact PTH levels. Serum intact PTH level was determined using the ElectroChemiLuminescence immunoassay (ECLIA; Cobas® e602 analyzer; Roche Diagnostics GmbH, Mannheim, Germany) (intra-assay CV 1.8% or less, and inter-assay CV 3.3% or less). Serum 25(OH)D was measured using radioimmunoassay (RIA; INCSTAR Corp., Stillwater, MN, USA; intra-assay CV 4.1% or less and inter-assay CV 7.0% or less). Serum 1,25(OH)2D3 level was determined via RIA (DIAsource ImmunoAssays S.A., Louvain-La-Neuve, Belgium; intra-assay CV 6.8–7.4%; and inter-assay CV 11.7–12.7%). Calcium, phosphate, creatinine levels, and 24-h urine collection at baseline were assayed using standard automated laboratory techniques. Estimated glomerular filtration rate (eGFR) was calculated using Chronic Kidney Disease Epidemiology Collaboration equation [14]. History of nephrolithiasis was defined based on Urolithiasis Guidelines of European Association of Urology (EAU) with use of non-contrast-enhanced computed tomography or defined by the retrospective review of medical record of subjects whom underwent extracorporeal shock wave lithotripsy [15]. Two dedicated pathologists reviewed the surgical specimen and assessed the content of chief cell and oxyphil cell of the hematoxylin–eosin stained slides of each culprit lesions.
Statistical analysis
Continuous variables were presented as mean ± standard deviation and median (interquartile range, IQR), while categorical variables are presented as numbers and percentages. To compare baseline characteristics between subjects who require PVS and those not needed, independent t test, Mann–Whitney U test, and Chi-square test were used to compare continuous and categorical variable correspondingly. Perioperative changes were compared using paired t test and Wilcoxon signed-rank test. Surgical confirmation of presence of culprit parathyroid lesions at each side (left or right; Supplementary Table 1) was listed in categorical variable as gold standard (positive or negative), while PTHmax to corresponding sides (left or right) were analyzed as continuous variable. The unit of diagnostic performance analysis was each lesion based on lateralization (two lesions per individual; left and right). To evaluate the discriminatory performance of PVS, the receiver-operating characteristics (ROC) analysis of respective variables were performed and area under the ROC curve (AUC) was calculated based on a total of 118 lesions [two-sided lesions (left and right) from 59 individuals]. All lateralized lesions had corresponding PTHmax value, PVS results depending on PTHmax value and pre-specified PVS threshold (PTHmax value above the threshold: positive; below the threshold: negative), and information of presence of culprit parathyroid lesions (presence of culprit lesion: positive; no existence of culprit lesion: negative) confirmed by surgery. (Supplementary Fig. 2) Optimal cutoff for PTHmax were selected based on threshold maximizing Youden (= sensitivity + specificity − 1) and Liu Index (= sensitivity × specificity) [16, 17]. Corresponding lesion-based sensitivity, specificity, and positive and negative predictive value (PPV and NPV) of PTHmax cutoff were calculated as follows: sensitivity = true positive (TP)/[TP + false negative (FN)]; specificity = true negative (TN)/[TN + false positive (FP)]; positive predictive value (PPV) = TP/(TP + FP); negative predictive value (NPV) = TN/(TN + FN). Additive diagnostic value of PVS to the conventional imaging modalities was assessed by comparison of AUC based on DeLong et al. [18]. We classified the results of US or MIBI as categorical variables (positive or negative) based on lateralized lesions. AUC was calculated using model-predicted probability of positive outcome (presence of culprit lesion) from logistic regression model with US and MIBI as binary independent variables using ‘predict’ and ‘lroc’ command in Stata. Each logistic predicted probability can be a possible cutoff point for classifying lesions [19]. ‘roccomp’ command was used to compare AUC between two models with or without PVS. Category-free net reclassification improvement (cfNRI = net proportion of culprit lesions reassigned to positive results + of non-culprit lesions reassigned to negative results) was calculated to examine additive discriminatory value of new PVS cutoff over conventional cutoff (2.0 ×) [20]. cfNRI > 0.01 indicate meaningful improvement of discrimination, with cfNRI 0.2–0.6 as moderate improvement and 0.6 or greater as strong contribution. All p values given are two-sided and p < 0.05 were considered significant. All statistical analyses were performed using STATA 14.2 S.E. (Stata Corp, College Station, TX, USA) [19].
Results
Baseline clinical characteristics
A total of 197 subjects with mean age of 58.2 ± 13.1 years and p redominant portion of female (n = 147/197, 74.6%) were analyzed. Subjects who underwent PVS tend to have lower median PTH level (119.8 pg/mL vs 133.7 pg/mL, p = 0.075). However, no additional significant differences in baseline characteristics including age, gender, serum corrected calcium, 25(OH)D, and bone mineral density were observed between subject groups with or without PVS (Table 1).
Surgical findings and pathology
Among a total of 118 lesions, 79 culprit parathyroid lesions (left 40 vs right 39) were confirmed by surgery from 59 patients (left vs right vs bilateral, 24 vs 26 vs 9) who underwent PVS. Majority of subjects contained a single culprit gland, but 11 patients showed multi-glandular feature at unilateral side (left 7 vs right 4) and 9 patients at bilateral side. All subjects showed more than 50% decrease in intra-operative PTH [− 83.5% (− 74.5 to − 88.9); supplementary Table 2], and post-operative serum calcium and PTH level were significantly reduced and normalized (calcium, 10.7 mg/dL to 8.6 mg/dL, p < 0.001; PTH, 167.1 pg/mL to 25.1 pg/mL, p < 0.001). Pathological diagnosis reported chief-cell dominance in surgical specimen (chief cell: oxyphil cell, 95%: 5%; supplementary Table 1).
Optimal PVS cutoff point
In ROC analysis, PTHmax at each left side and right side showed modest discrimination for the presence of culprit parathyroid lesions (AUC, 0.772; 95% CI 0.691–0.853). The optimal threshold for PTHmax was determined at 1.5-fold gradient (1.5 ×) based on both Youden and Liu methods. (Fig. 2) The sensitivity and specificity was 61.8% (95% CI 49.2–73.3) and 84.0% (95% CI 70.9–92.8), respectively. Compared with 2.0 × cutoff, applying 1.5 × improved the sensitivity (45.6–61.8%), while the specificity showed marginal decrease (92–84%). Besides, using 1.5 × cutoff, additional 14 lesions (37 vs 51) including 3 cases with unilateral multiglandular disease were further identified.
Discriminatory diagnostic value of 1.5 × cutoff in PVS in lateralization of parathyroid lesion
Conventional imaging modalities, US and MIBI, in parathyroid pre-operative localization have sensitivity and specificity of 57.4% and 88.0%, and 84.4% and 91.3%. Using 1.5 × cutoff, PVS significantly improved the discriminatory diagnostic performance when added to the combination of conventional diagnostic modality, US and MIBI [Fig. 3; AUC 0.892–0.942, p = 0.004; cfNRI 0.87 (0.49–1.25), p < 0.001]. Moreover, discriminative performance of 1.5 × cutoff was further enhanced when compared to 2.0 × cutoff [cfNRI 0.892 (0.51–1.27), p < 0.001].
Notable cases
Among a total 42 selected cases with culprit lesions (n = 51) correctly adjudicated by PVS using 1.5 × cutoff, seven cases still showed negative or equivocal results, particularly for chief-cell dominant lesions, and 20 cases were multiglandular diseases.
Two notable cases were identified. In case 1, a 67-year-old female with osteopenia and PHPT had negative US and equivocal MIBI findings due to diffuse uptake of thyroid gland by underlying Graves disease. PVS showed 1.5-fold increase at left inferior region and have not reached 2.0 × cutoff (Supplementary Fig. 3a). Neck exploration adjudicated parathyroid adenoma at left inferior pole of thyroid, which corresponded to PVS findings, and attained 89.1% decrease in intra-operative PTH level. Calcium and PTH levels were normalized from post-operative day 1 (calcium 11.3 to 8.9 mg/dL; PTH 139.4 to 19.5 pg/mL).
In case 2, a 76-year-old female with osteoporosis and PHPT had negative findings in conventional imaging. PVS showed 1.8-fold and 1.6-fold increase at left upper and lower pole of thyroid, respectively (Supplementary Fig. 3b). Surgery localized grossly enlarged parathyroid glands at left superior portion and left thymus, and diagnosed as parathyroid adenomas (chief cell 100%) by pathology. Intra-operative PTH decreased by 87.7% 15 min after parathyroidectomy, while post-operative calcium and PTH levels have decreased to normal range (calcium 12.5 to 9.4 mg/dL; PTH 165.4 to 6.8 pg/mL).
Discussion
In this retrospective cohort study, we sought to determine optimal cutoff of PTH gradient in PVS for the pre-operative localization of selected PHPT cases with elusive or discordant findings in the conventional imaging modalities. Based on unbiased statistical approach, 1.5 × was selected as an optimal cutoff with modest discriminatory performance. PVS with 1.5 × cutoff significantly improved discriminative performance for culprit parathyroid lesions when added to US and MIBI in these elusive, discordant cases (AUC 0.892 to 0.942, p = 0.004).
Previous studies reported that PVS could provide useful information for localization of lesions in selected indications, mainly in re-operation after prior neck surgery or known MEN1, familial hyperparathyroidism [7,8,9, 12, 21,22,23,24]. Surgical intervention currently being the only curative treatment of PHPT, PVS might provide further preoperative discrimination for lesions, as shown in notable cases 1 and 2, to minimize blind neck exploration and to determine multiplicity of lesions [1, 7, 24, 25]. However, studies regarding the PVS cutoff point were relatively limited. Currently 2.0 × cutoff is used as the standard threshold with sensitivity ranged from 75 to 96% [7, 9, 11, 20]. (Table 2) Nevertheless, several cutoffs, ranged from 1.4 × to 2.0 × , were considered in previous studies for optimal detection of the PHPT lesions but chosen arbitrarily (Table 2). For instance, Jones et al. proposed 2.0 × cutoff with sensitivity of 75%, while Ogilvie et al. proposed 1.5 × , 2.5 × and 3.0 × cutoffs with PPV of 72%, 75%, and 83%, respectively [7, 12]. Above all, Doppman et al., of note, suggested 1.4 × cutoff after thorough equilibration between maximization of diagnostic accuracy and minimization of equivocal findings of PVS [25]. In line with this study, despite the process in determination of cutoff could be arbitrary, our study aims to determine the optimal cutoff using unbiased statistical approach and suggested 1.5 × as the optimal cutoff based on following reasons [26]. First, we derived 1.5 × cutoff using Youden and Liu index on ROC curve, which may have stronger objectiveness as a data-driven approach. Second, applying 1.5 × cutoff significantly improved discrimination for culprit lesion compared to the conventional 2.0 × cutoff, as quantified by cfNRI. Third, compared with 2.0 × cutoff, applying 1.5 × improved the sensitivity (45.6% to 61.8%), while the specificity showed marginal decrease (92–84%). Our findings suggest that PVS 1.5 × might play as a better, sensitive threshold for correct lateralization of parathyroid lesions than conventional 2.0 × .
According to the guideline for management of PHPT by 4th International Workshop, the first-line imaging modalities for causative parathyroid localization are ultrasonography and MIBI [2]. Additionally, in this study, US and MIBI showed fairly good discrimination when used simultaneously (AUC 0.892) and justified the routine use of US and MIBI as the first-line pre-operative localization [2]. US with strong advantage of easy accessibility with no radiation hazard is widely used in diverse clinical field [8]. In Cheung et al., pooled sensitivity and PPV were 76.1% and 93.2% demonstrating US with moderate degree of diagnostic accuracy in localization of treatment naïve PHPT patients [27]. However, several pitfalls including lesions with small size and difficulties in differential diagnosis with cervical lymph node were reported [15]. Furthermore, due to the operator-dependent and tester-subjective nature of US, the sensitivity of US was lower in persistent/recurrent PHPT patients compared to treatment naïve patients (sensitivity 23–46% vs; PPV 43% vs 68.1–93.2%) which was speculated to be caused by the alteration of cervical anatomy after prior operation (Table 3) [28]. In accordance with the previous findings, sensitivity and PPV of US in this study were 57.4% and 86.7% showing relatively moderate discriminatory performance in lesion lateralization. MIBI could localize the culprit PHPT lesions based on the mechanism of accumulation of Technetium (99mTc) sestamibi, the lipophilic cation, in mitochondria abundant oxyphil cell [29]. Furthermore, several studies reported the utility of MIBI outweigh than that of US in diagnostic accuracy of localization of PHPT lesions (Table 3) [9, 27]. However, several limitations as in diffuse uptake in thyroid gland due to underlying thyroiditis or dominance in proportion of chief cell in culprit parathyroid lesion have contributed to the equivocal findings in MIBI [21]. In this study, subjects in 13 out of 42 cases were reported to have thyroiditis. Although US and MIBI were used complementarily, the presence of elusive or equivocal findings in these conventional imaging persists [30]. Elusive yet discrepant imaging findings demonstrated possible pitfall of conventional non-invasive modalities in PHPT localization most likely due to the chief-cell dominance in surgical specimen in the cases 1 and 2. Whereas, in this study, PVS showed additive discriminatory value in these selected cases and demonstrated 1.5 × as the optimal cutoff.
Using 1.5 × cutoff, 51 out of 79 culprit lesions were lateralized with sensitivity of and PPV of 61.8% and 84%, respectively, and were surgically concordant. Despite discriminative performance of PVS, several cases with insufficient increase in PTHmax at culprit parathyroid lesions were identified. In previous studies, PPV of PVS in persistent and recurrent PHPT patients ranged from 66.7 to 100% (Table 3). The underlying pitfall of PVS might be caused by the distortion of neck anatomy which leads to the variation of venous drainage pattern and result in false-positive increase in PTH gradient [28]. Besides, we speculate in studies using non-selective venous sampling protocol, where blood samples drawn from large vessel, IJV and BCV, and not collected super-selectively, and possible contamination of peripheral blood sample might have attributed to an insufficient increase in PTHmax at the site of culprit parathyroid lesions. Also, the influence of size of causative gland in PTH secretion cannot be neglected [21]. Nonetheless, robust enhancement of AUC with addition of PVS with 1.5 × cutoff suggested that the PVS could be considered as reliable method for localization with negative and equivocal non-invasive imaging modalities. In this context, our study provided complementary, quantifiable data and summary of individual cases which can form the basis of the discussion, although it needs to be further validated in prospective studies.
This study has several limitations. As our study is limited by retrospective nature, we could not completely rule out residual confounding. The cutoffs for PVS and diagnostic performance reported in this study could be influenced by selection bias inherent to the retrospective design, which need to be validated in further prospective studies. Most of our subjects did not undergo prior parathyroidectomy, which also limit the generalizability of cutoff derived from this population. The external validity of our findings need to be evaluated in well-designed, future prospective studies. Nevertheless, our study suggested potential utility of 1.5 × cutoff for PVS and improved diagnostic performance by PVS in patients with primary hyperparathyroidism and elusive focus, which may help to enrich diagnostic modalities for localizing culprit lesion in elusive PHPT with or without prior neck surgery. Because PVS is a technique-dependent procedure, our findings may not be generalizable to institutions with different volume of PHPT cases. We acknowledge that the prevalence of multiglandular disease in our study is at the higher end of reported range of 8.0–34.3% in previous literatures about sporadic multiglandular diseases in PHPT [31]. Previous studies showed that patients with elusive or inconsistent preoperative study results had high risk of multiglandular diseases [31]. These findings align with the relatively higher prevalence of multiglandular diseases in our cohort. Although genetic testing performed in 15 individuals with clinical suspicion of multiple endocrine neoplasia syndrome did not reveal any mutation in MEN1 gene, possibility for underlying genetic susceptibility for multiglandular diseases in this cohort cannot be excluded, because not all subjects with multiglandular diseases underwent genetic testing. Our results need to be interpreted in the context of potential confounding by mixture of hereditary PHPT with sporadic form, which requires further investigation on the clinical relevance of PVS in hereditary PHPT in prospective studies with genetic confirmation. Although PVS is a minimally invasive localization procedure, we did not observe any complications in our subjects which might be due to the usage of 4-French sized catheter throughout the procedure [8] Besides, PVS protocol in our institute was standardized based on the level of cervical spine, while blood samples were acquired from large vessel, IJV, and BCV, and not collected super-selectively, we expect lower operator-dependent than general cases. The median duration of PVS is about 20 min (IQR 15–24), and radiation exposure ranges from 1.23 to 5.8 mSv, which is consistent with previous study (0.8–19.6 mSv).[32] Although PVS confers the risk of radiation exposure, the radiation dosage of our protocol was half of 4-dimensional CT (10.4 mSv) and lower than MIBI (6.7–7.8 mSv) [32, 33]. Nonetheless, even though the degree of radiation exposure is not high, risk and benefit of the procedure need to be carefully weighted and considered according to the individual cases. For economical aspect, cost–benefit of PVS can be substantially different between countries according to medical financial system and reimbursement policy. Kunstman et al. illustrated total cost of cervical US and MIBI in the United States to be $671.86 (US $125.10 and MIBI $546.76) per patient based on Medicare reimbursement [8]. Contrastingly, in Korea, due to national insurance coverage, approximated medical expense for PVS is about $300.[34] Therefore, cost-effectiveness of the PVS as localization study needs to be determined based on the context of the cost of each country. Although interpretation of true positive results is variable and study-dependent among correct laterality or quadrant of location, we followed laterality method, because correct laterality determined by preoperative localization studies can have clinical relevance in deciding surgical procedures. However, direct comparison of diagnostic performance of PVS in this cohort can be limited with studies with different localization methods (e.g., quadrant analysis) where external validation is needed.
Our results need to be interpreted with caution. First, in clinical practice, increase in sensitivity would be mediated by considering the other localization studies. The difference in the risk of false negative operations in practice would not be the same extent as the difference in sensitivity (16.2%), because findings from imaging studies would be helpful to avoid missing responsible lesions even with 2.0 × cutoff value. In addition, lowering threshold for positive lesion might lead to increase the chance of unnecessary neck exploration. In our study, the false-positive rate (1-specificity) increased from 8 to 16%, which need to be debated whether this increase would be negligible or not in further prospective studies.
In conclusion, our findings suggest that applying 1.5 × cutoff in interpretation of PVS results might improve diagnostic performance of this adjunct test in PHPT localization as an adjunct test, particularly in cases with elusive or equivocal results by the conventional modalities, which merits validation in further prospective studies.
References
Bilezikian JP, Bandeira L, Khan A, Cusano NE (2017) Hyperparathyroidism. Lancet. https://doi.org/10.1016/S0140-6736(17)31430-7
Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JJT (2014) Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab 99:3561–3569. https://doi.org/10.1210/jc.2014-1413
Eastell R, Brandi ML, Costa AG, D'Amour P, Shoback DM, Thakker RV (2014) Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab 99:3570–79. https://doi.org/10.1210/jc.2014-1414
Khan AA, Hanley DA, Rizzoli R, Bollerslev J, Young JEM et al (2017) Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int 28:1–19. https://doi.org/10.1007/s00198-016-3716-2
Rubin MR, Bilezikian JP, McMahon DJ, Jacobs T, Shane E, Siris E, Udesky J, Silverberg SJ (2008) The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab 93:3462–3470. https://doi.org/10.1210/jc.2007-1215
Jung KW, Won YJ, Kong HJ, Lee ES (2018) Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015 (in eng). Cancer Res Treatm 50:303–316. https://doi.org/10.4143/crt.2018.143
Ogilvie CM, Brown PL, Matson M, Dacie J, Reznek RH, Britton K, Carpenter R, Berney D, Drake WM, Jenkins PJ, Chew SL, Monson JP (2006) Selective parathyroid venous sampling in patients with complicated hyperparathyroidism (in eng). Eur J Endocrinol 155:813–821. https://doi.org/10.1530/eje.1.02304
Kunstman JW, Kirsch JD, Mahajan A, Udelsman R (2013) Clinical review: parathyroid localization and implications for clinical management. J Clin Endocrinol Metab 98:902–912. https://doi.org/10.1210/jc.2012-3168
Ibraheem K, Toraih EA, Haddad AB, Farag M, Randolph GW, Kandil E (2018) Selective parathyroid venous sampling in primary hyperparathyroidism: A systematic review and meta-analysis. Laryngoscope. https://doi.org/10.1002/lary.27213
Taslakian B, Trerotola SO, Sacks B, Oklu R, Deipolyi A (2017) The essentials of parathyroid hormone venous sampling (in eng). Cardiovasc Intervent Radiol 40:9–21. https://doi.org/10.1007/s00270-016-1481-4
Morris LF, Loh C, Ro K, Wiseman JE, Gomes AS, Asandra A, Wariri S, Yeh MW (2012) Non–super-selective venous sampling for persistent hyperparathyroidism using a systemic hypocalcemic challenge. J Vasc Interv Radiol 23:1191–1199. https://doi.org/10.1016/j.jvir.2012.06.005
Jones JJ, Brunaud L, Dowd CF, Duh QY, Morita E, Clark OH (2002) Accuracy of selective venous sampling for intact parathyroid hormone in difficult patients with recurrent or persistent hyperparathyroidism (in eng). Surgery 132:944–950. https://doi.org/10.1067/msy.2002.128477
Gauger PG, Agarwal G, England BG, Delbridge LW, Matz KA, Wilkinson M, Robinson BG, Thompson NW (2001) Intraoperative parathyroid hormone monitoring fails to detect double parathyroid adenomas: a 2-institution experience. Surgery 130:1005–1010. https://doi.org/10.1067/msy.2001.118385
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Gambaro G, Croppi E, Coe F, Lingeman J, Moe O et al (2016) Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: a consensus statement. J Nephrol 29:715–734. https://doi.org/10.1007/s40620-016-0329-y
Liu X (2012) Classification accuracy and cut point selection (in eng). Stat Med 31:2676–2686. https://doi.org/10.1002/sim.4509
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach (in eng). Biometrics 44:837–845
Cleves MA (2002) From the help desk: comparing areas under receiver operating characteristic curves from two or more probit or logit models. Stata J 2:301–313. https://doi.org/10.1177/1536867x0200200307
Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond (in eng). Stat Med 27:157–172. https://doi.org/10.1002/sim.2929
Yamada T, Ikuno M, Shinjo Y, Hiroishi A, Matsushita S, Morimoto T, Kumano R, Yagihashi K, Katabami T (2017) Selective venous sampling for primary hyperparathyroidism: how to perform an examination and interpret the results with reference to thyroid vein anatomy. Jpn J Radiol 35:409–416. https://doi.org/10.1007/s11604-017-0658-3
Udelsman R, Åkerström G, Biagini C, Duh Q-Y, Miccoli P, Niederle B, Tonelli F (2014) The surgical management of asymptomatic primary hyperparathyroidism: proceedings of the fourth international workshop. J Clin Endocrinol Metab 99:3595–606. https://doi.org/10.1210/jc.2014-2000
Reidel MA, Schilling T, Graf S, Hinz U, Nawroth P, Buchler MW, Weber T (2006) Localization of hyperfunctioning parathyroid glands by selective venous sampling in reoperation for primary or secondary hyperparathyroidism (in eng). Surgery 140:907–913. https://doi.org/10.1016/j.surg.2006.06.037
Sun PY, Thompson SM, Andrews JC, Wermers RA, McKenzie TJ, Richards ML, Farley DR, Thompson GB (2016) Selective parathyroid hormone venous sampling in patients with persistent or recurrent primary hyperparathyroidism and negative, equivocal or discordant noninvasive imaging (in eng). World J Surg 40:2956–2963. https://doi.org/10.1007/s00268-016-3621-z
Doppman JL, Skarulis MC, Chang R, Alexander HR, Bartlett D, Libutti SK, Marx SJ, Spiegel AM (1998) Hypocalcemic stimulation and nonselective venous sampling for localizing parathyroid adenomas: work in progress (in eng). Radiology 208:145–151. https://doi.org/10.1148/radiology.208.1.9646806
Berrar D, Flach P (2011) Caveats and pitfalls of ROC analysis in clinical microarray research (and how to avoid them). Brief Bioinform 13:83–97. https://doi.org/10.1093/bib/bbr008
Cheung K, Wang TS, Farrokhyar F, Roman SA, Sosa JA (2012) A meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism (in eng). Ann Surg Oncol 19:577–583. https://doi.org/10.1245/s10434-011-1870-5
Witteveen JE, Kievit J, van Erkel AR, Morreau H, Romijn JA, Hamdy NA (2010) The role of selective venous sampling in the management of persistent hyperparathyroidism revisited (in eng). Eur J Endocrinol 163:945–952. https://doi.org/10.1530/eje-10-0654
Lavely WC, Goetze S, Friedman KP, Leal JP, Zhang Z, Garret-Mayer E, Dackiw AP, Tufano RP, Zeiger MA, Ziessman HA (2007) Comparison of SPECT/CT, SPECT, and planar imaging with single- and dual-phase (99m)Tc-sestamibi parathyroid scintigraphy (in eng). J Nucl Med 48:1084–1089. https://doi.org/10.2967/jnumed.107.040428
Eloy JA, Mitty H, Genden EM (2006) Preoperative selective venous sampling for nonlocalizing parathyroid adenomas (in eng). Thyroid 16:787–790. https://doi.org/10.1089/thy.2006.16.787
Barczynski M, Branstrom R, Dionigi G, Mihai R (2015) Sporadic multiple parathyroid gland disease—a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg 400:887–905. https://doi.org/10.1007/s00423-015-1348-1
Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M (2008) Effective doses in radiology and diagnostic nuclear medicine: a catalog (in eng). Radiology 248:254–263. https://doi.org/10.1148/radiol.2481071451
Mahajan A, Starker LF, Ghita M, Udelsman R, Brink JA, Carling T (2012) Parathyroid four-dimensional computed tomography: evaluation of radiation dose exposure during preoperative localization of parathyroid tumors in primary hyperparathyroidism (in eng). World J Surg 36:1335–1339. https://doi.org/10.1007/s00268-011-1365-3
Health Insurance Review and Assessment service of Korea (2019) website. pp 287–288. https://www.hira.or.kr/ebooksc/ebook_535/ebook_535_201904190107289950.pdf
Habibollahi P, Shin B, Shamchi SP, Wachtel H, Fraker DL, Trerotola SO (2018) Eleven-year retrospective report of super-selective venous sampling for the evaluation of recurrent or persistent hyperparathyroidism in 32 patients (in eng). Cardiovasc Intervent Radiol 41:63–72. https://doi.org/10.1007/s00270-017-1757-3
Lebastchi AH, Aruny JE, Donovan PI, Quinn CE, Callender GG, Carling T, Udelsman R (2015) Real-Time Super Selective Venous Sampling in Remedial Parathyroid Surgery (in eng). J Am Coll Surg 220:994–1000. https://doi.org/10.1016/j.jamcollsurg.2015.01.004
Ginsburg M, Christoforidis GA, Zivin SP, Obara P, Wroblewski K, Angelos P, Grogan RH, Kaplan EL (2015) Adenoma localization for recurrent or persistent primary hyperparathyroidism using dynamic four-dimensional CT and venous sampling (in eng). J Vasc Interv Radiol 26:79–86. https://doi.org/10.1016/j.jvir.2014.09.019
Fayet P, Hoeffel C, Fulla Y, Legmann P, Hazebroucq V, Luton JP, Chapuis Y, Richard B, Bonnin A (1997) Technetium-99m sestamibi scintigraphy, magnetic resonance imaging and venous blood sampling in persistent and recurrent hyperparathyroidism (in eng). Br J Radiol 70:459–464. https://doi.org/10.1259/bjr.70.833.9227226
Nilsson BE, Tisell LE, Jansson S, Zackrisson BF, Lindstedt G, Lundberg PA (1994) Parathyroid localization by catheterization of large cervical and mediastinal veins to determine serum concentrations of intact parathyroid hormone (in eng). World J Surg 18:605–610
Sugg SL, Fraker DL, Alexander R, Doppman JL, Miller DL, Chang R, Skarulis MC, Marx SJ, Spiegel AM, Norton JA (1993) Prospective evaluation of selective venous sampling for parathyroid hormone concentration in patients undergoing reoperations for primary hyperparathyroidism (in eng). Surgery 114:1004–1009
Nafisi Moghadam R, Amlelshahbaz AP, Namiranian N, Sobhan-Ardekani M, Emami-Meybodi M, Dehghan A, Rahmanian M, Razavi-Ratki SK (2017) Comparative diagnostic performance of ultrasonography and 99mTc-Sestamibi scintigraphy for parathyroid adenoma in primary hyperparathyroidism; systematic review and meta-analysis. Asian Pac J Cancer Prev 18:3195–3200. https://doi.org/10.22034/APJCP.2017.18.12.3195
Suh YJ, Choi JY, Kim SJ, Chun IK, Yun TJ, Lee KE, Kim JH, Cheon GJ, Youn YK (2015) Comparison of 4D CT, ultrasonography, and 99mTc sestamibi SPECT/CT in localizing single-gland primary hyperparathyroidism (in eng). Otolaryngol Head Neck Surg 152:438–443. https://doi.org/10.1177/0194599814562195
Mortenson MM, Evans DB, Lee JE, Hunter GJ, Shellingerhout D, Vu T, Edeiken BS, Feng L, Perrier ND (2008) Parathyroid exploration in the reoperative neck: improved preoperative localization with 4D-computed tomography (in eng). J Am Coll Surg 206:888–895. https://doi.org/10.1016/j.jamcollsurg.2007.12.044
Acknowledgements
The authors would like to thank Dong-Su Jang, MFA (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his support with the medical illustration. We thank SENTINEL (Severance endocrinology data science platform) team members Doori Cho and Minheui Yoo for supporting literature review and data extraction process.
Funding
This research was funded by Severance Hospital Research fund for Clinical excellence (SHRC C-2019-0032).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Etical approval
This study was approved by the Institutional Review Board (IRB) of Severance Hospital (4-2018-0050).
Informed consent
The IRB waived the requirement for obtaining informed consent based on study design of retrospective medical record review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lee, J., Hong, N., Kim, B.M. et al. Evaluation of an optimal cutoff of parathyroid venous sampling gradient for localizing primary hyperparathyroidism. J Bone Miner Metab 38, 570–580 (2020). https://doi.org/10.1007/s00774-020-01085-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01085-2